多元水鹽體系冰鹽共晶點的測定和圖形表達
王雪瑩,黃文婷,黃雪莉
(新疆煤炭潔凈轉化與化工過程重點實驗室,新疆大學化學化工學院,新疆 烏魯木齊 830046)
?
多元水鹽體系冰鹽共晶點的測定和圖形表達
王雪瑩,黃文婷,黃雪莉
(新疆煤炭潔凈轉化與化工過程重點實驗室,新疆大學化學化工學院,新疆 烏魯木齊 830046)
水鹽體系共晶點的數據可以為鹽湖鹵水低溫加工工藝開發提供必要的理論依據,采用低溫冷卻法,針對四元同離子體系和四元交互體系及其相關的6個三元子體系,測定研究了其冰點、共晶點以及析鹽規律,繪制出了共晶點溫度-液相組成圖。研究結果表明:通過測定多元水鹽體系降溫過程中的時間-溫度圖,可以判斷鹽類析出規律、測定冰和鹽的共晶點溫度;上述體系中,常溫下存在的復鹽鉀芒硝,在共晶點溫度下均不再出現,水鹽體系相關系得以簡化;用棱柱圖可以簡單直觀地表達三元體系、四元同離子體系和四元交互體系的相、冰點或共晶點的溫度和溶液組成的關系;在三元體系的共晶點溫度-液相組成圖中,存在兩條單鹽與冰的共晶線、一個兩種鹽與冰的共晶點、一個冰析出面;在四元同離子體系和四元交互體系共晶點溫度-液相組成圖中,分別有3個和4個單鹽與冰的共晶面、3條和5條兩種鹽與冰的共晶線、1個和2個3種鹽與冰的共晶點。
水鹽體系;共晶點;溫度;相
DOI:10.11949/j.issn.0438—1157.20151485
引 言
鹽湖資源的利用,大多采用自然或強制蒸發的方式分離鹽類,能耗水耗較高。開發新技術實現高效節能、節水、益于環境的鹽湖化工生產非常有必要。鹵水冷凍至冰點或共晶點溫度時冰、鹽分別析出,進行分離后可獲得純水和相應的鹽類,也即共晶冷凍結晶技術[1-6]。該技術和蒸發相比,能耗低[7],可獲得純水,并可以利用冬季冷能。不過能否采用該技術,還取決于不同鹵水的冷凍性質,也即冰點或共晶點數據和鹵水組成、降溫析鹽規律等。因此有必要針對不同鹵水進行此類研究,為探索鹽湖化工低溫分離工藝提供理論依據。
對于二元水鹽體系的冰點和共晶點的研究數據較多[8-9]。但三元體系的數據較少[10-14],四元及以上的體系鮮有報道。對于多元水鹽體系,一些研究者進行了模型預測。Sander等[15]研究了一種改進的擴展UNIQUAC模型;Thomsen等[16-17]提出了對多組分溶液冰點的預測模型;Peralta等[18-19]提出了一個用過量Gibbs自由能來預測溶液的熱容、密度和冰點的模型。
目前國內對于多元水鹽體系低溫下的相平衡的研究已有相關報道[20-21],但對水鹽體系冰點和共晶點的實驗測定和表達方法的研究未見相關報道。硝酸鹽類鹽湖,是新疆特有的干鹽湖化學類型,其中一類雜硝礬礦用水溶浸后的體系屬于五元體系,研究其基礎數據可以為綜合利用硝酸鹽資源提供技術支持和保證,具有重要的意義,而對此五元體系的冰點及共晶點的研究,需要相關的次級體系(四元、三元及二元體系)的共晶點數據。故本文以其子體系四元同離子體系和四元交互體系及其相關的6個三元子體系為例,進行了冰點及共晶點研究。測定了共晶點的溫度和組成,分析鑒定相應的固相,研究并繪制出共晶點溫度-液相組成圖、干鹽投影圖和共晶點溫度投影圖,并加以分析。
1 實驗部分
1.1實驗試劑和儀器
試劑:均為分析純或基準試劑;儀器:DHJF-4010低溫恒溫攪拌反應浴(范圍-40℃~99℃,精度±0.2℃);精密電子溫差測量儀(范圍-50℃~100℃,精度±0.001℃);754型紫外可見分光光度計等。
1.2實驗裝置
本實驗裝置由低溫恒溫攪拌反應浴、精密溫差測量儀和計算機組成,實驗裝置如圖1所示。為檢驗裝置及實驗方法的可靠性,對不同濃度的多種單鹽和三元體系的冰點和共晶點,進行了多次的實驗測定,實驗結果與文獻值[8-9]吻合良好。
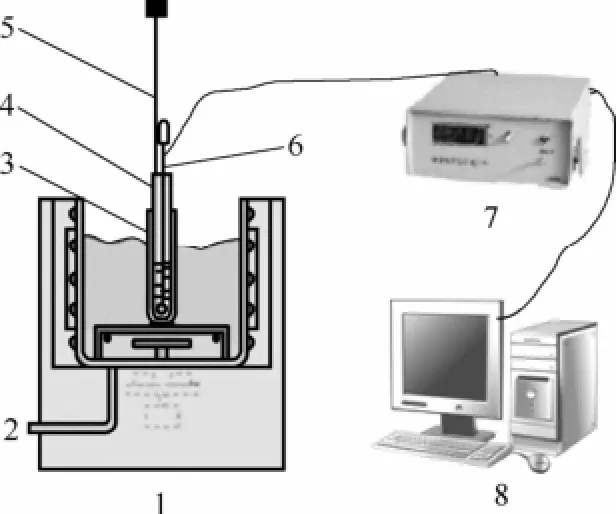
圖1 冰點測量實驗裝置Fig.1 Sketch of freezing point measurement 1—reaction bath struction; 2—liquid outlet; 3—casing; 4—loading test tube;5—agitator ;6—thermometer probe; 7—electronic temperature measuring instrument; 8—computer
1.3實驗方法
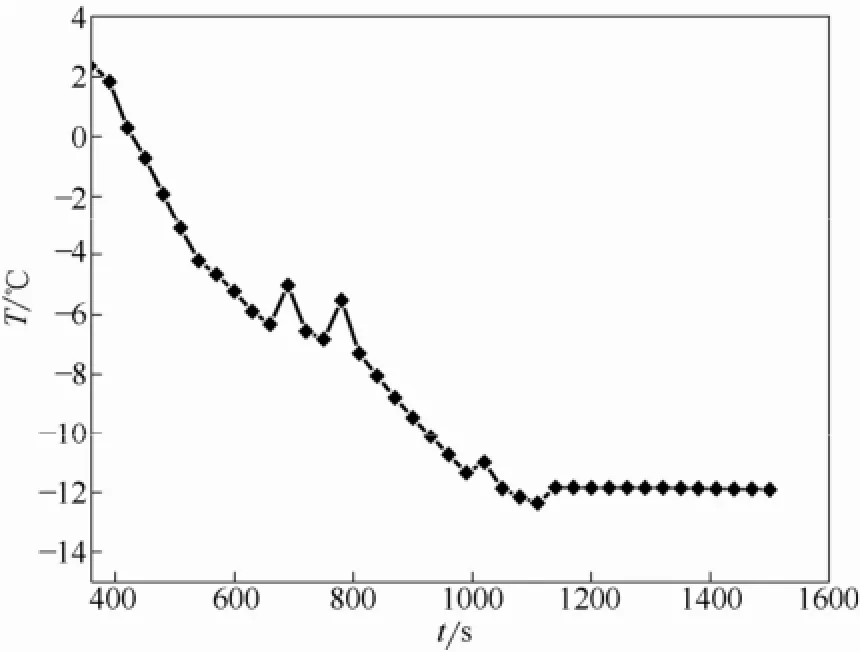
圖2 實驗體系時間-溫度圖Fig.2 Time-temperature diagram of experimental system
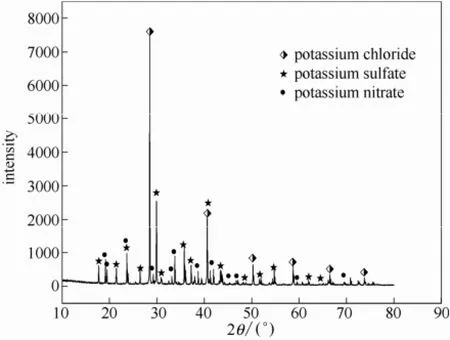
圖3 K+//Cl-,NO-3,SO24--H2O 體系共晶點XRD譜圖Fig.3 XRD pattern of system
1.3.2多元體系共晶點溶液組成的測定取上述留用的溶液300 g左右,加入到燒瓶中,置于低溫恒溫裝置中,溫度調至略低于已測出的共晶點或冰點溫度下,攪拌,使其緩慢析出較多冰鹽時,取液相進行分析,并鑒定固相(測出的XRD如圖3所示),從而得到共晶點溶液的組成。實驗證明,由于傳熱溫差很小,冰鹽析出速度較慢且穩定,此時溶液組成維持不變。
1.4化學分析方法
Cl-:硝酸銀容量法;K+:四苯硼鈉重量法;SO42-:比濁法;NO3-:重鉻酸鉀氧化法;Na+:差減法,偏差小于0.4%。固相鑒定采用X射線晶體衍射法綜合進行。
2 結果與討論
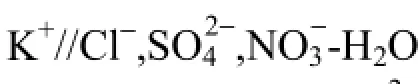
2.1三元體系研究結果
上述6個三元體系的共晶點的液相組成和溫度數據列于表1中。
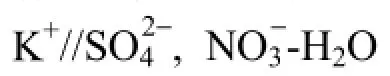
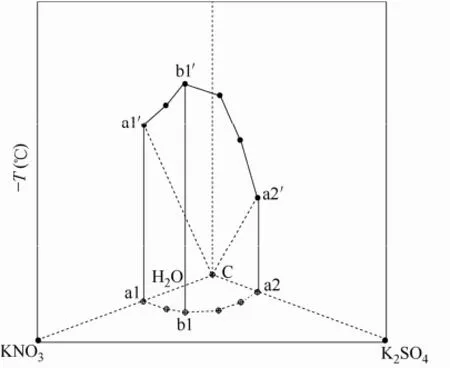
圖4 體系共晶點溫度-液相組成(局部放大圖)Fig.4 Eutectic point temperature-liquid composition diagram of system(enlarged partial)
底面正三角形的3個頂點分別為KNO3、K2SO4和水;三條邊表示3個二元體系分別為KNO3-H2O、K2SO4-H2O和KNO3-K2SO4;三角形內部為三元體系組成的點。任一實驗數據點在該圖中的標繪方法是:將液相各組分的百分含量作為坐標,在底面上標出共晶點的液相組成點,垂直升高到相應的共晶點溫度。將表1中的數據按此方法繪制,再依次把單鹽-冰的共晶點連接起來,形成兩條單鹽-冰的共晶點線,相交于兩鹽-冰的共晶點,可得到共晶點溫度-液相組成圖。圖4中,三棱柱底面上a1、b1、a2三點分別為 KNO3-H2O、KNO3-K2SO4-H2O和K2SO4-H2O體系的共晶點液相組成,a1′、b1′、a2′為各對應的共晶點溫度,C為純水的冰點。曲線C-a1′為KNO3-H2O冰點線、C-a2′為K2SO4-H2O冰點線;a1′-b1′為不同K2SO4的含量下,KNO3與冰的共晶點曲線;a2′-b1′為不同的KNO3含量下,K2SO4與冰的共晶點曲線。空間曲面 a1′-b1′-a2′-C為KNO3-K2SO4-H2O體系的冰點溫度曲面,在此組成范圍內的溶液,冰點均在 a1′-b1′-a2′-C曲面上。超過此組成范圍的溶液,共晶點溫度均為b1′點。
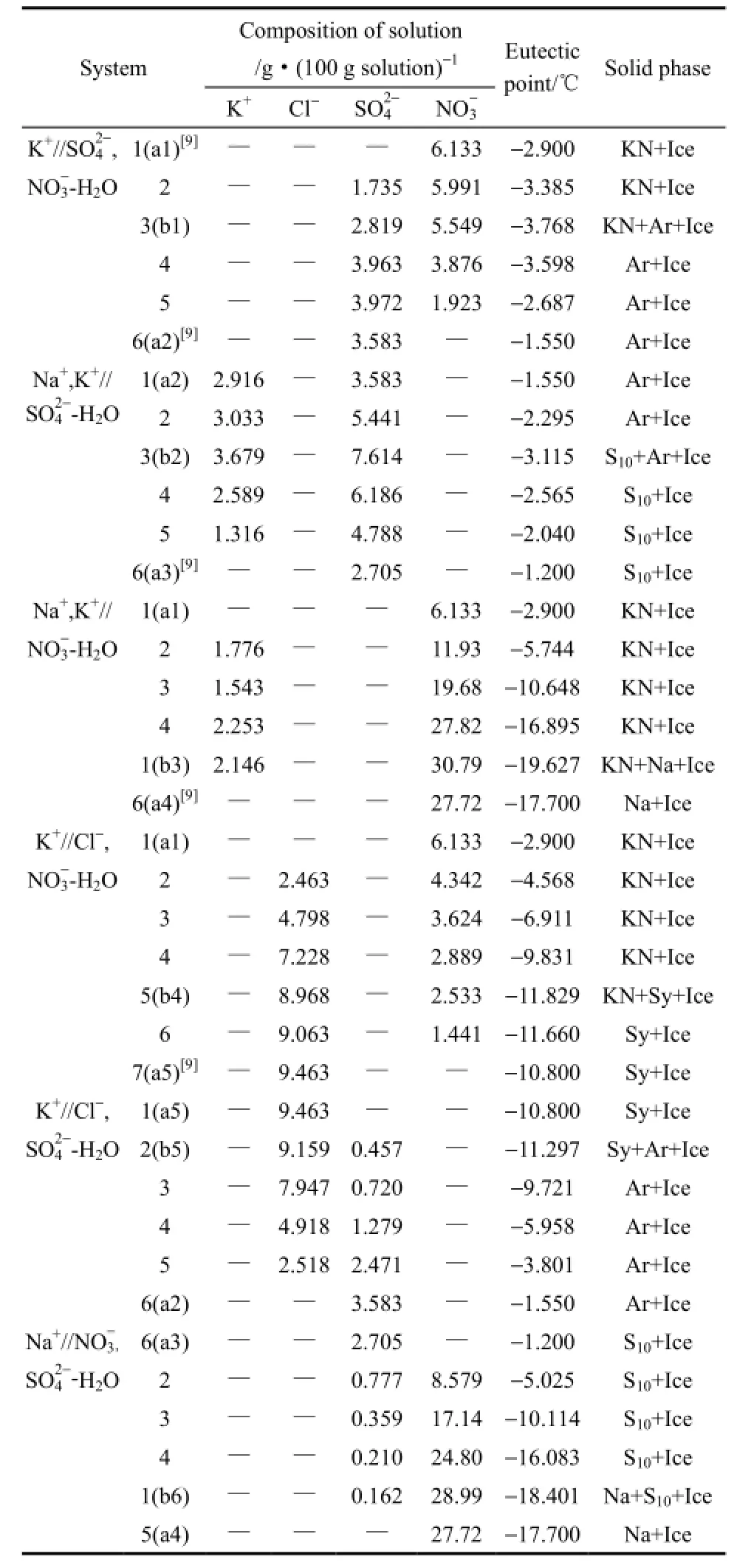
表1 R三元體系共晶點溫度及液相組成Table 1 Liquid compositions and temperatures of eutectic points of ternary systems
本文所研究的三元體系,無論常溫下是否存在復鹽,在共晶點或冰點下均為簡單體系,即復鹽不再存在,共晶點溫度-液相組成圖中存在兩條單鹽與冰的共晶線、一個兩種鹽與冰的共晶點以及一個冰點面。
2.2四元體系實驗研究結果
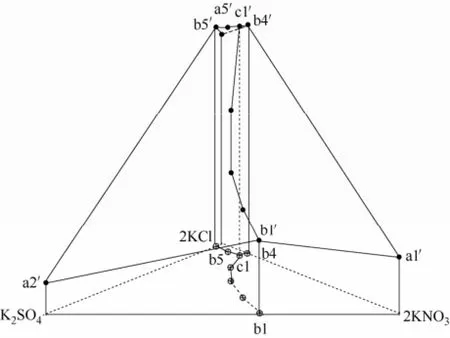
圖5 K+//Cl-,NO-3,SO24--H2O 體系共晶點溫度-液相組成Fig.5 Eutectic point temperature-liquid composition diagram of system
同離子四元體系的冰點、共晶點相圖的表達方法和三元體系類似,采用三棱柱法。三棱柱的棱表示溫度的絕對值,底面為干鹽組成投影圖,其坐標為相應的J?necke指數,列于表2。據此繪制出相應的共晶點溫度-液相組成圖、干鹽投影圖和共晶點溫度投影圖,見圖5~圖7。圖5中,底面正三角形的3個頂點分別為二元體系 KNO3-H2O、K2SO4-H2O和 KCl-H2O,三條邊表示 3個三元體系分別為KNO3-KCl-H2O、K2SO4-KCl-H2O和KNO3-K2SO4-H2O,三角形內部表示四元體系,標繪方法是把每一個實驗點的液相組成按J?necke指數在底面上標出,再垂直升高到相應的共晶點溫度,最后再連線獲得共晶點溫度-液相相圖。三棱柱底面b1、b4、b5點為各三元體系的兩鹽共晶點液相組成點,b1′、b4′、b5′為各對應的共晶點溫度;c1為此體系的三鹽共晶點液相組成點,c1′為其對應的溫度;a1′、a2′、a5′為各單鹽和冰的共晶點溫度。b4′-c1′為 KCl+ KNO3+Ice的共晶曲線、b5′-c1′為 KCl+K2SO4+Ice的共晶曲線、b1′-c1′為K2SO4+ KNO3+Ice的共晶曲線。a5′-b5′-c1′-b4′-a5′為 KCl和冰的共晶曲面;a2′-b1′-c1′-b5′-a2′為 K2SO4和冰的共晶曲面;a1′-b1′-c1′-b4′-a1′為KNO3和冰共晶曲面。
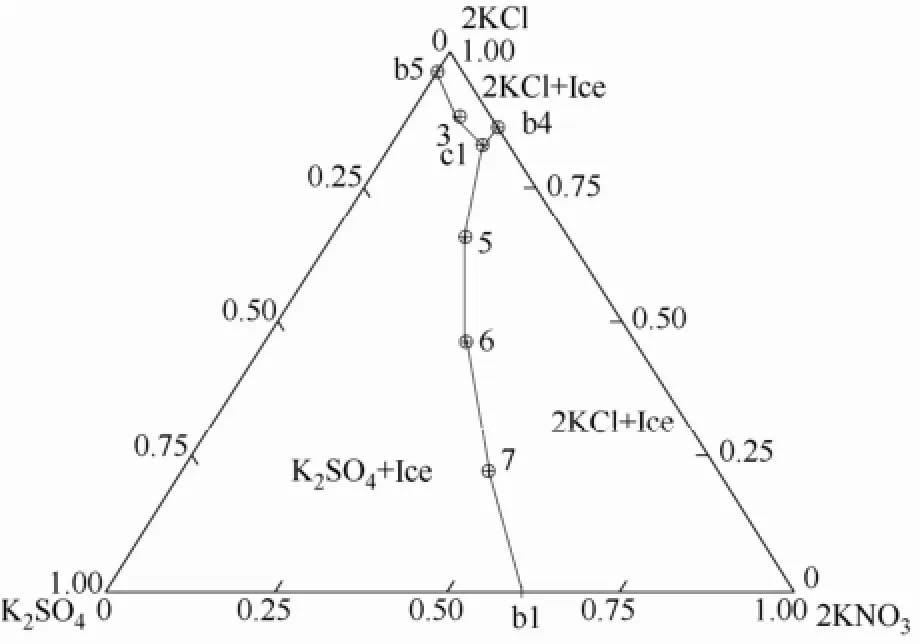
圖6 體系干鹽投影Fig.6 Dry salt projection diagram of system
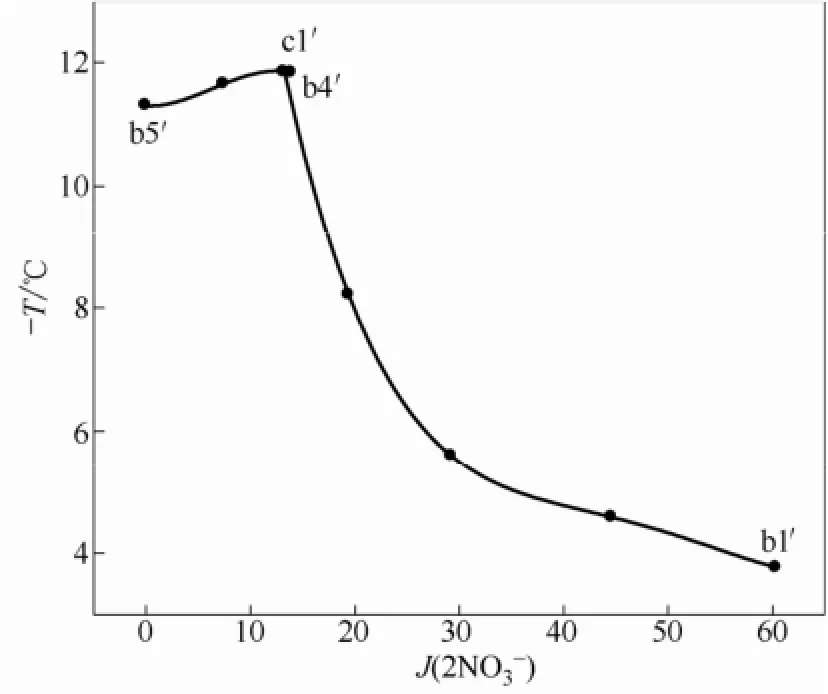
圖7 K+//Cl-,NO-3,SO24--H2O 體系共晶點溫度投影Fig.7 Eutectictemperature projection diagram of system
本文所研究的同離子四元體系,共晶點溫度-液相組成圖中存在3個單鹽與冰的共晶面、3條兩種鹽與冰的共晶線以及1個3種鹽與冰的共晶點。
由圖可直觀發現KCl的濃度對共晶點溫度的影響最大,其與冰相共結晶區最小,表明KCl和冰不易共晶析出,相比之下,硫酸鉀溶液在很大的組成范圍內,都有可能與冰共晶。
四元交互體系的共晶點溫度-液相組成圖的表達方法與三元體系類似,可以采用四棱柱法表達,在此不再詳述,相應的J?necke指數列于表3,據此繪制出相應的共晶點溫度-液相組成圖、干鹽投影圖和共晶點溫度投影圖,見圖8~圖10。圖8中,底面四邊形的4個頂點分別為二元體系NaNO3-H2O、KNO3-H2O、K2SO4-H2O和Na2SO4-H2O,四條邊表示 4個三元體系分別為 NaNO3-Na2SO4-H2O、KNO3-NaNO3-H2O、K2SO4-KNO3-H2O和 Na2SO4-K2SO4-H2O,四邊形內部表示四元體系。此四棱柱底面b1、b2、b3、b6點為各三元體系的兩鹽共晶點液相組成,b1′、b2′、b3′、b6′為各對應的共晶點溫度;c2、c3為此體系的三鹽共晶點液相組成,c2′、c3′為其對應的溫度;a1′、a2′、a3′、a4′為各單鹽和冰的共晶點溫度。兩鹽和冰的共晶曲線 b6′-c2′、b3′-c2′、b1′-c3′、b2′-c3′、c2′-c3′分別對應為Na2SO4·10H2O+NaNO3+Ice、NaNO3+KNO3+Ice、K2SO4+KNO3+Ice、K2SO4+Na2SO4·10H2O+Ice和Na2SO4·10H2O+KNO3+Ice。a4′-b3′-c2′-b6′-a4′為NaNO3和冰的共晶曲面;a1′-b1′-c3′-c2′-b3′-a1′為KNO3和冰的共晶曲面;a2′-b2′-c3′-b1′-a2′為K2SO4和 冰 的 共 晶 曲 面 ; a3′-b2′-c3′-c2′-b6′-a3′為Na2SO4·10H2O和冰的共晶曲面。
表2 R四元體系共晶點液相組成和溫度Table 2 Liquid compositions and temperatures of eutectic points of quaternary system
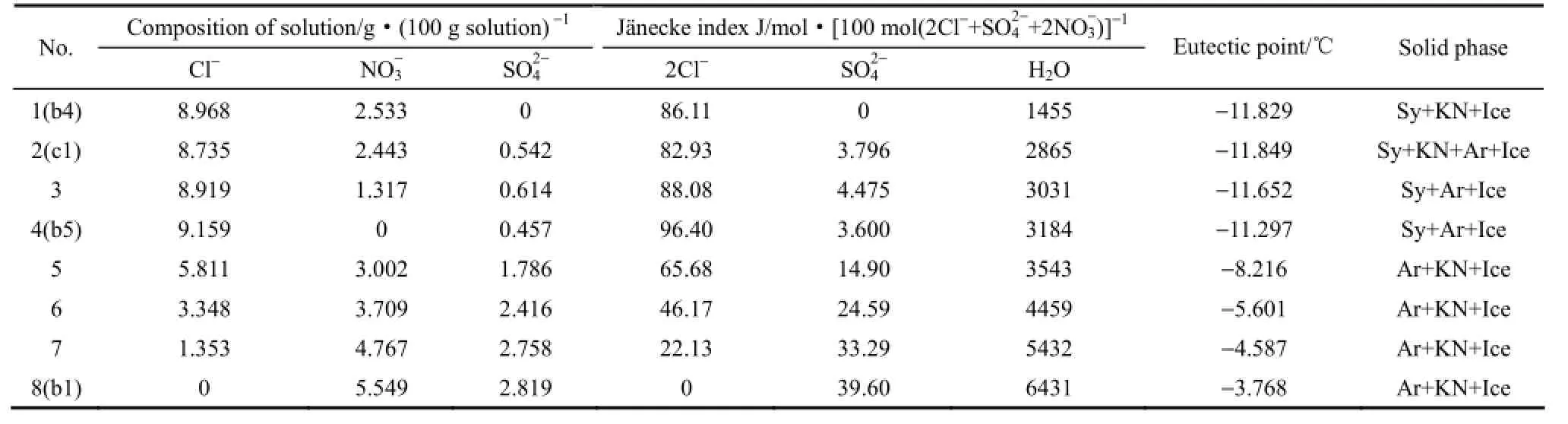
表2 R四元體系共晶點液相組成和溫度Table 2 Liquid compositions and temperatures of eutectic points of quaternary system
Composition of solution/g·(100 g solution)-1 J?necke index J/mol·[100 mol(2Cl-+SO42-+2NO3-)]-1No. Cl- NO3- SO42- 2Cl- SO42- H2O Eutectic point/℃ Solid phase1(b4) 8.968 2.533 0 86.11 0 1455 -11.829 Sy+KN+Ice 2(c1) 8.735 2.443 0.542 82.93 3.796 2865 -11.849 Sy+KN+Ar+Ice 3 8.919 1.317 0.614 88.08 4.475 3031 -11.652 Sy+Ar+Ice 4(b5) 9.159 0 0.457 96.40 3.600 3184 -11.297 Sy+Ar+Ice 5 5.811 3.002 1.786 65.68 14.90 3543 -8.216 Ar+KN+Ice 6 3.348 3.709 2.416 46.17 24.59 4459 -5.601 Ar+KN+Ice 7 1.353 4.767 2.758 22.13 33.29 5432 -4.587 Ar+KN+Ice 8(b1) 0 5.549 2.819 0 39.60 6431 -3.768 Ar+KN+Ice
表3 R四元體系Na+,K+//NO-3,SO24--H2O 共晶點液相組成和溫度Table 3 Liquid compositions and temperatures of eutectic points of quaternary system
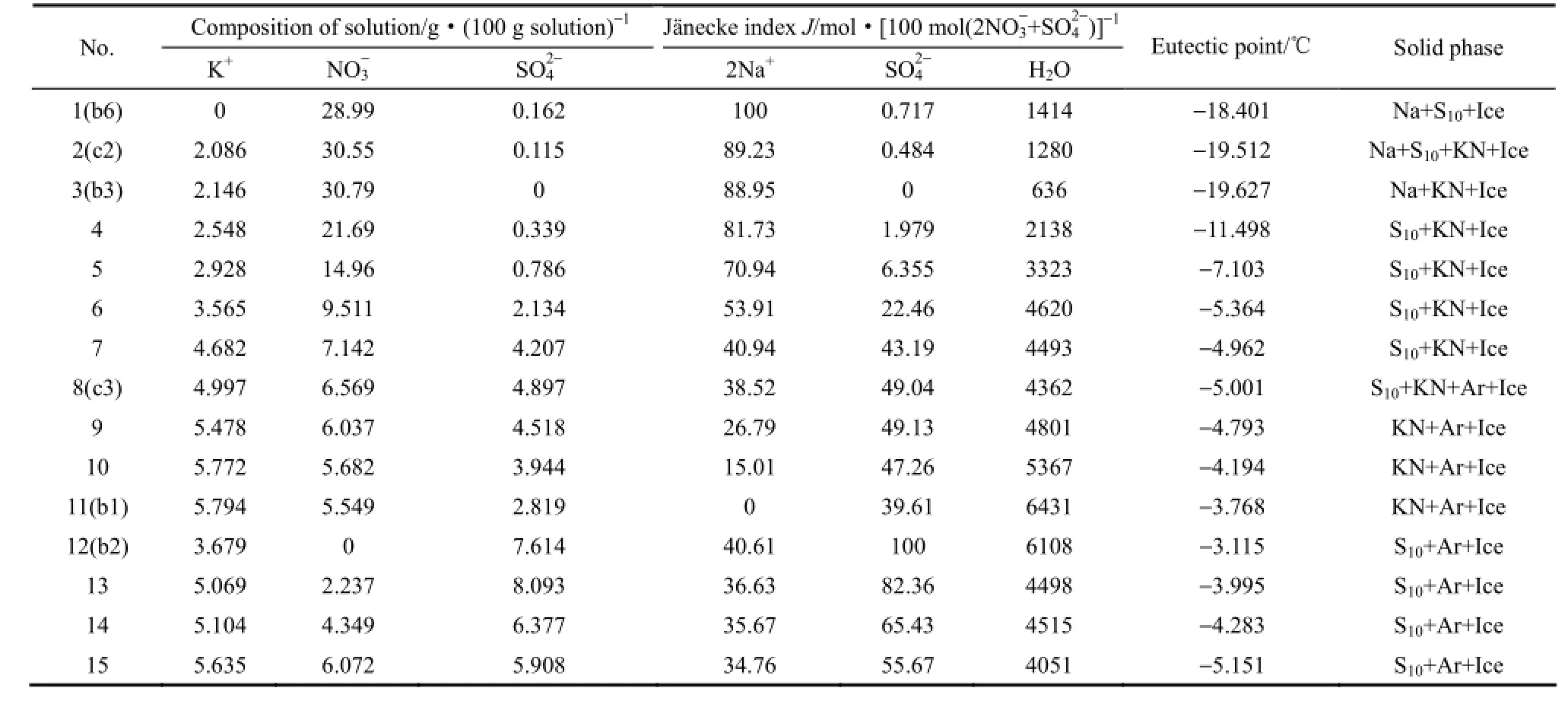
表3 R四元體系Na+,K+//NO-3,SO24--H2O 共晶點液相組成和溫度Table 3 Liquid compositions and temperatures of eutectic points of quaternary system
Composition of solution/g·(100 g solution)J?necke index J/mol·[100 mol(2NO3+SO4)]No. K+ NO3- SO42- 2Na+ SO42- H2O Eutectic point/℃ Solid phase 1(b6) 0 28.99 0.162 100 0.717 1414 -18.401 Na+S10+Ice 2(c2) 2.086 30.55 0.115 89.23 0.484 1280 -19.512 Na+S10+KN+Ice 3(b3) 2.146 30.79 0 88.95 0 636 -19.627 Na+KN+Ice 4 2.548 21.69 0.339 81.73 1.979 2138 -11.498 S10+KN+Ice 5 2.928 14.96 0.786 70.94 6.355 3323 -7.103 S10+KN+Ice 6 3.565 9.511 2.134 53.91 22.46 4620 -5.364 S10+KN+Ice 7 4.682 7.142 4.207 40.94 43.19 4493 -4.962 S10+KN+Ice 8(c3) 4.997 6.569 4.897 38.52 49.04 4362 -5.001 S10+KN+Ar+Ice 9 5.478 6.037 4.518 26.79 49.13 4801 -4.793 KN+Ar+Ice 10 5.772 5.682 3.944 15.01 47.26 5367 -4.194 KN+Ar+Ice 11(b1) 5.794 5.549 2.819 0 39.61 6431 -3.768 KN+Ar+Ice 12(b2) 3.679 0 7.614 40.61 100 6108 -3.115 S10+Ar+Ice 13 5.069 2.237 8.093 36.63 82.36 4498 -3.995 S10+Ar+Ice 14 5.104 4.349 6.377 35.67 65.43 4515 -4.283 S10+Ar+Ice 15 5.635 6.072 5.908 34.76 55.67 4051 -5.151 S10+Ar+Ice
同樣,本文所研究的四元體系,無論常溫下是否存在復鹽,在共晶點或冰點溫度下均為簡單體系,即復鹽不再存在,共晶點溫度-液相組成圖中存在4個單鹽與冰的共晶面、5條兩種鹽與冰的共晶線以及兩個3種鹽與冰的共晶點。
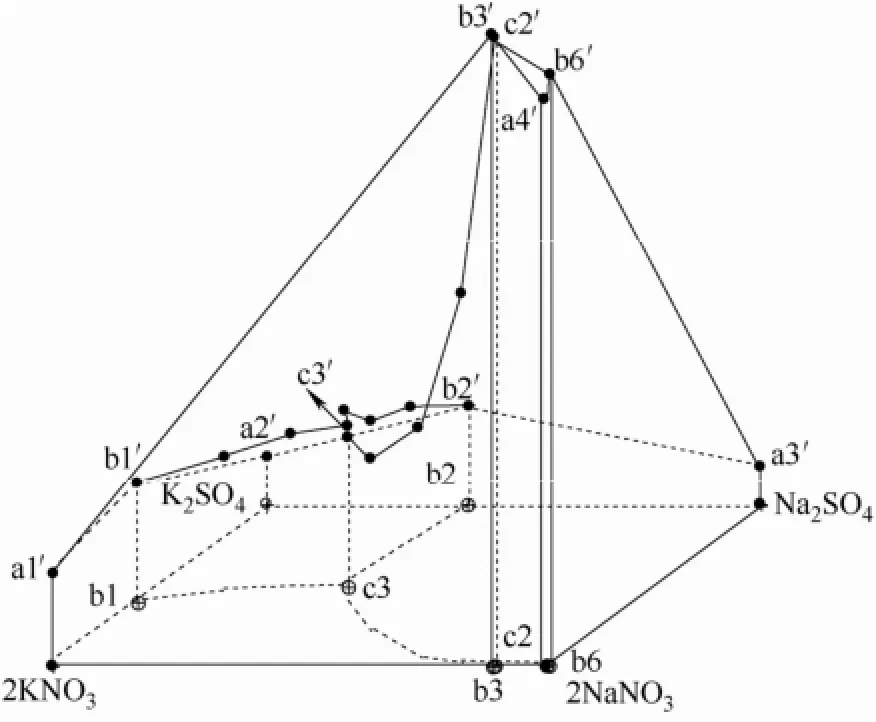
圖8 Na+,K+//NO-3,SO24--H2O 體系共晶點溫度-液相組成(為清晰所見,未給出線上的點)Fig.8 Eutectic point temperature-liquid composition diagram of systemNa+,K+// NO-3,SO24--H2O
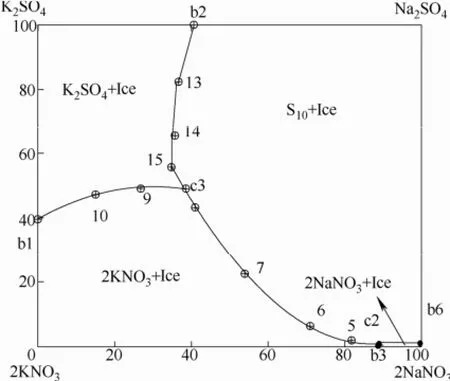
圖9 體系干鹽投影Fig.9 Dry salt projection diagram of system
同樣由圖可直觀發現 NaNO3的濃度對共晶點溫度的影響最大,其單鹽和冰的共晶區最小,不易共晶析出;Na2SO4·10H2O與冰的共晶區最大,較易共晶析出。
3 結 論
(1)通過測定多元水鹽體系降溫過程中的時間-溫度圖,可以判斷鹽類析出規律,測定冰鹽的共晶點溫度與溶液組成;
(2)用棱柱圖可以簡單直觀表達三元體系、四元同離子體系和四元交互體系的相、冰點或共晶點的溫度和溶液組成的關系;
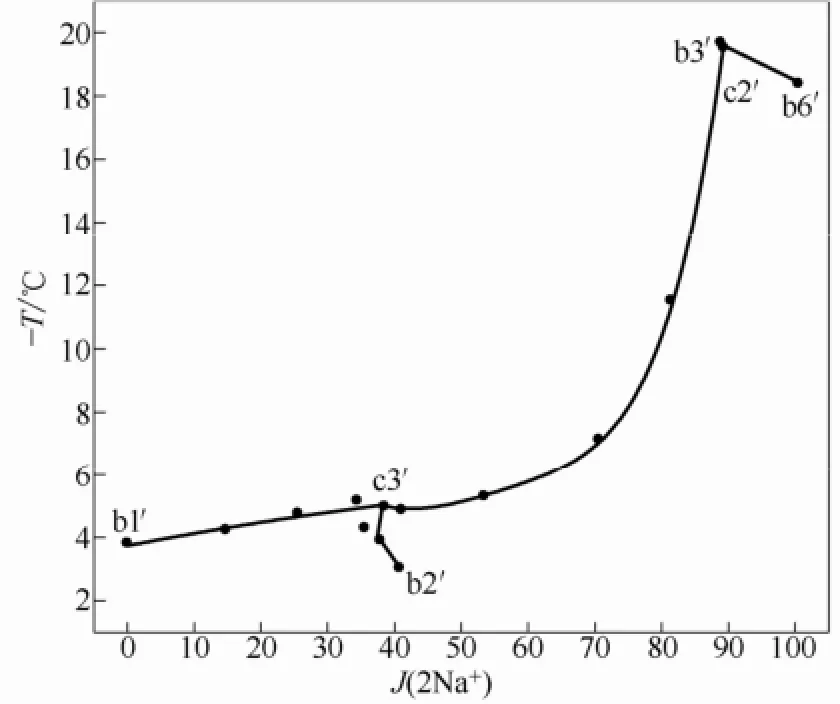
圖10 體系共晶點溫度投影Fig.10 Eutectictemperature projection diagram of system
(3)無復鹽存在的簡單三元體系共晶點溫度-液相組成圖中,存在兩條單鹽與冰的共晶線、一個兩種鹽與冰的共晶點、一個冰析出面;無復鹽存在的四元同離子體系和交互體系共晶點溫度-液相組成圖中,分別有3和4個單鹽與冰的共晶面、3和5條兩種鹽與冰的共晶線、1和2個3種鹽與冰的共晶點。
References
[1] STEPAKOFF G L, SIEGELMAN D, JOHNSON R, et al. Development of an eutectic freeze process for brine disposal [J]. Desalination, 1974, 15(1): 25-38.
[2] VAN DER HAM F, SECKLER M M, WITKAMP G J. Eutectic freeze crystallization in a new apparatus: the cooled disk column crystallizer [J]. Chem. Eng. Process., 2004, 43(2): 161-167.
[3] HIMAWAN C, KRAMER H J M, WITKAMP G J. Study on the recovery of purified MgSO4·7H2O crystals from industrial solution by eutectic freezing [J]. Sep. Purif. Technol., 2006, 50(2): 240-248.
[4] VAN SPRONSEN J, PASCUAL M R, GENCELI F E, et al. Eutectic freeze crystallization from the ternary Na2CO3-NaHCO3-H2O system:a novel scraped wall crystallizer for the recovery of soda from an industrial aqueous stream [J]. Chem. Eng. Res. Des., 2009, 88(9):1259-1263.
[5] REDDY S T, LEWIS A E, WITKAMP G J, et al. Recovery of Na2SO4·10H2O from a reverse osmosis retentate by eutectic freeze crystallization technology [J]. Chem. Eng. Res. Des., 2010, 88(9):1153-1157.
[6] LEWIS A E, NATHOO J, THOMSEN K, et al. Design of a eutectic freeze crystallization process for multicomponent waste water stream[J]. Chem. Eng. Res. Des., 2010, 88(9): 1290-1296.
[7] 李卜義, 王建友. 濃海水處理及綜合利用技術的新進展 [J]. 化工進展, 2014, 33(11): 3067-3074. LI B Y, WANG J Y. Progress on treatment and comprehensive utilization of concentrated seawater [J]. Chemical Industry and Engineering Progress, 2014, 33(11): 3067-3074.
[8] SILCOK H. Solubililies of Inorganic and Organic Compounds[M]. 3rd ed. Oxford: New York Pergamon Press, 1979.
[9] 牛自得, 程芳琴. 水鹽體系相圖及應用[M]. 天津: 天津大學出版社, 2002. NIU Z D, CHENG F Q. The Phase Diagram of Salt-water System and Its Application[M]. Tianjin: Tianjin University Press, 2002.
[10] WEAST R C. CRC Handbook of Chemistry and Physics[M]. BocaRaton, USA: CRC Press, 1974.
[11] HALL D L, STERNER S M , BODNAR R J. Freezing point depression of NaCl-KCl-H2O solutions [J]. Economic Geology, 1988,83(1): 197-202.
[12] OAKES C S, BODNAR R J, SIMONSON J M. The system NaCl-CaCl2-H2O(Ⅰ): The ice liquidus at 1 atm total pressure [J]. Geochimica et Cosmochimica Acta, 1990, 54(3): 603-610.
[13] GIBBARD JR H F, GOSSMANN A F. Freezing points of electrolyte mixtures (Ⅰ): Mixtures of sodium chloride and magnesium chloride in water [J]. Journal of Solution Chemistry, 1974, 3(5): 385-393.
[14] DUBOIS M, MARIGNAC C. The H2O-NaCl-MgCl2ternary phase diagram with special application to fluid inclusion studies [J]. Economic Geology, 1997, 92(1): 114-119.
[15] SANDER B, FREDENSLUND A, RASMUSSEN P. Calculation of vapor-liquid equilibria in mixed solvent/salt systems using an extended UNIQUAC equation [J]. Chemical Engineering Science,1986, 41(5): 1171-1183.
[16] THOMSEN K, RASMUSSEN P, GANI R. Correlation and prediction of thermal properties and phase behavior for a class of aqueous electrolyte systems [J]. Chemical Engineering Science, 1996, 51(14):3675-3683.
[17] THOMSEN K. Aqueous electrolytes: model parameters and process simulation[D]. Denmark: Technical University of Denmark, 1997.
[18] ALONSO H A T, PERALTA J M, RUBIOLO A C, et al. Prediction of the freezing point of multicomponent liquid refrigerant solutions [J]. Journal of Food Engineering, 2011, 104(1): 143-148.
[19] PERALTA J M, RUBIOLO A C, ZORRILLA S E. Prediction of heat capacity, density and freezing point of liquid refrigerant solutions using an excess Gibbs energy model [J]. Journal of Food Engineering,2007, 82(4): 548-558.
Determination and graphics expression of ice-salt eutectic points of multicomponent salt-water systems
WANG Xueying, HUANG Wenting, HUANG Xueli
(Key Laboratory of Cleaner Transition of Coal & Chemicals Engineering of Xinjiang Uyghur Autonomous Region, College of Chemistry and Chemical Engineering, Xinjiang University, Urumqi 830046, Xinjiang, China)
For the quaternary salt-water systems, such as the homo-ion systemand interaction system, as well as their six ternary subsystems, the freezing points, eutectic points and the crystallization regularities of salts were studied by cooling method. According to experimental data,the eutectic point temperature-liquid composition diagrams for these systems were plotted. The results were as follows: (1) The crystallization regularity of salts and the eutectic point temperatures can be obtained by measuring the time-temperature curves for multicomponent salt-water systems during cooling process; (2) In these systems above, 3K2SO4·Na2SO4existing under normal temperature did not appear at the eutectic point temperature. As a result, the phase relationships in these systems were simplified; (3) The prism can be used to describe the relationships among the phases, freezing points or eutectic points and the compositions of liquids for the ternary systems, the quaternary homo-ion systems and interaction systems concisely and visually; (4) In the eutectic point temperature-liquid composition diagrams, for ternary systems without double-salts, there were twoeutectic curves with single salt and ice, one eutectic point with two salts and ice, and one ice crystallizing region. For the quaternary homo-ion systems and interaction systems, there were three and four eutectic regions with single salt and ice, three and five eutectic curves with two salts and ice, and one and two eutectic points with three salts and ice, respectively.
date: 2015-09-23.
Prof. HUANG Xueli, xuelih@163.com
supported by the National Natural Science Foundation of China (21166022).
salt-water system; eutectic point; temperature; phase
O 642.5+4
A
0438—1157(2016)05—1687—07
2015-09-23收到初稿,2016-01-25收到修改稿。
聯系人:黃雪莉。第一作者:王雪瑩(1988—),女,碩士研究生。
國家自然科學基金項目(21166022)。

