磁共振氨基質子轉移成像技術原理和應用
吳仁華
磁共振氨基質子轉移成像技術原理和應用
吳仁華*

本文概述了氨基質子轉移(amide proton transfer, APT)技術與化學交換飽和轉移(chem ical exchange saturation transfer, CEST)技術的關系、APT技術的原理、影響APT技術的諸多因素,特別是在體APT成像需研究核奧氏效應(nuc lear Overhauser effect, NOE)的重要性。闡述了APT技術的應用,主要有pH成像、溫度成像、APT加權像在各器官組織的臨床應用。
氨基質子轉移;化學交換飽和轉移;核奧氏效應;酸堿度成像;溫度成像
Received 17 Feb 2016, Accepted 3 Apr 2016
ACKNOW LEDGM ENTSThis work was part of National Natural Science Foundation of China (No. 81471730).
磁共振氨基質子轉移(am ide proton transfer, APT)成像[1-4]是化學交換飽和轉移(chem ical exchange saturation transfer, CEST)技術[5-6]的一種,CEST技術與磁化傳遞技術[7-8]密切相關,從某種意義來說,CEST技術是特異性預飽和的磁化傳遞技術,而臨床上應用的磁化傳遞成像是非特異性飽和的磁化傳遞技術。盡管CEST成像目前還有許多問題尚待解決,特別是在體CEST成像主要會有直接水飽和(溢出效應)、大分子交換池(磁化傳遞)和核奧氏效應(nuclear overhauser effect, NOE)等的影響[9-10],由于CEST成像具有高空間和時間分辨率的優點[11-13],可檢測蛋白和代謝物中的可交換質子,用于無創傷的酸堿度(pH值)成像[1, 4]、內源性蛋白分子成像[14-15]、代謝物成像[16-17]、細胞標記[18-19]、報告基因的研究[20]等,目前是磁共振成像研究的熱點。
CEST技術中的APT成像在模型、動物和人體層面上有過非常多的研究[9, 11, 14, 16, 21],學術界有過廣泛的討論,目前認為在優化技術的前提下,在人體層面上可以獲得APT加權像[22],APT加權像具有重要的臨床應用價值,特別是應用于腦腫瘤的診斷和鑒別診斷[3, 23-25]、腦卒中的早期診斷[26-27]、神經變性疾病的定性[28-29]、肝糖原檢測[30]等,本文主要綜述APT成像的技術原理和臨床應用價值。
1 氨基質子轉移成像技術原理
1.1 氨基質子的預飽和頻率
APT成像的技術原理也基于CEST技術的原理,只是APT成像的預飽和脈沖頻率是特異性飽和氨基質子。Forsen等[31]在1963年利用雙共振方法開創性地研究了快速化學反應現象。Guivel-Scharen等[32]在1998年研究小分子溶液的磁化傳遞現象時最早觀察到靠近水共振頻率Z譜的不對稱性,為了與既往的磁化傳遞名詞相區分,將這一磁共振現象命名為 CEST。這些學者發現水的氫質子(4.7 ppm)與內源的游離蛋白質和多肽上的酰胺基(-NH)質子(8.3 ppm)存在一個快速的化學交換(氫交換)的過程,而且此過程與環境的pH值相互依賴,這一磁共振現象隨之被命名為氨基質子轉移[13, 33-35]。
1.2 CEST技術原理的二池、三池模型
對CEST技術原理最經典的解釋是二池模型(圖1)[13, 27, 36],假設人體內環境中有如下兩池(A、B池),A池代表體內水質子信號,B池代表體內某游離大分子的某個(或幾個)質子信號,在主磁場中(B0)二者會有著不同的共振進動頻率,通過施加選擇性的脈沖(預飽和脈沖B1)飽和B池信號,在適當的條件下(適宜的溫度、pH范圍),這些質子會和周圍的水質子發生化學交換,進而將部分飽和傳遞到A池,通過特定磁共振技術采集到的A池信號可以反映出該CEST效應的強弱效果[13, 33-35]。
也有學者提出磁共振CEST技術原理的三池模型及其它模型,所謂三池模型即是將組織中的質子分為三個池。第一個池為自由水池,為磁共振成像提供大量信號。第二個池為結合池,包含與蛋白質、其他大分子或者細胞膜束縛的氫質子,主要是人體中的半固態組織,這些氫質子的信號在常規磁共振成像技術中是無法被檢測的。第三池為特定分子池,系游離大分子(某些內源游離大分子或CEST對比劑)上的氫質子(包括某些羥基、胺基、酰胺基及亞胺基等)為可交換的質子。CEST技術主要研究的是第三池與自由水池的交換效應,但結合池的影響不可忽視[27, 33-37]。
1.3 外源性化合物的研究
APT成像主要依賴內源性的氨基質子,當應用某些外源性化合物時,如鑭系螯合物[38]、CT掃描的對比劑[39]等,氨基質子的交換會發生變化,這對于酸堿度的研究有一定意義。
1.4 核奧氏效應(NOE)
應用化合物模型對APT成像進行研究時,可以得到非常規整的CEST譜(Z譜)和效果非常好的APT圖,但在體研究時,特別是人體研究時,Z譜不可能規整,其中主要是受到NOE的影響,因而目前認為在人體層面上獲得的是APT加權像[22]。NOE的定義可以這樣表述:當某一自旋的核磁共振(nuclear magnetic resonance, NMR)吸收得到飽和時,另一自旋的NMR吸收強度積分值的改變[40]。在分析Z譜時,認為氨基質子對側的譜線為NOE效應[41-43]。要深入研究APT成像,必須研究NOE的影響及其NOE成像。
2 氨基質子轉移成像技術的應用
2.1 在體器官組織的pH測定
APT成像技術已有很廣泛的應用,其中一個主要方向是在體器官組織的pH測定[1, 4, 44-46]。相對于磁共振譜方法的pH成像,APT成像方法具有高空間和時間分辨率。正常的人體機能有賴于正常的穩定的內環境,組織pH的改變是提示許多病理變化的指標。腫瘤細胞內pH(pH i)及細胞外pH (pHe)存在梯度差。與正常組織相比,pHe通常較低,而pHi可以較高或基本無變化。因此,通過檢測pH的變化可評估腫瘤病人的預后及治療反應[45]。APT成像的pH研究大部分在3 T以上場強磁共振機器上進行,筆者研究在臨床1.5 T機器上也可以實現pH的檢測[4](圖2)。
對于pH的測定,目前的研究方向是全面考慮影響pH測定的各種因素[47],進而精準無損傷地在體量化器官組織的pH值[48],不僅量化細胞內pH (pHi),結合外源性對比劑[39, 49],也量化細胞外pH (pHe)值,有助于良性腫瘤與惡性腫瘤的鑒別,也有助于腫瘤異質性的判定,觀察一些疾病的治療效果。
2.2 在體器官組織的溫度測定
結合外源性對比劑[50],應用氨基質子轉移技術可以在體測定pH值的同時,測定組織的溫度差異。結合水交換譜(w ater exchange spectrum, WEX),不應用外源性對比劑也可以獲得相似的pH和溫度信息,該技術也有在體應用的潛力[51],無損傷的在體器官組織磁共振溫度測定相對于紅外線溫度測定,其內部結構更清楚,有望應用于腫瘤射頻治療的溫度監測。
2.3 在各器官組織的應用
2.3.1 在腦的應用
APT技術早期應用于腦梗塞的研究,認為APT成像比彌散加權像在顯示梗死組織方面更有優勢[1],在判斷腦梗死半暗帶方面也有優勢[52],能更精確判斷腦梗死半暗帶的范圍,這對于是否選擇溶栓治療非常有幫助。APT成像在腦梗死方面的研究一直持續至今[52-55],在超急性期腦卒中的動物中,可簡便地區分開出血性腦中和缺血性腦卒中[55]。APT技術同時也較多地應用于腦腫瘤方面的研究[3, 24, 56],研究認為APT技術有助于腦腫瘤的診斷、鑒別診斷和治療,應用APT技術顯示膠質瘤的內部結構,可以與應用對比劑時才能獲取的信息相媲美,可應用于轉移瘤和高級別神經上皮腫瘤的鑒別診斷。在神經變性疾病方面,APT技術有助于神經變性疾病的早期診斷,可作為帕金森病臨床診斷和病情監測的有效工具,為帕金森病的病理生理研究提供重要的信息[29]。
2.3.2 在腎臟的應用
結合外源性對比劑,APT技術可應用于腎臟pH的測定,目前在小鼠腎臟獲得了較好的pH成像[49],有望應用于腎臟損傷程度的判定、腎臟腫瘤的診斷和鑒別診斷,以及腎移植的效果評價等。
2.3.3 在肝臟的應用
結合APT技術和CEST技術,肝APT加權像和肝糖原成像已在動物和人體成功獲得。在動物實驗中,禁食前后和四氯化碳中毒模型前后肝APT加權像和肝糖原成像均有明顯的差異。人體肝APT加權像和肝糖原成像也有好的重復性。為常規磁共振肝成像提供了有益的補充,特別是有益于早期肝纖維化的認識,這對于今后肝臟疾病的處理會有影響[30]。
2.3.4 在乳腺的應用
APT技術也應用到乳腺組織[57-58]。在動物乳腺癌模型研究中,結果表明APT技術提供癌細胞在腫瘤組織內的分布信息,有望了解腫瘤對周邊正常組織侵襲的情況[57]。應用APT技術在人乳腺正常纖維腺樣組織成功成像,今后也可應用于研究病理組織,有益于乳腺疾病的診斷和預后判定[58]。
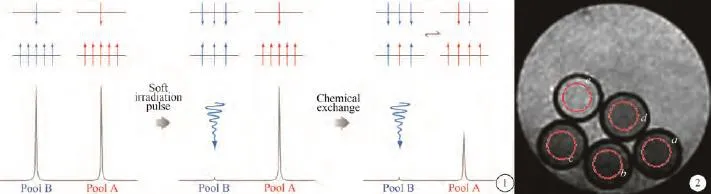
圖1 化學交換飽和轉移的二池模型 圖2 4個pH值不同的離心試管(a~d)與裝有等量水的試管(e)的磁化傳遞成像,施加了預報和脈沖,紅圓圈所標定的是感興趣區,測定結果a~d各試管信號強度不同Fig. 1 Model of Two pools for CEST Fig. 2 MT imaging of four centrifuge tubes with different pH values(a—d) and a tube w ith water (e) obtained w ith saturation pulse. The red circles denote regions of interest with different signal intensities for tubes a—d.
[References]
[1]Zhou J, Payen JF, W ilson DA, et al. Using the am ide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Medicine, 2003, 9(8): 1085-1090.
[2]Sun PZ, Zhou J, Sun W, et al. Suppression of lipid artifacts in amide p roton transfer imaging. M agnetic Resonance in Medicine, 2005, 54(1): 222-225.
[3]W en Z, Hu S, Huang F, et al. MR im aging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuro Image, 2010, 51(2): 616-622.
[4]Wei MB, Shen ZW, Xiao G, et al. Study of magnetic resonance im aging at 1.5 Tesla based on pH-sensitive m agnetization transfer technolog. Chin J Magn Reson Imaging, 2012, 3(1): 40-43.
韋茂彬, 沈智威, 肖剛, 等. 基于pH值敏感的磁化傳遞技術在1.5 T磁共振成像上的研究. 磁共振成像, 2012, 3(1): 40-43.
[5]W olff SD, Balaban RS. NMR imaging of labile p roton exchange. Journal of Magnetic Resonance, 1990, 86(1): 164-169.
[6]W ard KM, A letras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). Journal of Magnetic Resonance, 2000, 143(1): 79-87.
[7]Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magnetic Resonance in Medicine, 1989, 10(1): 135-144.
[8]Zhang TJ, Gong QY. Current status of magnetization transfer imaging in psychiatric disorders. Chin J Magn Reson Imaging,2010, 1(6): 468-472.
張體江, 龔啟勇. 磁化傳遞成像及其在精神疾病中的研究現狀. 磁共振成像, 2010, 1(6): 468-472.
[9]Zaiss M, Bachert P. Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: a review of theoretical approaches and m ethods. Physics in M edicine and Biology, 2013, 58(22): 221-269.
[10]Wu R, Xiao G, Zhou IY, et al. Quantitative chemical exchange saturation transfer(qCEST) MRI - omega plot analysis of RF-spillover-corrected inverse CEST ratio asymm etry for simultaneous determination of labile proton ratio and exchange rate. NMR in Biomedicine, 2015, 28(3): 376-383.
[11]Shen ZW, W ang LH, Dai ZZ, et al. Chem ical exchange saturation transfer (CEST)im aging of pH. Neuroscience and Biomedical Engineering, 2013, 1(2): 111-115.
[12]Wu RH, Shen ZW, Ning LB, et al. Magnetic resonance pH imaging. International Journal of Medical Radiology, 2010, 33(5): 409-410.
吳仁華, 沈智威, 寧立波, 等. 磁共振pH成像的研究. 國際醫學放射學雜志, 2010, 33(5): 409-410.
[13]N ing LB, Qiu QC, X iao YU, et al. Chenm ical ex change saturation transfer and its app lication on MR pH imaging. Zhonghua Fang She Xue Za Zhi, 2011, 45(4): 414-416.
寧立波, 邱慶春, 肖葉玉, 等. 化學交換飽和轉移在MR pH成像方面的應用. 中華放射學雜志, 2011, 45(4): 414-416.
[14]Zhou JY, Hong XH. Molecular imaging using endogenous cellular protein. Chinese Journal of Magnetic Resonance, 2013, 30(3): 307-321.
周進元, 洪曉華. 內源性蛋白質分子成像的研究進展. 波譜學雜志, 2013, 30(3): 307-321.
[15]Teng GJ, Cui Y. Research progress in molecular magnetic resonance imaging. Chin J Magn Reson Imaging, 2014, 5(S1): 31-36.
滕皋軍, 崔瑩. 磁共振分子影像學研究進展. 磁共振成像, 2014, 5(S1): 31-36.
[16]Kogan F, Hariharan H, Reddy R. Chem ical exchange saturation transfer (CEST)imaging: description of technique and potential clinical applications. Current Radiology Reports, 2013, 1(2): 102-114.
[17]Zhang T, Jia YL, Nie TT, et al. Study of a new method for imaging of GABA based on chemical exchange saturation transfer using a 7.0 T MR scanner. Chin J M agn Reson Imaging, 2015, 6(5): 385-389.
章桃, 賈巖龍, 聶婷婷, 等. 7.0 T MR γ-氨基丁酸化學交換飽和轉移成像的新技術研究. 磁共振成像, 2015, 6(5): 385-389.
[18]Aime S, Carrera C, Delli Castelli D, et al. Tunable imaging of cells labeled w ith MRI-PARACEST agents. Angewandte Chemie, 2005, 44(12): 1813-1815.
[19]Srivastava AK, Kadayakkara DK, Bar-Shir A, et al. Advances in using MRI probes and sensors for in vivo cell tracking as app lied to regenerative medicine. Disease M odels & Mechanisms, 2015, 8(4): 323-336.
[20]Gilad AA, M cMahon MT, Walczak P, et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nature Biotechnology, 2007, 25(2): 217-219.
[21]Xiao H, X iao G, Wu RH. M odeling and simulation of chem ical exchange saturation transfer m agnetic resonance im aging. Journal of Lanzhou University of Technology, 2014, 40(4): 71-75.
肖紅, 肖剛, 吳仁華. 化學交換飽和傳遞磁共振成像建模與仿真. 蘭州理工大學學報, 2014, 40(4): 71-75.
[22]Zhou J, Hong X, Zhao X, et al. APT-weighted and NOE-weighted image contrasts in glioma w ith different RF saturation powers based on magnetization transfer ratio asymmetry analyses. M agnetic Resonance in Medicine, 2013, 70(2): 320-327.
[23]Zaiss M, W indschuh J, Paech D, et al. Relaxation-compensated CEST-MRI of the human brain at 7 T: Unbiased insight into NOE and am ide signal changes in human gliob lastoma. NeuroImage, 2015, 112: 180-188.
[24]Yu H, Wang XL, Jiang SS, et al. A prelim inary study on identification of the brain metastatic tumors and the high-grade neuroepithelial tumors w ith am ide proton transfer m agnetic resonance im aging. Chinese Journal of Neurosurgery, 2015, 31(10): 1042-1046.
于昊, 王顯龍, 蔣山姍, 等. 磁共振氨基質子轉移成像鑒別腦轉移瘤與高級別神經上皮腫瘤的初步探討. 中華神經外科雜志, 2015, 31(10): 1042-1046.
[25]Ma B, Blakeley JO, Hong X, et al. Applying amide proton transfer-w eighted MRI to distinguish pseudoprogression from true progression in m alignant gliomas. J M agn Reson Imaging, 2016 Jan 20. doi: 10.1002/jm ri.25159.
[26]Sun PZ, Benner T, Kumar A, et al. Investigation of optim izing and translating pH-sensitive pulsed-chem ical exchange saturation transfer (CEST) imaging to a 3 T clinical scanner. Magnetic Resonance in Medicine, 2008, 60(4): 834-841.
[27]Cao Z, Yang ZX, Zhou ZH, et al. MR pH imaging. In Book: Edited by Lang ZJ, M iao YW, W u RH, et al. MR new techniques. Shanghai: Shanghai Sci Tech Publisher, 2015: 192-194.
曹震, 楊忠現, 趙芝弘, 等. MR酸堿度成像. 郎志謹, 苗延巍,吳仁華, 等. MRI 新技術, 上海: 上海科學技術出版社, 2015: 192-194.
[28]Wang R, Li SY, Chen M, et al. Amide proton transfer magnetic resonance im aging of A lzheimer's d isease at 3.0 Tesla: a prelim inary study. Chinese Medical Journal, 2015, 128(5): 615-619.
[29]Wang L, Li CM, Zhang C, et al. A preliminary study on amide proton transfer MR imaging at 3.0 T of the substantia nigra and red nucleusinParkinsondisease. Zhonghua Fang She Xue Za Zhi, 2015, 49(2): 81-84.
王蕊, 李春媚, 張晨, 等. 帕金森病患者黑質和紅核的3.0 T M R氨基質子轉移成像初步研究. 中華放射學雜志, 2015, 49(2): 81-84.
[30]Chen SZ, Yuan J, Deng M, et al. Chemical exchange saturation transfer (CEST)MR technique for in-vivo liver imaging at 3.0 tesla. Eur Radiol, 2015 Sep 3.[Epub ahead of print].
[31]Forsen S, Hoffman RA. Study of moderately rapid chemical exchange reactions by m eans of nuclear m agnetic double resonance. The Journal of Chem ical Physics, 1963, 39(11): 2892-2901.
[32]Guivel-Scharen V, Sinnwell T, Wolff SD, et al. Detection of proton chemical exchange between metabolites and water in biological tissues. Journal of Magnetic Resonance, 1998, 133(1): 36-45.
[33]Ning LB. pH imaging pulse sequence designin the clinical m agnetic resonance scannerbased on chem ical exchange saturation transfer. Shantou: Shantou University, 2010.
寧立波. 基于化學交換飽和轉移機制的磁共振pH成像脈沖序列設計. 汕頭: 汕頭大學, 2010.
[34]Liu TZ. Study on detection of intracranial tumors with magnetization transfer sequence. Shantou: Shantou University, 2011.
劉天柱. 應用磁化傳遞序列檢測顱內腫瘤的研究. 汕頭: 汕頭大學, 2011.
[35]Lin TF. Chem ical exchange saturation transfer imaging of hyperacute brain infarction using 1.5 T scanner. Shantou: Shantou University, 2011.
林泰鋒. 超急性期腦梗塞1.5 T磁共振化學交換飽和轉移成像.汕頭: 汕頭大學, 2011.
[36]Sherry AD, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annual Review of Biomedical Engineering, 2008, 10(10): 391-411.
[37]Desmond KL, Stanisz GJ. Understanding quantitative pulsed CEST in the presence of MT. Magnetic Resonance in Medicine, 2012, 67(4): 979-990.
[38]W oods M, Pasha A, Zhao P, et al. Investigations into whole water, prototropic and am ide proton exchange in lanthanide(III) DOTA-tetraamide chelates. Dalton Transactions, 2011, 40(25): 6759-6764.
[39]Sun PZ, Longo DL, Hu W, et al. Quantification of iopamidol multi-site chemical exchange properties for ratiometric chemical exchange saturation transfer (CEST) imaging of pH. Physics in Medicine and Biology, 2014, 59(16): 4493-4504.
[40]Zhou YL, Li HL. Applications of nuclear overhauser effect. Orgnic Chem istry, 1986(1): 1-18.
周原朗, 李華立. 核OVERHAUSER效應(NOE)的應用. 有機化學, 1986(1): 1-18.
[41]Dai Z, Ji J, Xiao G, et al. Magnetization transfer prepared gradient echo MRI for CEST imaging. PloS One, 2014, 9(11): 112219.
[42]Lin Z, Oostenbrink C, van Gunsteren WF. On the use of onestep perturbation to investigate the dependence of NOE-derived atom-atom distance bound violations o f pep tides upon a variation of force-field param eters. European Biophysics Journal, 2014, 43(2-3): 113-119.
[43]Zaiss M, W indschuh J, Goerke S, et al. Dow nfield-NOE-suppressed am ide-CEST-MRI at 7 Tesla provides a unique contrast in human gliob lastoma. Magnetic Resonance in Medicine, 2016, in press. DOI: 10.1002/m rm.26100.
[44]Sun PZ, Farrar CT, Sorensen AG. Correction for artifacts induced by B(0) and B(1) field inhomogeneities in pH-sensitive chemical exchange saturation transfer (CEST) imaging. Magnetic Resonance in M edicine, 2007, 58(6): 1207-1215.
[45]Cheng XF, W u RH. MR-based methods for pH measurement in brain tumors: current status and clinical potential. Journal of Gannan University, 2011, 31(4): 506-509.
成小芳, 吳仁華. 基于磁共振技術的腦腫瘤pH檢測方法的現狀及臨床應用前景. 贛南醫學院學報, 2011, 31(4): 506-509.
[46]Cheng XF, Wu RH. MR-based methods for pH measurement in brain tumors: current status and clinical potential. In Book: Edited by Ana L. Abujam ra: Brain Tumors, 2011, in Tech 287-302.
[47]Sun PZ, Xiao G, Zhou IY, et al. A method for accurate pH mapping w ith chemical exchange saturation transfer (CEST) MRI. Contrast M edia & M olecular Imaging, 2016, in press. DOI: 10.1002/cmmi.1680.
[48]Sun PZ, Wang Y, Dai Z, et al. Quantitative chemical exchange saturation transfer(qCEST) MRI--RF spillover effect-corrected omega plot for simultaneous determination of labile proton fraction ratio and exchange rate. Contrast Media & Molecular Imaging, 2014, 9(4): 268-275.
[49]Longo DL, Sun PZ, Consolino L, et al. A general MRI-CEST ratiometric approach for pH imaging: demonstration of in vivo pH m apping w ith iobitridol. Journal of the American Chem ical Society, 2014, 136(41): 14333-14336.
[50]M cVicar N, Li AX, Suchy M, et al. Sim ultaneous in vivo pH and temperature mapping using a PARACEST-MRI contrast agent. M agnetic Resonance in M edicine, 2013, 70(4): 1016-1025.
[51]Bodet O, Goerke S, Behl NG, et al. Am ide proton transfer of carnosine in aqueous solution studied in vitro by WEX and CEST experiments. NMR in Biomedicine, 2015, 28(9): 1097-1103.
[52]Tietze A, Blicher J, M ikkelsen IK, et al. Assessment of ischemic penumbra in patients w ith hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI. NMR in Biomedicine, 2014, 27(2): 163-174.
[53]Sun PZ, Cheung JS, Wang E, et al. Association between pH-w eighted endogenous am ide p ro ton chem ical ex change saturation transfer MRI and tissue lactic acidosis during acute ischem ic stroke. Jou rnal of Cereb ral B lood Flow and Metabolism, 2011, 31(8): 1743-1750.
[54]Huang D, Li S, Dai Z, et al. Novel gradient echo sequencebased am ide p roton transfer m agnetic resonance im aging in hyperacute cerebral infarction. M olecular M edicine Reports, 2015, 11(5): 3279-3284.
[55]Wang M, Hong X, Chang CF, et al. Simultaneous detection and separation of hyperacute intracerebral hemorrhage and cerebral ischemia using amide proton transfer MRI. Magnetic Resonance in Medicine, 2015, 2(19): 1608-1612.
[56]Lee DH, Heo HY, Zhang K, et al. Quantitative assessment of the effects of water proton concentration and w ater T changes on am ide p roton transfer (APT) and nuclear overhauser enhancement (NOE) MRI: The origin of the APT imaging signal in brain tumor. Magnetic Resonance in Medicine, 2016, in press. DOI: 10.1002/m rm.26131.
[57]Wu KW, W u RH, Zhang MM. Preliminary study of breast cancer in magnetic resonance im aging based on am ide proton transfer. Functional and M olecular Medical Imaging, 2013, 2(1): 35-39.
伍康偉, 吳仁華, 張苗苗. 基于氨基轉移機制的乳腺癌磁共振成像初步研究. 功能與分-r醫學影像學(電子版), 2013, 2(1): 35-39.
[58]Dula AN, Dewey BE, Arlinghaus LR, et al. Optim ization of 7 T chem ical exchange saturation transfer param eters for validation o f g lycosam inog lycan and am ide proton transfer of fibroglandular breast tissue. Radiology, 2015, 275(1): 255-261.
M agnetic resonance am ide p roton transfer (APT) imaging: description of technical principles and potential applications
WU Ren-hua*
Department of M edical Imaging, the 2nd A ffiliated Hospital, Shantou University M edical College, Shantou 515041, China
Am ide proton transfer (APT) imaging is related to chem ical exchange saturation transfer (CEST) technique. This review focuses on description of APT technical principles, factors influencing APT imaging, and relationship between in vivo APT imaging and nuc lear Overhauser effect (NOE). Potential clinical applications of APT technique, such as pH imaging, temperature imaging, as well as APT-weighted imaging for organs and tissues, are discussed.
Am ide proton transfer; Chem ical exchange saturation transfer; Nuclear Overhauser effect; pH imaging; Tem perature imaging
國家自然科學基金項目(編號:81471730)
汕頭大學醫學院第二附屬醫院,汕頭515041
ail: cjr.wurenhua@vip.163.com
2016-02-17
接受日期:2016-04-03
R445.2
A
10.12015/issn.1674-8034.2016.04.003
吳仁華. 磁共振氨基質子轉移成像技術原理和應用. 磁共振成像, 2016, 7(4): 254–258.
*Correspondence to: Wu RH, E-mail: cjr.wurenhua@vip.163.com