基于mtDNA Cytb基因序列的我國北方地區甜菜夜蛾遺傳多樣性與種群歷史分析
王興亞,周俐宏
1 遼寧省農業科學院植物保護研究所,沈陽 110161 2 遼寧省農業科學院花卉研究所,沈陽 110161
?
基于mtDNACytb基因序列的我國北方地區甜菜夜蛾遺傳多樣性與種群歷史分析
王興亞1,*,周俐宏2
1 遼寧省農業科學院植物保護研究所,沈陽110161 2 遼寧省農業科學院花卉研究所,沈陽110161
摘要:為了明確我國北方不同地理種群甜菜夜蛾Spodoptera exigua遺傳多樣性與種群遺傳結構,闡明該種害蟲的種群歷史動態,首次對采自我國北方8省17縣(市)304頭甜菜夜蛾樣品進行mtDNA Cytb基因序列測定與分析,利用DnaSP 5.0和Arlequin 3.0軟件分析種群遺傳多樣性、遺傳結構、遺傳分化與分子變異,基于MP、ML與貝葉斯法構建單倍型系統發育樹,與此同時,基于Median-joining法對所有個體構建單倍型網絡關系圖。結果表明,在所分析的304個序列樣本中,共檢測出19個單倍型,其中,包括9個共享單倍型, 單倍型Hap6為所有種群所共享。總群體具有較低的遺傳多樣性(Hd=0.422±0.035,π=0.00119±0.00011)與較小的遺傳分化(FST=0.108,P<0.001)。單倍型系統發育分析與網絡關系圖結果表明,雖然19個單倍型被分為2個分支,但各單倍型相互散布在不同種群中,未形成明顯譜系地理格局。AMOVA分析表明,甜菜夜蛾遺傳變異主要來自種群內(89.18%),種群間變異水平較低(10.82%)。中性檢驗(Tajima′s D = -1.897, P<0.05; Fu′s FS = -4.424, P<0.05)與錯配分布分析表明,我國北方地區甜菜夜蛾種群曾經歷過種群的近期擴張。
關鍵詞:甜菜夜蛾;細胞色素b(Cytb);線粒體DNA;種群歷史;遺傳分化
甜菜夜蛾Spodopteraexigua(Hübner)屬鱗翅目Lepidoptera夜蛾科Noctuidae灰夜蛾屬Spodoptera,是我國一種重要雜食性害蟲,可危害玉米、大豆、棉花、蔬菜等多種作物,造成嚴重的經濟損失[1- 3]。該種害蟲起源于南亞,廣泛分布于亞洲、歐洲、非洲及北美洲等熱帶與溫帶地區[4]。19世紀90年代,我國甜菜夜蛾首次在北京有發生與危害記錄。近年來,隨著全球氣候轉暖,甜菜夜蛾已迅速擴散到我國北方蔬菜產區,成為危害大蔥等蔬菜的最重要害蟲。通常,該種害蟲幼蟲取食寄主葉片,導致作物減產,如不及時防治,最終可造成寄主死亡[5- 6]。另外,甜菜夜蛾亦是一種高繁殖率、具有長距離遷移能力的害蟲[7- 9]。尤其是,近年來由于長期大量的使用殺蟲劑,使得甜菜夜蛾對有機磷類、氨基甲酸酯等殺蟲劑產生嚴重抗藥性,給該種害蟲的防治帶來更大困難[10- 11]。
近年來,許多分子標記被廣泛應用于物種系統發育和生物地理學等研究方面[12- 13]。由于線粒體基因(mtDNA)具有嚴格的母系遺傳、缺少基因重組以及較快的進化速率等特點,而被廣泛用于研究種群進化歷史、譜系地理學及物種形成等方面[13- 17]。其中,細胞色素b(cytochrome b,Cytb)基因是目前昆蟲線粒體基因組13個編碼蛋白質基因中結構與功能研究最為清楚的基因[18- 19]。并且,由于該基因進化速率適中,適合昆蟲種及種下階元的分類鑒定以及群體遺傳變異[20- 24]。迄今,國內外在DNA水平上探討甜菜夜蛾的種群遺傳學研究甚少。張艷研究了甜菜夜蛾抗性遺傳及AFLP體系的建立[25];孫小潔等建立了甜菜夜蛾cDNA表達文庫[26];牛成偉等利用AFLP研究了我國北方甜菜夜蛾種群內遺傳多樣性高于南方種群,不同種群間不存在明顯遺傳分化[27]。
鑒于此,本研究利用mtDNACytb基因序列作為分子標記,對我國北方不同地理種群甜菜夜蛾的種群遺傳多樣性、遺傳結構、遺傳分化及種群歷史進行深入分析,為闡明該種害蟲在我國北方的種群歷史動態,進而為制定合理的害蟲防治策略提供理論依據。
1材料與方法
1.1實驗材料與采集方法
本研究選取2012年采自我國8省17縣(市)的304頭甜菜夜蛾樣品,該樣品采用實地采集幼蟲與性信息素誘捕雄性成蟲方法獲得(表1)。為了盡量避免采集來自同一個父母本的后代個體,采集時盡量保持一定距離。采集到的所有樣品均浸泡在- 20 ℃,95%乙醇中,并保存于遼寧省農業科學院植物保護研究所(中國,沈陽)。另外,在構建系統發育樹時,采用Spodopteraandrogea(Stoll)、西部黃條粘蟲夜蛾Spodopterapraefica(Grote)和Spodopteralatifascia(Walker)作為外群。
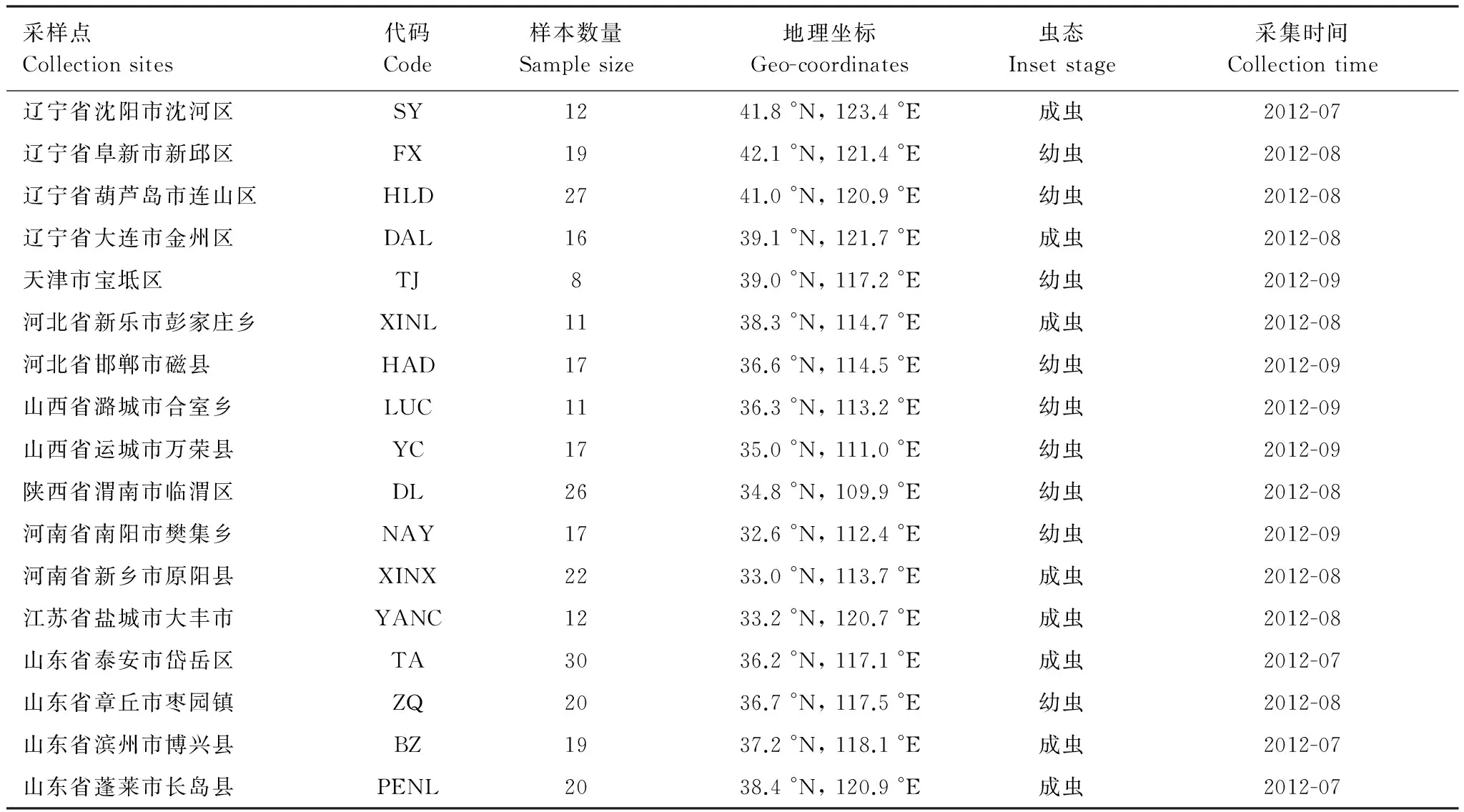
表1 我國北方地區不同地理種群甜菜夜蛾樣品的采集信息
1.2基因組總DNA的提取、PCR擴增與測序
利用Qiagen′s DNEasy提取試劑盒(Qiagen,Valencia,CA)進行甜菜夜蛾總DNA提取,選用mtDNACytb部分序列作為分子標記,其擴增引物為CP1(5′-GATGATGAAATTTTGGATC-3′)[28]和TRs(5′-TATTTCTTTATTATGTTTTCAAAAC-3′)[29]。PCR反應均為25 μL反應體系,包含0.25 μL EasyTaq DNA Polymerase(5U/μL);2.5 μL 10×Easy Taq Buffer(+Mg2+);0.5 μL dNTP (2.5 mmol/L);正反引物各0.5 μL (10 μmol/L);1 μL DNA模板;19.75 μL滅菌水。35個PCR循環參數為:94 ℃預變性5 min;94 ℃變性30 s,46 ℃退火30 s,72 ℃延伸45 s,30個循環;72 ℃終延伸5 min。PCR產物在4 ℃保存。PCR反應在Applied Biosystems ABI 3730(Applied Biosystem,USA)上進行。所得雙向測序結果通過DNAStar 5.0(DNASTAR,Inc. 1996)軟件包中的Seqman程序進行校對及雙向測序結果的拼接。
1.3序列處理與數據分析
運用Clustal X 1.81[30]軟件進行多重序列比對,使用MEGA 5.2[31]軟件基于Kimura雙參模型(Kimura 2-parameter)計算全部堿基替換情形下的序列間遺傳距離(K2P-distance)[32]。計算公式:
式中,P和Q分別代表轉換和顛換類型的同源位點概率值。
利用最大簡約法(MP)、最大似然法(ML)和貝葉斯法(BI)構建系統發育樹,估計單倍型之間的系統發育關系。MP樹在PAUP*4.10b[33]上進行,采用啟發式搜索,隨機加樣,重復1000次,構建最大簡約樹。用Bootstrapping分析,對各分支點進行評價,檢測1000次,每次檢測隨機加樣重復100次。獲得的50%合意樹,如超過1個最大簡約樹,利用Modeltest 3.7[34]和Akaike information criterion(AIC)[35]進行最適進化模型估算。ML樹也在PAUP*4.10b上執行,采用啟發式搜索,隨機加樣,重復100次,構建最大似然樹。用bootstrapping分析,對各分支點進行評價,檢測100次,每次檢驗隨機加樣重復10次。BI系統樹分析,以隨機樹為起始樹,根據其最適模型,4條馬爾可夫鏈運行1000000代,每200代抽樣并保存1次數據,保存樹的分支長度。拋棄前2500代,將抽樣得到的前500棵樹舍棄,以保證樹的似然率-LnL達到最大并趨于穩定。
使用NETWORK 2.0[36]進行基于Median-joining法對所有個體構建單倍型之間的網絡關系圖。使用DnaSP 5.0統計單倍型多態度(Hd)、核苷酸多態度(π),以及分子標記的變異速率的中性檢驗[37]。應用Arlequin 3.0[38]進行分子變異分析(analysis of molecular variance, AMOVA)與基于Kimura 2-parameter遺傳距離的種群遺傳分化FST值統計,FST值計算公式如下[39]:
式中,ni代表在第i種群樣本中的基因拷貝數。
2結果與分析
2.1Cytb序列變異及遺傳多樣性
通過實驗得到8省17個地理種群的304頭甜菜夜蛾的測序結果,Cytb基因序列長度為798 bp。其中,776個堿基序列為保守位點,22個變異位點(占序列總長度的2.8%),9個簡約信息位點。沒有出現堿基插入和缺失現象。平均堿基組成分別為42.7% A,33.1% T,12.6% G和11.5% C,A+T含量(75.8%)明顯高于G+C含量(24.2%),嚴重A+T偏向性與其它鱗翅目昆蟲相一致[40- 41]。
由表2可以看出,17個地理種群甜菜夜蛾的總群體單倍性多態度(Hd)為0.422 ± 0.035,核苷酸多態度(π)為0.00119 ± 0.00011。其中,BZ種群的單倍型多態度(Hd)和核苷酸多態度(π)最高,分別為0.643 ± 0.106和0.00214 ± 0.00039。與之相比較,SY和HAD種群的單倍型多態度(Hd)和核苷酸多態度(π)最低,皆為0。
2.2單倍型分布與系統發育分析
在304頭甜菜夜蛾Cytb基因序列中,共檢測出19個單倍型(GenBank登錄號:KF589828-KF589846)。各地理種群單倍型分布見表2。各地理種群單倍型數量范圍為1—5個,平均具有單倍型3.11個,其中,DL、YANC、BZ和PENL種群單倍型數量最豐富,分別在26、12、19、20個檢測個體中皆共定義了5個單倍型。在此19個單倍型中,包括9個共享單倍型(Hap1、Hap3、Hap5、Hap6、Hap8、Hap12、Hap14、Hap16和Hap17)。其中,Hap1廣泛分布于所有5個種群的14個樣本中;Hap6廣泛分布于所有全部17個種群的228個樣本中;Hap14廣泛分布于所有12個種群的31個樣本中。另外,還包括10個獨有單倍型,其中,Hap2、Hap4、Hap7、Hap13、Hap15、Hap18和Hap19分別為LUC、DL、XINX、TA、BZ、YANC和SY種群所特有,Hap9、Hap10、Hap11為PENL種群所特有。
以S.androgea、S.praefica和S.latifascia作為外群,基于最大簡約樹(MP)、最大似然樹(ML)與貝葉斯樹(BI)構建單倍型系統發育樹(圖1),結果表明,盡管MP/ML系統樹的后驗概率較低,系統發育樹被分為2個分支(Clade),但也存在其他尚未解決的分支。Clade I中包括Hap6—Hap12,Clade II中包括Hap13—Hap19。與此同時,基于Median-joining法構建的單倍型網絡關系的研究結果表明,單倍型Hap6廣泛分布于全部17個地理種群中。單倍型之間通常僅由1—2個突變所聯系,其中,Hap6分別與Hap7、Hap8、Hap9、Hap10和Hap13之間僅有1個突變聯系(圖2)。該單倍型網絡關系與系統發育結果一致,各單倍型都相互散布在不同的地理種群中,未形成明顯的系統地理格局。
表2我國北方不同地理種群甜菜夜蛾的單倍型分布、遺傳多樣性及中性檢測
Table 2Distribution of the haplotypes, genetic diversity and neutral test among different geographic populations ofSpodopteraexiguain North China
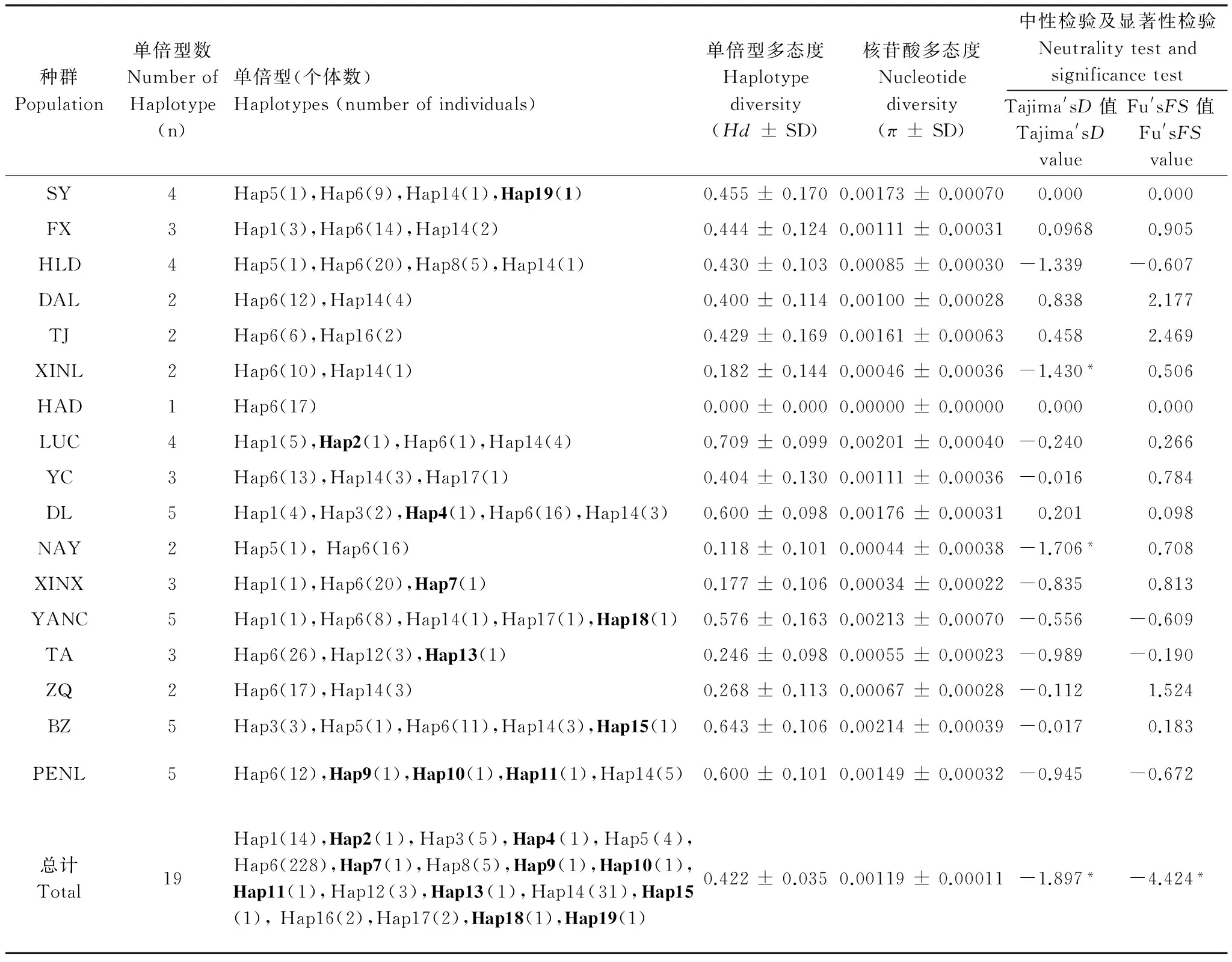
種群Population單倍型數NumberofHaplotype(n)單倍型(個體數)Haplotypes(numberofindividuals)單倍型多態度Haplotypediversity(Hd±SD)核苷酸多態度Nucleotidediversity(π±SD)中性檢驗及顯著性檢驗NeutralitytestandsignificancetestTajima'sD值Tajima'sDvalueFu'sFS值Fu'sFSvalueSY4Hap5(1),Hap6(9),Hap14(1),Hap19(1)0.455±0.1700.00173±0.000700.0000.000FX3Hap1(3),Hap6(14),Hap14(2)0.444±0.1240.00111±0.000310.09680.905HLD4Hap5(1),Hap6(20),Hap8(5),Hap14(1)0.430±0.1030.00085±0.00030-1.339-0.607DAL2Hap6(12),Hap14(4)0.400±0.1140.00100±0.000280.8382.177TJ2Hap6(6),Hap16(2)0.429±0.1690.00161±0.000630.4582.469XINL2Hap6(10),Hap14(1)0.182±0.1440.00046±0.00036-1.430*0.506HAD1Hap6(17)0.000±0.0000.00000±0.000000.0000.000LUC4Hap1(5),Hap2(1),Hap6(1),Hap14(4)0.709±0.0990.00201±0.00040-0.2400.266YC3Hap6(13),Hap14(3),Hap17(1)0.404±0.1300.00111±0.00036-0.0160.784DL5Hap1(4),Hap3(2),Hap4(1),Hap6(16),Hap14(3)0.600±0.0980.00176±0.000310.2010.098NAY2Hap5(1),Hap6(16)0.118±0.1010.00044±0.00038-1.706*0.708XINX3Hap1(1),Hap6(20),Hap7(1)0.177±0.1060.00034±0.00022-0.8350.813YANC5Hap1(1),Hap6(8),Hap14(1),Hap17(1),Hap18(1)0.576±0.1630.00213±0.00070-0.556-0.609TA3Hap6(26),Hap12(3),Hap13(1)0.246±0.0980.00055±0.00023-0.989-0.190ZQ2Hap6(17),Hap14(3)0.268±0.1130.00067±0.00028-0.1121.524BZ5Hap3(3),Hap5(1),Hap6(11),Hap14(3),Hap15(1)0.643±0.1060.00214±0.00039-0.0170.183PENL5Hap6(12),Hap9(1),Hap10(1),Hap11(1),Hap14(5)0.600±0.1010.00149±0.00032-0.945-0.672總計Total19Hap1(14),Hap2(1),Hap3(5),Hap4(1),Hap5(4),Hap6(228),Hap7(1),Hap8(5),Hap9(1),Hap10(1),Hap11(1),Hap12(3),Hap13(1),Hap14(31),Hap15(1),Hap16(2),Hap17(2),Hap18(1),Hap19(1)0.422±0.0350.00119±0.00011-1.897*-4.424*
加粗單倍型為獨有單倍型,SD表示標準差,*表示差異顯著(P<0. 05)
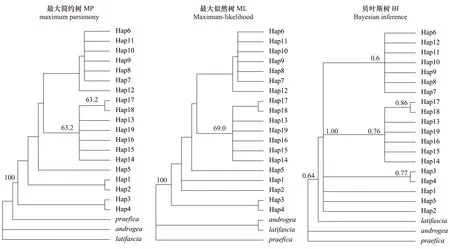
圖1 基于mtDNA Cytb基因的單倍型序列的甜菜夜蛾系統發育分析Fig.1 Phylogenetic trees of Spodoptera exigua based on haplotype sequence variation of partial mtDNA Cytb sequences MP/ML分析的自舉檢驗與BI分析的后驗概率分析表示在枝的上部 [MP樹:一致性參數 (Consistent index, CI),0.862;保留指數 (Retention index, RI),0.840;校正一致性指數 (Rescaled consistency, RC),0.725;樹長,138;GTR+I為ML和Bayesian分析的最優模型];Spodoptera androgea (HQ177620)、Spodoptera praefica (HQ177719)和Spodoptera latifascia (HQ177678) 作為外群

圖2 基于mtDNA Cytb基因序列的19個單倍型的Median-joining網絡關系圖Fig.2 Median-joining network of 19 haplotypes of Spodoptera exigua based on mtDNA Cytb gene sequences每個單倍型用一個圓圈表示,分別代表單倍型Hap1—Hap19;每個圓圈代表一個單倍型,圓的面積與單倍型頻率成正比
2.3不同地理種群間遺傳距離與遺傳分化
研究結果表明,我國北方地區甜菜夜蛾不同地理種群間的平均遺傳距離為0.001(0—0.003)。總體上,LUC種群與其它8個地理種群(HLD、SY、HAD、NAY、XINX、YANC、TA和LUC)遺傳距離最遠,皆為0.003(表3)。另外,19個單倍型之間的遺傳距離為0.005(0—0.009)。
遺傳分化指數(F-statistic,FST)可表示不同群體間等位基因頻率的變異,是反映群體進化歷史的重要參數,可在一定程度上揭示種群間基因流和遺傳漂變的程度。而基因流則可以揭示出群體間可能的基因滲透及影響遺傳分化的遺傳現象。Arlequin 3.0計算結果顯示,17個地理種群間的遺傳分化指數FST為0.108,各地理種群間的遺傳分化指數為-0.049—0.666(表3),LUC與其它各地理種群間存在明顯的遺傳分化(P< 0.05),其余地理種群間的遺傳分化程度還較低。
2.4種群遺傳結構與種群歷史分析
我國北方不同地理種群甜菜夜蛾遺傳變異的分子變異分析(AMOVA)結果見表4,不同地理種群間的遺傳分化(FST)變異較小(FST= 0.108,P<0.001)。89.18%遺傳變異主要發生在種群內,而種群間的變異僅為10.82%,說明我國北方地區甜菜夜蛾的遺傳變異主要來自于種群內部,種群間的遺傳變異水平較低。
表3基于Kimura 2-parameter遺傳距離的種群遺傳分化FST值(下三角)和遺傳距離(上三角)
Table 3Population pairwiseFSTvalues (below diagonal) and genetic distance (above diagonal) between the populations ofSpodopteraexigua
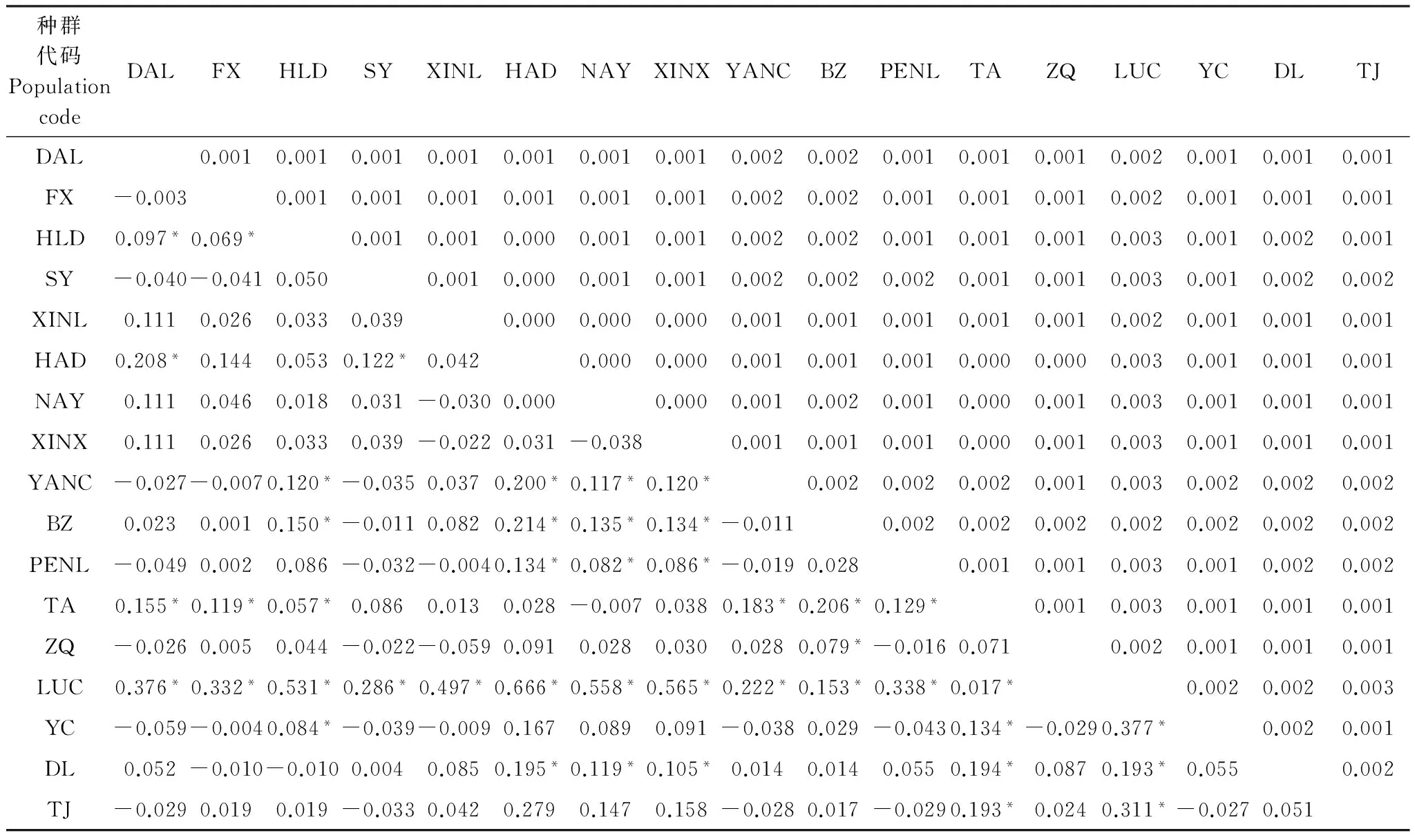
種群代碼PopulationcodeDALFXHLDSYXINLHADNAYXINXYANCBZPENLTAZQLUCYCDLTJDAL0.0010.0010.0010.0010.0010.0010.0010.0020.0020.0010.0010.0010.0020.0010.0010.001FX-0.0030.0010.0010.0010.0010.0010.0010.0020.0020.0010.0010.0010.0020.0010.0010.001HLD0.097*0.069*0.0010.0010.0000.0010.0010.0020.0020.0010.0010.0010.0030.0010.0020.001SY-0.040-0.0410.0500.0010.0000.0010.0010.0020.0020.0020.0010.0010.0030.0010.0020.002XINL0.1110.0260.0330.0390.0000.0000.0000.0010.0010.0010.0010.0010.0020.0010.0010.001HAD0.208*0.1440.0530.122*0.0420.0000.0000.0010.0010.0010.0000.0000.0030.0010.0010.001NAY0.1110.0460.0180.031-0.0300.0000.0000.0010.0020.0010.0000.0010.0030.0010.0010.001XINX0.1110.0260.0330.039-0.0220.031-0.0380.0010.0010.0010.0000.0010.0030.0010.0010.001YANC-0.027-0.0070.120*-0.0350.0370.200*0.117*0.120*0.0020.0020.0020.0010.0030.0020.0020.002BZ0.0230.0010.150*-0.0110.0820.214*0.135*0.134*-0.0110.0020.0020.0020.0020.0020.0020.002PENL-0.0490.0020.086-0.032-0.0040.134*0.082*0.086*-0.0190.0280.0010.0010.0030.0010.0020.002TA0.155*0.119*0.057*0.0860.0130.028-0.0070.0380.183*0.206*0.129*0.0010.0030.0010.0010.001ZQ-0.0260.0050.044-0.022-0.0590.0910.0280.0300.0280.079*-0.0160.0710.0020.0010.0010.001LUC0.376*0.332*0.531*0.286*0.497*0.666*0.558*0.565*0.222*0.153*0.338*0.017*0.0020.0020.003YC-0.059-0.0040.084*-0.039-0.0090.1670.0890.091-0.0380.029-0.0430.134*-0.0290.377*0.0020.001DL0.052-0.010-0.0100.0040.0850.195*0.119*0.105*0.0140.0140.0550.194*0.0870.193*0.0550.002TJ-0.0290.0190.019-0.0330.0420.2790.1470.158-0.0280.017-0.0290.193*0.0240.311*-0.0270.051
*表示差異顯著(P<0. 05),Inf代表無窮大
各地理種群進行中性檢測結果見表2。檢測結果表明,各地理種群無顯著差異(P> 0.05),表明大多數地理種群內的序列在進化上遵循中性模型。而將所有個體作為一個整體進行分析,兩個中性檢驗參數(Tajima′sD和Fu′sFS)皆為負值,且達到差異顯著水平,說明我國8省甜菜夜蛾作為一個整體種群偏離中性進化,因此,推測我國甜菜夜蛾曾經有種群擴張事件。另外,通過對所有地理種群序列的單倍型錯配分布進行分析(圖3),表明所有的Cytb單倍型呈現為一條雖不完整平滑但只有一個明顯頂峰的單峰型曲線,進一步揭示了甜菜夜蛾曾作為一個整體經歷過種群擴張。
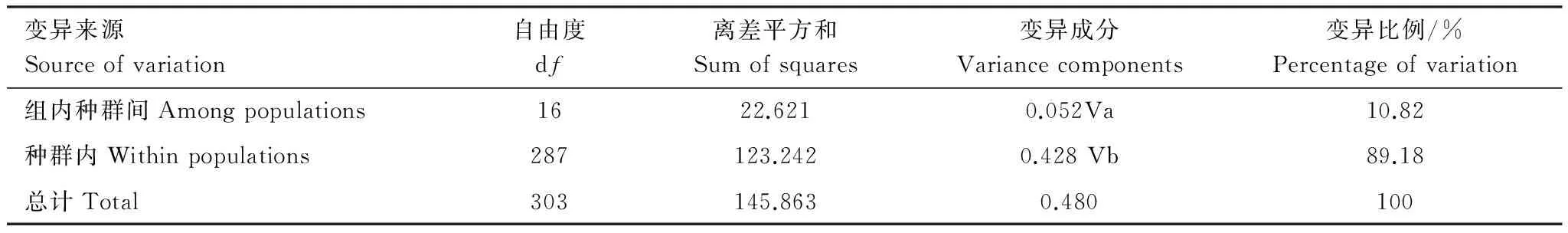
表4 不同地理種群甜菜夜蛾遺傳變異的分子變異分析(AMOVA)
固定系數 Fixation index,FST= 0.108,***P<0.001
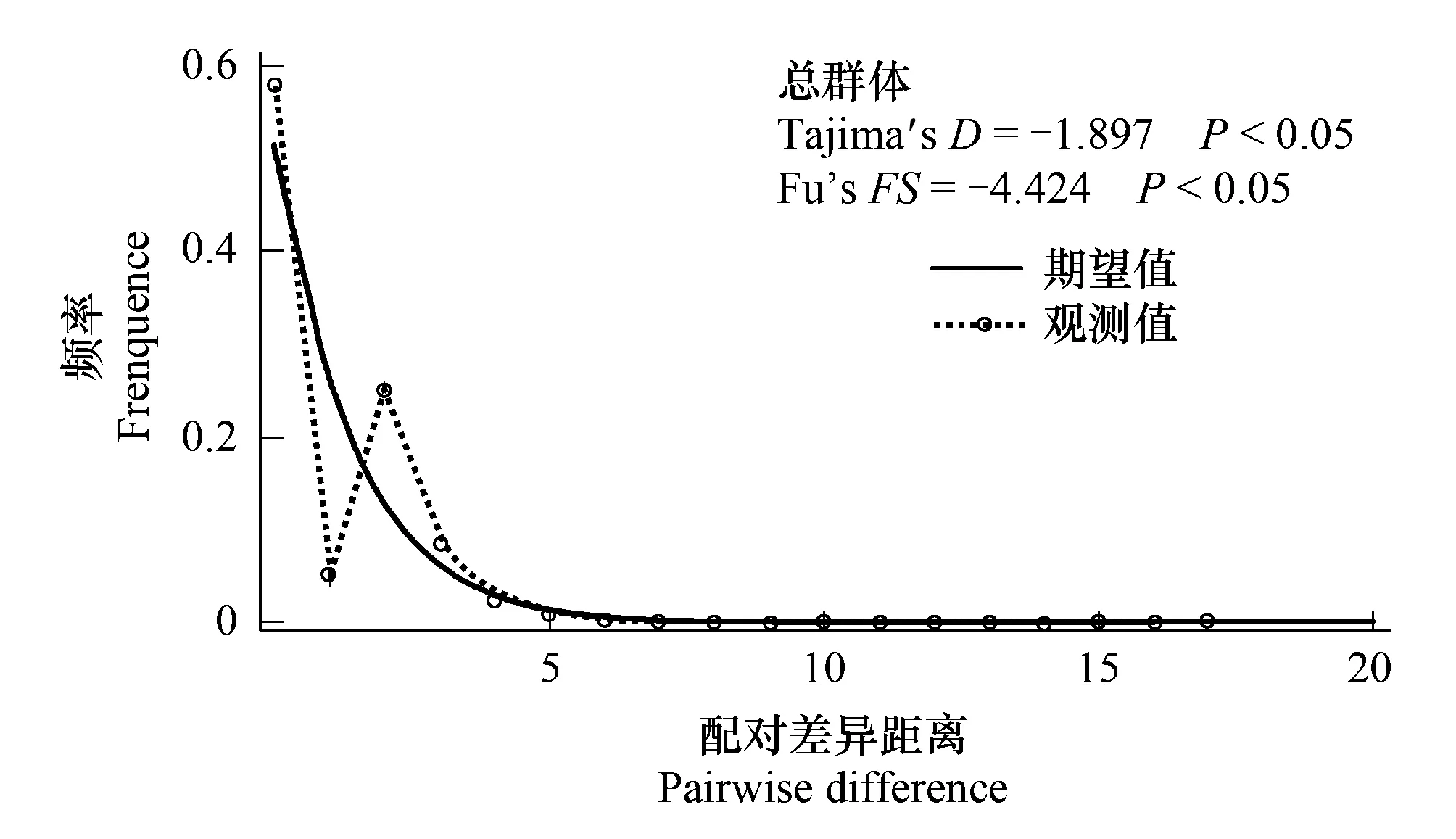
圖3 歧點分布與Tajima′s D 和Fu′s FS的中性檢驗 Fig.3 Mismatch distribution and the results of Tajima′s D and Fu′s FS tests with associated probability for all sampling localities in China實線代表期望值;帶有圓環的虛線代表觀察值。
3討論
3.1mtDNACytb基因序列變異與遺傳多樣性
研究物種遺傳變異有助于揭示該物種起源與進化歷史[42]。本研究通過分析我國北方不同地理種群甜菜夜蛾Cytb基因片段的遺傳變異情況,發現該地區甜菜夜蛾種群呈現出較低遺傳多態性,并且,在所研究的Cytb目的片段基因中,僅有22個變異位點。甜菜夜蛾各地理種群間平均遺傳距離僅為0.001。與之相一致的是,在其它夜蛾科昆蟲中,豹燈蛾Arctiacaja和曲紋灰蝶Lampidesboeticus也得到了相同的結果[34, 43]。較低水平的遺傳多樣性表明,甜菜夜蛾種群可能經過嚴重的瓶頸效應或奠基者效應,可能與甜菜夜蛾在我國北方具有較短的種群擴張歷史,尚未積累過多的遺傳變異有關。而值得注意的是,BZ種群具有相對較高的遺傳多樣性,可能與該地區甜菜夜蛾沒有經過嚴重的瓶頸效應、奠基者效應或者較小的選擇壓力(殺蟲劑等)有關。
3.2單倍型分布與系統發育分析
基于最大簡約樹(MP)、最大似然樹(ML)與貝葉斯樹(BI)構建的單倍型系統發育樹,以及基于Median-joining法構建的單倍型網絡關系的結果表明,兩者結果基本一致,但單倍型網絡關系可直接看出單倍型之間的進化關系,盡管MP/ML系統樹的后驗概率較低,單倍型系統發育樹表現出2個分支,但各單倍型中并沒有按照地理分布形成明顯的族群,各單倍型都相互散布在不同的地理種群中,未形成明顯的系統地理結構。LUC、DL、XINX、TA、BZ、YANC、SY和PENL種群所特有的10個獨有單倍型,說明各地理種群存在一定基因交流的同時,也具有一定程度的遺傳分化。值得注意的是,在所確定的19個單倍型中,單倍型 Hap6是各地理種群中普遍共享的單倍型。通常認為,共享單倍型源于共同的祖先,是一種較為穩定、能夠適應環境選擇的單倍型[44- 45]。
3.3遺傳距離與遺傳分化
通常,具有較強擴散能力的物種在不同地區間具有較小的遺傳分化。在本研究中,大部分地理種群間沒有顯著的遺傳分化,具有較小序列差異,其結果與牛成偉等一致[27]。這可能歸因于群體分化程度主要是對所在生態條件相適應的結果,環境作用強度及方向大體相同,則會造成各分布區內的種群在遺傳上將難以形成顯著的分化[46]。由于山脈的隔離,例如太行山山脈的隔離可能造成了LUC種群與其它地理種群存在顯著的遺傳分化。我國8省甜菜夜蛾地理種群間的遺傳分化指數FST為0.108,基因流Nm為4.130。當Nm>4時,種群間的基因交流就更為充分,遺傳分化更小[47]。可見我國北方甜菜夜蛾各地理種群間基因交流充分,制約了地理種群間的遺傳分化。本研究在一定程度上為甜菜夜蛾的系統演化研究提供了分子水平證據,為該種害蟲的合理有效控制提供了分子生物學方面的基礎資料。
3.4種群遺傳結構與種群歷史分析
群體遺傳結構是指遺傳變異在物種或群體中的一種非隨機分布,這種非隨機分布是由不同過程共同作用產生的,包括物種長期進化歷史、分布區改變、生境破碎、群體隔離、突變、遺傳漂變、繁育系統、基因流與選擇等[43, 46]。因此,深入理解害蟲種群遺傳結構,有助于提供給我們重要的生物學信息、遷飛規律和地理變異格局[48- 49]。另外。昆蟲的擴散能力、地理隔離以及近來人類活動(如苗木運輸等)皆可影響物種的種群結構。寄主分布可直接影響專食性昆蟲的分布[50]。寄主專化有幫助于保持昆蟲種內的高度的種內變異[51- 52],展現出高水平的遺傳結構[53- 54]。總體上,除了我國北方甜菜夜蛾沒有明顯的棲息地丟失、棲息地片段化、寄主不連續分布、氣候屏障等影響甜菜夜蛾的存活外,特別是該種害蟲的遷飛擴散導致充分的基因流的產生也會使得我國北方不同地理種群的甜菜夜蛾表現出具有較小的遺傳分化。
在本研究中,Tajima′sD和Fu′sFS中性進化檢測以及歧點分布分析來研究甜菜夜蛾種群歷史,顯著的兩個中性檢驗參數的負值以及錯配分析所呈現的單峰分布都表明甜菜夜蛾近期發生過種群擴張。另外,通常單倍型的星狀網絡圖被認為是種群擴張的證據。在本研究中,單倍型網絡圖呈星狀分布特征,分布格局表明甜菜夜蛾群體過去由于瓶頸效應經歷過種群擴張。
3.5害蟲防治啟示與未來工作
目前,甜菜夜蛾廣泛分布于我國各蔬菜主產區。鑒于該種害蟲的嚴重危害性,有必要了解其擴散風險與潛在地理分布。因此,應積極對其進行有效監控,采取一些措施盡量避免其快速擴散到其它蔬菜主產區。抗藥性的產生是昆蟲適應環境與長期進化的結果,同時也是人為自然選擇的結果[52- 53]。在過去的幾十年,一些地區大量使用單一殺蟲藥劑來防治甜菜夜蛾,結果造成了嚴重的抗藥性[13, 54]。同時,也造成一些地區的甜菜夜蛾經歷了嚴重的種群瓶頸效應[55]。大多數敏感性個體被殺死,僅有少量具有抗性的個體存活下來。此外,不同地理種群間充分的基因流可以用來解釋抗藥性的快速擴散,同時,許多抗性特征也通過長距離的遷移被積累下來[56]。因此,在較大時間和空間尺度調查甜菜夜蛾的抗藥性,結合抗性基因與遺傳結構分析,將有助于了解甜菜夜蛾的抗性水平與抗藥類別,最終指導化學藥劑的合理使用。例如,甜菜夜蛾不同地理種群間遺傳距離小、分化程度低,說明蟲源相似,在進行化學防治時,盡量避免施用與蟲源地使用相同的藥劑類型,以避免產生的抗藥性影響防治效果;對遺傳多樣性豐富的地區,通常認為甜菜夜蛾具有較高適應環境變化的能力,防治比較困難,需采取輪換施藥,降低抗性上升速度。
此外,近年來研究表明,通過母系mtDNA與核基因組中mtDNA假基因間,以及父系滲漏引起的不同單倍型的雙親mtDNA間發生基因重組[57- 58],這對以mtDNA嚴格母系遺傳為基礎的許多應用領域產生重要影響[59]。例如,在群體遺傳、進化與系統發育研究方面,mtDNA 重組可能影響關于群體大小變化的推論[60]。與此同時,mtDNA重組還會影響系統發育關系構建和分子定時的準確性[61]。因此,為了更好的理解甜菜夜蛾的種群進化歷史和種群遺傳結構,有必要使用更長、更多的基因序列,甚至是更快的進化標記,如微衛星等[62- 63]。
參考文獻(References):
[1]Dingha B N, Moar W J, Apple A G. Effects ofBacillusthuringiensisCry 1C toxin on the metabolic rate of Cry 1C resistant and susceptibleSpodopteraexigua(Lepidoptera: Noctuidae). Physiological Entomology, 2004, 29(5): 409- 418.
[2]Rizwan-ul-Haq M, Hu Q B, Hu M Y, Lin S Q, Zhang W L. Biological impact of harmaline, ricinine and their combined effects withBacillusthuringiensisonSpodopteraexigua(Lepidoptera: Noctuidae). Journal of Pest Science, 2009, 82(4): 327- 334.
[3]Guo J Y, Wu G, Wan F H. Activities of digestive and detoxification enzymes in multiple generations of beet armyworm,Spodopteraexigua(Hübner), in response to transgenic Bt cotton. Journal of Pest Science, 2010, 83(4): 453- 460.
[4]魏娟, 陳浩濤, 崔璟輝, 周生海. 1989—2010年我國甜菜夜蛾文獻計量分析. 長江蔬菜: 學術版, 2010, (18): 124- 127.
[5]鄭霞林, 王攀, 王小平, 雷朝亮. 大蔥甜菜夜蛾主要生物學習性、暴發成因及防治. 長江蔬菜: 學術版, 2009, (18): 4- 7.
[6]朱國仁, 古希樹, 王少麗, 張友軍, 胡霞, 徐維紅. 天津地區大蔥甜菜夜蛾發生規律和綜合治理. 長江蔬菜: 學術版, 2010, (18): 96- 10.
[7]Adamczyk Jr J J, Williams M R, Reed J T, Hubbard D W, Hardee D D. Spatial and temporal occurrence of beet armyworm (Lepidoptera: Noctuidae) moths in Mississippi. Florida Entomologist, 2003, 86(3): 229- 232.
[8]Feng H Q, Wu K M, Cheng D F, Guo Y Y. Radar observation of the autumn migration of the beet armyworm,Spodopteraexigua, and other moths in northern China. Bulletin of Entomological Research, 2003, 93(2): 115- 124.
[9]Jiang X F, Luo L Z, Sappington T W. Relationship of flight and reproduction in beet armyworm,Spodopteraexigua(Lepidoptera: Noctuidae), a migrant lacking the oogenesis-flight syndrome. Journal of Insect Physiology, 2010, 56(11): 1631- 1637.
[10]Meinke L J, Ware G W. Tolerance of three beet armyworm strains in Arizona to methomyl.Journal of Economic Entomology, 1978, 71(4): 645- 646.
[11]Chaufaux J, Ferron P. Sensibilité différente de deux populations deSpodopteraexiguaHüb. (Lépid.,Noctuidae) aux baculovirus et aux pyréthrino?des de synthèse. Agronomie, 1986, 6(1): 99- 104.
[12]Chatterjee S N, Tanushree T. Molecular profiling of silkworm biodiversity in India. Genetika, 2004, 40(12): 1618- 1627.
[13]Behura S K. Molecular marker systems in insects: current trends and future avenues. Molecular Ecology, 2006, 15(11): 3087- 3113.
[14]Avise J C. Ten unorthodox perspectives on evolution prompted by comparative population genetic findings on mitochondrial DNA. Annual Review of Genetics, 1991, 25: 45- 69.
[15]Kim Y, Kim N. Cold hardiness inSpodopteraexigua(Lepidoptera: Noctuidae). Environmental Entomology, 1997, 26(5): 1117- 1123.
[16]Sunnucks P. Efficient genetic markers for population biology. Trends in Ecology& Evolution, 2000, 15(5): 199- 203.
[17]Hurst G D D, Jiggins F M. Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proceedings of the Royal Society B: Biological Sciences, 2005, 272(1572): 1525- 1534.
[18]Gray M W. Origin and evolution of mitochondrial DNA. Annual Review of Cell Biology, 1989, 5: 25- 50.
[19]Morit A C, Dowling T E, Brown W M. Evolution of animal mitochondrial DNA: Relevance for population biology and systematics. Annual Review of Ecology and Systematics, 1987, 18: 269- 292.
[20]Pirounakis K, Koulianos S, Schmid- Hempel P. Genetic variation among European populations ofBombuspascuorum(Hymenoptera, Apidae) from mitochondrial DNA sequence data. European Journal of Entomology, 1998, 95(1): 27- 33.
[21]Morrow J L, Scott L, Congdon B, Yeates D K,Frommer M, Sved J A. Close genetic similarity between two sympatric species of tephritid fruit fly reproductively isolated by mating time. Evolution: International Journal of Organic Evolution, 2000, 54(3): 899- 910.
[22]Birungi J, Arctander P. Large sequence divergence of mitochondrial DNA genotypes of the control region within populations of the African antelope, kob (Kobuskob). Molecular Ecology, 2000, 9(12): 1997- 2008.
[23]dela Cruz K D, Whiting M F. Genetic and phylogeographic structure of populations ofPulexsimulans(Siphonaptera) in Peru inferred from two genes (CytbandCoII). Parasitology Research, 2003, 91(1): 55- 59.
[24]Ren Z M, Ma N B, Guo Y P. The studies of the phylogeny of acridoidea based on mtDNA sequences. Acta Genetica Sinica, 2002, 29(4): 314- 321.
[25]張艷. 甜菜夜蛾Spodopteraexigua抗性遺傳研究及AFLP體系的建立[D]. 長春: 吉林農業大學, 2005.
[26]孫小潔, 李朝飛, 于航, 裘雪梅, 李慶, 張文慶. 甜菜夜蛾cDNA文庫的構建. 昆蟲知識, 2006, 43(3): 404- 407.
[27]牛成偉, 張青文, 葉志華, 羅禮智. 不同地區甜菜夜蛾種群的遺傳多樣性分析. 昆蟲學報, 2006, 49(5): 867- 873.
[28]Sezonlin M, Dupas S, LeRü B, LeGall P, Moyal P, Calatayud P A, Giffard I, Silvain J F. Phylogeography and population genetics of the maize stalk borerBusseolafusca(Lepidoptera, Noctuidae) in sub-Saharan Africa. Molecular Ecology, 2006, 15(2): 407- 420.
[29]Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America, 1994, 87(6): 651- 701.
[30]Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G, 1997. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25(24): 4876- 4882.
[31]Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 2011, 28(10): 2731- 2739.
[32]Kimura M. A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 1980, 16(2): 111- 120.
[33]Swofford D L. Paup*: Phylogenetic analysis using parsimony (*and other methods), 4.0 beta 10 ed. Sinauer, Sunderland, Mass, 2002.
[34]Posada D, Crandall K A. MODELTEST: Testing the model of DNA substitution. Bioinformatics, 1998, 14(9): 817- 818.
[35]Posada D, Buckley T R. Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology, 2004, 53(5): 793- 808.
[36]Bandelt H J, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution, 1999, 16(1): 37- 48.
[37]Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics, 2009, 25(11): 1451- 1452.
[38]Excoffier L, Laval G, Schneider S. Arlequin (version 3. 0): an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online, 2005, 1: 47- 50.
[39]Weir B S, Hill W G. EstimatingF- statistics. Annual Review of Genetics, 2002, 36: 721- 750.
[40]Taylor M F J, McKechnie S W, Pierce N, Kreitman M. The lepidopteran mitochondrial control region: structure and evolution. Molecular Biology and Evolution, 1993, 10(6): 1259- 1272.
[41]Cameron S L, Whiting M F. The complete mitochondrial genome of the tobacco hornworm,Manducasexta(Insecta: Lepidoptera: Sphingidae), and an examination of mitochondrial gene variability within butterflies and moths. Gene, 2008, 408(1/2): 112- 123.
[42]Lohman D J, Peggie D, Pierce N E, Meier R. Phylogeography and genetic diversity of a widespread Old World butterfly,Lampidesboeticus(Lepidoptera: Lycaenidae). BMC Evolutionary Biology, 2008, 8: 301.
[43]Anderson S J, Conrad K F, Gillman M P, Woiwod I P, Freeland J R. Phenotypic changes and reduced genetic diversity have accompanied the rapid decline of the garden tiger moth (Arctiacaja) in the UK. Ecological Entomology, 2008, 33(5): 638- 645.
[44]張立勛, 阮祿章, 安蓓, 劉迺發. 西藏雪雞青海亞種的種群遺傳結構和地理變異. 動物學報, 2005, 51(6): 1044- 1049.
[45]周志軍, 張艷霞, 常巖林, 楊明茹. 暗褐蟈螽不同地理種群間的遺傳分化. 遺傳, 2011, 33(1): 75- 80.
[46]Armstrong K F, Wratten S D. The use of DNA analysis and the polymerase chain reaction in the study of introduced pests in New Zealand // Symondson W O C, Liddell J E, eds. The Ecology of Agricultural Pests. Melbourne: Chapman and Hall, 1996: 231- 263.
[47]Boivin T, Bouvier J C, Beslay D, Suphanor B. Variability in diapause propensity within populations of a temperate insect species: interactions between insecticide resistance genes and photoperiodism. Biological Journal of the Linnean Society, 2004, 83(3): 341- 351.
[48]Miller N J, Birley A J, Overall A D J, Tatchell G M. Population genetic structure of the lettuce root aphid,Pemphigusbursarius(L.), in relation to geographic distance, gene flow and host plant usage. Heredity, 2003, 91(3): 217- 223.
[49]Timm A E, Pringle K L, Warnich L. Genetic diversity of woolly apple aphidEriosomalanigerum(Hemiptera: Aphididae) populations in the Western Cape, South Africa. Bulletin of Entomological Research, 2005, 95(3): 187- 191.
[50]Mendelson T C, Shaw K L. Use of AFLP markers in surveys of arthropod diversity. Methods in Enzymology, 2005, 395: 161- 177.
[51]Avise J C, Arnold J, Ball R M, Bermingham E, Lamb T, Neigel J E, Reeb C A, Saunders N C. Intraspecific phylogeography: the mitochondrial DNA bridge between population genetics and systematics. Annual Review of Ecology Evolution and Systematics, 1987, 18: 489- 522.
[52]Karunaratne S H P P. Insecticide resistance in insects: a review. Ceylon Journal of Science (Biological Sciences), 1998, 25: 72- 99.
[53]Ffrench-Constant R H, Daborn P J, Le Goff G. The genetics and genomics of insecticide resistance. Trends in Genetics, 2004, 20(3): 163- 170.
[54]Feyereisen J. Molecular biology of insecticide resistance. Toxicology Letters, 1995, 82- 83: 83- 90.
[55]Saeed Q, Saleem M A, Ahmad M. Toxicity of some commonly used synthetic insecticides againstSpodopteraexigua(Fab) (Lepidoptera: Noctuidae). Pakistan Journal of Zoology, 2012, 44(5): 1197- 1201.
[56]Bouvier J C, Buès R, Boivin T, Boudinhon L, Beslay D, Sauphanor B. Deltamethrin resistance in the codling moth (Lepidoptera: Tortricidae): inheritance and number of genes involved. Heredity, 2001, 87(4): 456- 462.
[57]McVean G A T. What do patterns of genetic variability reveal about mitochondrial recombination? Heredity, 2001, 87(6): 613- 620.
[58]Guo X H, Liu S J, Liu Y. Evidence for recombination of mitochondrial DNA in triploid crucian carp. Genetics, 2006, 172(3): 1745- 1749.
[59]于曉麗, 黃原. 動物線粒體DNA重組的研究進展. 動物學雜志, 2008, 43(2): 145- 149.
[60]Piganeau G, Gardner M, Eyre-Walker A. A broad survey of recombination in animal mitochondria. Molecular Biology and Evolution, 2004, 21(12): 2319- 2325.
[61]Hagelberg E. Recombination or mutation rate heterogeneity? Implications for Mitochondrial Eve. Trends in Genetics, 2003, 19(2): 84- 90.
[62]Posada D, Crandall K A. Intraspecific gene genealogies: trees grafting into networks. Trends in Ecology & Evolution, 2001, 16(1): 37- 45.
[63]Downie D A. Evidence for multiple origins of grape phylloxera (DaktulosphairavitifoliaeFitch) (Hemiptera: Phylloxeridae) in South African vineyards. African Entomology, 2005, 13(2): 359- 365.
Genetic diversity and population history among geographic populations ofSpodopteraexiguain North China based on mtDNACytbgene sequences
WANG Xingya1,*, ZHOU Lihong2
1InstituteofPlantProtection,LiaoningAcademyofAgriculturalSciences,Shenyang110161,China2InstituteofFlowerResearch,LiaoningAcademyofAgriculturalSciences,Shenyang110161,China
Abstract:The beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae), is an important and cosmopolitan pest that attacks cultivated crops, including maize, cotton, soybeans, beet, tomato, cabbage, and alfalfa, causing serious economic losses in some of the main crop-producing areas. This species originated in South Asia and is distributed throughout the tropical and temperate regions of Asia, Europe, Africa, and North America. In China, S. exigua was first recorded in Beijing in the 1890s and has been widely distributed in the southern provinces in recent years. However, recently, with global climate warming and the adjustment of agricultural planting structures, S. exigua has quickly spread to the main crop-producing areas of North China. Genetic diversity and population genetic structure are important aspects of the population genetics of agricultural insects, and provide essential information for understanding local adaptation and dispersal patterns, and for clarifying the relationships between genetic variation and outbreaks of pest species. Cytochrome b (Cytb) has a moderate evolutionary rate and a clear evolutionary pattern, suitable for the studies of phylogenetic evolution at the intra- and inter-specific levels. To identify the genetic diversity and population history among geographic populations of S. exigua in North China, and clarify its population demographic history and genetic structure, the genetic diversity, structure, differentiation, and molecular variance were analyzed using DnaSP 5.0 and Arlequin 3.0. In the present study, 798 bases of mtDNA Cytb genes were obtained from 304 individuals of S. exigua, collected from 17 geographic populations in 8 provinces of North China in 2012. Of the 798 characters, 776 sites were conserved and 22 sites were variable (2.8% of the total length), including 9 parsimonious informative sites. The average base frequencies were 42.7% A, 33.1% T, 12.6% G, and 11.5% C. Within-locality diversity was estimated in terms of haplotype diversity (h) and nucleotide diversity (π) from all surveyed localities. Low genetic diversity (Hd = 0.422 ± 0.035,π = 0.00119 ± 0.00011) in the total populations among different geographic populations was detected. The highest estimate (Hd = 0.643 ± 0.106, π = 0.00214 ± 0.00039) was detected in Binzhou (BZ). Nineteen haplotypes, including 9-shared haplotypes were identified. Hap6 was shared in 228 individuals of all populations. Phylogenetic analysis was conducted to determine the relationships between S. exigua haplotypes, and detect discernible groups related to geographic distribution. High congruence was observed between the phylogenies derived from Maximum parsimony (MP), Maximum-likelihood (ML) and Bayesian analyses, and these analyses generated only two inclusive clades. Moreover, the median-joining network was similar to the topology of the phylogenetic tree with 19 haplotypes, and revealed no obvious phylogeographic pattern. The pairwise FSTvalues between the populations varied from -0.049—0.666. Generally, little genetic differentiation (FST= 0.108, P<0.001) among different geographic populations was detected, with the only significant differentiation between the Lucheng (LUC) and other S. exigua populations. The analysis of molecular variance (AMOVA) showed that the percentage of variation within a population (89.18%) was greater than that between the populations (10.82%). The results of neutrality tests on the S. exigua data set of the total population are: Tajima′s D (D = -1.897, P<0.05) and Fu′s Fs (Fs = -4.424, P<0.05), combined with the unimodal mismatch distribution, indicated recent population expansion of S. exigua in large spatial scales.
Key Words:Spodoptera exigua; cytochrome b (Cytb); mtDNA; population history; genetic differentiation
基金項目:國家自然科學基金項目(31101626)
收稿日期:2014- 10- 04; 網絡出版日期:2015- 08- 21
*通訊作者
Corresponding author.E-mail: wangxingya20081@163.com
DOI:10.5846/stxb201410041952
王興亞,周俐宏.基于mtDNACytb基因序列的我國北方地區甜菜夜蛾遺傳多樣性與種群歷史分析.生態學報,2016,36(8):2337- 2347.
Wang X Y, Zhou L H.Genetic diversity and population history among geographic populations ofSpodopteraexiguain North China based on mtDNACytbgene sequences.Acta Ecologica Sinica,2016,36(8):2337- 2347.

