紫外激發Ba2SiO4:Gd3+,Tb3+的發光性能
肖林久,耿艷麗,謝 穎,姜新東,崔永強,汪書東
(沈陽化工大學遼寧省稀土化學及應用重點實驗室,遼寧沈陽 110142)
紫外激發Ba2SiO4:Gd3+,Tb3+的發光性能
肖林久*,耿艷麗,謝穎,姜新東,崔永強,汪書東
(沈陽化工大學遼寧省稀土化學及應用重點實驗室,遼寧沈陽 110142)
采用高溫固相法合成了可被紫外光激發的Ba2SiO4:Gd3+,Tb3+熒光粉。考察了激活離子摻雜量等因素對發光性能的影響。通過X射線衍射(XRD)、熒光(FL)光譜和熒光壽命曲線對所合成樣品的結構和發光性能進行表征,研究了Gd3+和Tb3+的特征吸收波長激發Ba2SiO4:Gd3+,Tb3+的發光性能。在275 nm(Gd3+:8S7/2→6IJ)激發下,檢測到了Tb3+的特征發射。通過對比不同Tb3+摻雜量下Gd3+:6P7/2能級的衰減曲線,發現隨著Tb3+摻雜濃度的增加,該能級的熒光壽命不斷縮短,表明樣品中存在Gd3+→Tb3+的能量傳遞,傳遞方式為無輻射共振能量傳遞。在244 nm(Tb3+:4f8→4f75d1)激發下,Gd3+的摻入使得Tb3+的5D3能級的發射逐漸減弱,5D4能級的發射增強。Gd3+的摻入使得544 nm(5D4→7F5)處的特征發射增強了59%~128%,結合熒光衰減曲線得出Gd3+的摻入對Tb3+能級中5D3→5D4與7F6→7F0交叉馳豫有促進作用。
熒光粉;能量傳遞;交叉馳豫;Ba2SiO4:Gd3+,Tb3+
1 引 言
稀土摻雜的無機材料在照明光源、太陽能電池、X射線影像、閃爍體、生物探針等諸多領域有著廣闊的應用前景,自其進入發光材料領域以來一直受到研究者們的重視[1]。白光LED以其高效節能等優點[2]吸引了世界目光,被譽為21世紀的新一代照明光源[3-5]。隨著LED芯片的發展,紫外/近紫外型熒光粉越來越受到關注[6-7]。其中用于光轉換的綠色熒光粉的發光效率對總的光通量影響很大,是紫外發光二極管用三基色熒光粉開發中的一個重要環節[6,8]。
以硅酸鹽為基質的熒光粉由于具有良好的化學穩定性和熱穩定性,而且高純度的二氧化硅原料具有價廉、易得、燒結溫度比鋁酸鹽體系低的優點,長期以來受到人們的重視。發射綠光的典型離子代表是Tb3+,研究發現Gd3+可有效地敏化Tb3+而增強其發光。盡管Gd3+和Tb3+共摻雜的熒光粉的發光性能及Gd3+→Tb3+的能量傳遞已有一些研究,如:Na3GdSiO2O7:Tb3+[9]、δ-Gd2Si2O7:Eu3+,Tb3+[10]、CdSiO3:Tb3+,Re3+(Re3+=Gd3+,Y3+,La3+)[11]等,但該離子對在Ba2SiO4基質中的研究尚鮮有報道,并且Gd3+對Tb3+間的交叉馳豫的影響也未見報道。
本文采用高溫固相法制備了可被紫外光激發的Ba2SiO4:Gd3+,Tb3+熒光粉,研究了Ba2SiO4:Gd3+,Tb3+熒光粉在Gd3+特征吸收光激發下和Tb3+特征吸收光激發下的光譜性能,探討了Gd3+→Tb3+的能量傳遞以及Tb3+間的交叉馳豫現象。
2 實 驗
2.1樣品制備
合成樣品使用了氧化釓(Gd2O3,99.99%)、氧化鋱(Tb4O7,99.99%)、碳酸鋇(BaCO3,AR)、二氧化硅(SiO2,AR)和硼酸(H3BO3,AR)等原料。制備過程采用高溫固相法,按照實驗考察確定的適宜制備條件,將不同的稀土元素摻雜量按化學計量比稱量放入瑪瑙研缽中,充分研磨使其均勻,而后裝入瓷舟放入高溫管式爐中,升溫至1 200℃并保溫2 h,然后冷卻至室溫,取出樣品并用瑪瑙研缽研細,即得所需的Ba2-x-ySiO4:xGd3+,yTb3+樣品。
2.2樣品表征
樣品采用Bruker D8 X射線衍射儀(XRD)確定物相,儀器經過退火的Al2O3校正,采用銅靶(λ=0.154 06 nm),電壓為40 kV,電流為40 mA,掃描范圍為10°~80°,步長設置為0.05°。采用Hitach公司的F-4600熒光光譜儀測試樣品的激發光譜和發射光譜。采用Horiba公司的FL-3穩態/瞬態熒光光譜儀測試樣品的熒光衰減曲線。所有測試都在室溫條件下進行。
3 結果與討論
3.1晶相分析
1 200℃下焙燒2 h制得的Ba1.85SiO4:0.1Gd3+,0.05Tb3+的XRD譜如圖1所示。從圖中可看出,樣品為單一純相,符合基質材料Ba2SiO4的JCPDS標準卡片(JCPDS No.26-1403)圖譜數據,屬于正交晶系。

圖1 Ba2SiO4:Gd3+,Tb3+的X射線衍射譜及Ba2SiO4的標準卡片(JCPDS No.26-1403)Fig.1 XRD patterns of Ba2SiO4:Gd3+,Tb3+samples and the standard data of Ba2SiO4(JCPDS No.26-1403)
3.2激發光譜
圖2(a)給出了單摻Gd3+樣品的激發光譜,監測波長為313 nm(Gd3+:6P7/2→8S7/2躍遷發射),激發峰在275 nm處,來源于Gd3+的銳線型8S7/2→6I7/2能級躍遷吸收。圖2(b)為單摻Tb3+樣品的激發光譜,監測波長為544 nm(Tb3+:5D4→7F5躍遷發射),激發峰在244 nm處,來源于Tb3+的銳線型4f8→4f75d1躍遷吸收。圖2(c)為雙摻Gd3+/Tb3+樣品的激發光譜。實線為313 nm(Gd3+特征發射)監測下的激發光譜,激發峰在275 nm處,來源于Gd3+的8S7/2→6I7/2能級躍遷;虛線為544 nm(Tb3+特征發射)監測下的激發光譜,從圖中可同時觀測到244 nm處Tb3+的4f8→4f75d1躍遷吸收和275 nm處Gd3+的8S7/2→6I7/2能級躍遷吸收,說明樣品中存在Gd3+到Tb3+的能量傳遞。
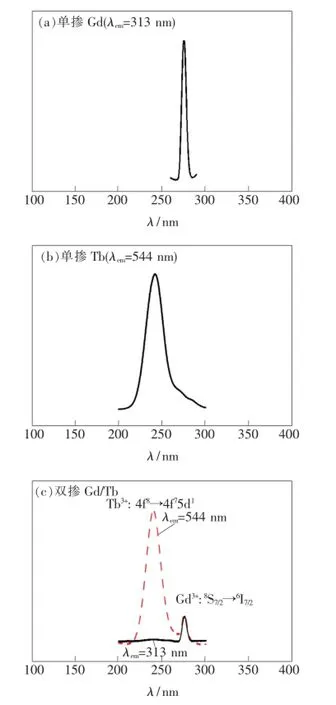
圖2 單摻Gd3+(a)、單摻Tb3+(b)及雙摻Gd3+/Tb3+(c)樣品的激發光譜。Fig.2 Excitation spectra of single doped Gd3+(a),single doped Tb3+(b),and double doped Gd3+/Tb3+(c)samples,respectively.
3.3發射光譜
3.3.1275 nm(Gd3+特征吸收光)激發下的發射光譜
在Gd3+特征吸收275 nm的激發下,Ba1.9-y-SiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)的發射光譜如圖3所示。當Ba2SiO4中只摻Gd3+(y=0)時,僅能觀測到313 nm處Gd3+的6P7/2→8S7/2躍遷發射峰;當Ba2SiO4中同時摻有Gd3+和Tb3+時,在Gd3+特征吸收光275 nm激發產生的發射光譜中,既出現了Gd3+的特征發射,還觀測到了Tb3+的特征發射,證明樣品中存在Gd3+到Tb3+的能量傳遞。隨著Tb3+摻雜濃度的增大,Gd3+的發射強度降低,Tb3+的特征發射增強。當Tb3+的摩爾分數y=0.05時,Tb3+的發射達到最強,繼續增加Tb3+的濃度,Gd3+和Tb3+的發光均減弱,發生了濃度猝滅。
由Gd3+的能級躍遷可知,圖中313 nm的發射峰對應于Gd3+的6P7/2→8S7/2能級躍遷,381,416,437 nm的發射峰對應于Tb3+的5D3→7FJ(J=6,5,4)能級躍遷,488,544,584,616 nm的發射峰對應于Tb3+的5D4→7FJ(J=6,5,4,3)能級躍遷。
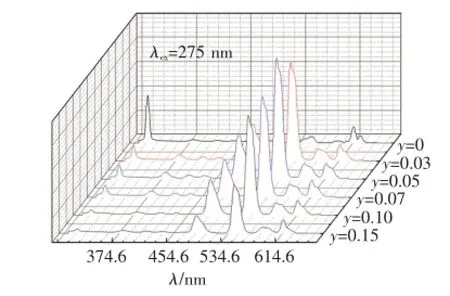
圖3 在275 nm的Gd3+特征吸收光激發下的Ba1.9-ySiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)的發射光譜Fig.3 Emission spectra of Ba1.9-ySiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)under 275 nm excitation of Gd3+characteristic absorption
為進一步分析Gd3+→Tb3+的能量傳遞,對比圖3中Gd3+的特征發射(313 nm)和Tb3+的特征發射(544 nm)熒光強度隨Tb3+摻雜濃度的變化情況,得到圖4。隨著Tb3+濃度的增加,Gd3+的特征發射峰的強度(實線)逐漸降低,而Tb3+的特征發射峰的強度(虛線)先增大后減小,由此可看出Gd3+發射出的能量并未轉換成光釋放出來,而是通過無輻射共振的方式作為Tb3+的吸收光譜,從而被Tb3+吸收。而隨著Tb3+摻入量的增加,較多的Tb3+原子吸收的能量也就越多,從而使得Gd3+以發光形式放出的能量進一步減少,所以Gd3+的313 nm發射峰的強度隨著Tb3+摻入量的增加而逐漸降低,Tb3+的544 nm特征發射峰的強度增加[12]。但是當y(Tb3+)>0.05時,Tb3+的544 nm(5D4→7F5)處的發光強度反而降低。Tb3+的能級結構表明,5D4沒有能量匹配的交叉弛豫途徑,因此5D4(544 nm)發光的濃度猝滅主要是由于Tb3+離子間交換相互作用引起的,使得能量的損失超過了能量的發射,發生濃度猝滅,繼而發光強度降低[13]。
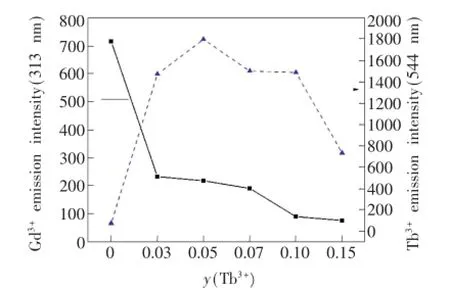
圖4 Ba1.9-ySiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)在313 nm和544 nm處的發射強度(λex=275 nm)Fig.4 Emission intensity of Ba1.9-ySiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)at 313 nm and 544 nm(λex=275 nm)
圖5是不同Tb3+摻雜濃度樣品在275 nm激發下,監測Gd3+:6P7/2→8S7/2(313 nm)處的衰減曲線。從擬合曲線得出,Gd3+單摻樣品的熒光壽命為63.80 μs。隨著樣品中Tb3+摻雜濃度的增加,Gd3+的衰減明顯變快,當y(Tb3+)為10%時,Gd3+:6P7/2的壽命為46.40 μs。熒光壽命縮短的原因是隨著樣品中Tb3+的加入,Gd3+存在了一種新的衰減路徑,在6P7/2能級布居的電子通過能量傳遞,將能量傳遞給Tb3+使其達到5H7能級,造成了Gd3+:6P7/2能級衰減加速。根據Dexter能量傳輸理論[14],這說明Gd3+和Tb3+之間存在的能量傳遞是無輻射能量傳遞。
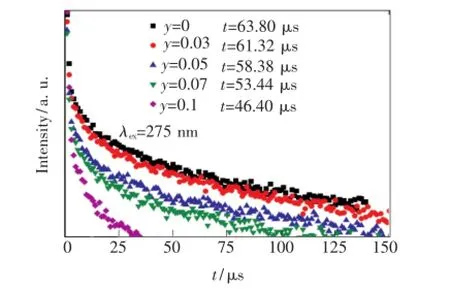
圖5 275 nm激發下的Gd3+:6P7/2→8S7/2(313 nm)的發光衰減曲線與Tb3+摻雜量的關系Fig.5 Decay time of Gd3+:6P7/2emission(313 nm)under the excitation of 275 nm as a function of Tb3+content
根據Gd3+和Tb3+能級數據畫出Gd3+→Tb3+的能量傳遞示意圖,如圖6所示。由于Gd3+的8S7/2→6P7/2與Tb3+的5H7→7F6能量間距相近,均約為32 000 cm-1,因此Gd3+→Tb3+的能量傳遞可通過共振無輻射形式實現。
在275 nm激發下,Gd3+的基態8S7/2能級吸收能量激發到6IJ能級,然后快速無輻射弛豫(NR)到6P7/2能級。6P7/2能級的電子可躍遷回到基態產生Gd3+的特征發射(313 nm),或者通過共振能量傳遞將激發能傳遞給Tb3+,使得Tb3+從基態躍遷到5H7能級,再無輻射弛豫到Tb3+的較低激發態5D3和5D4能級,最后躍遷至不同的基態能級產生Tb3+的特征發射[15]。
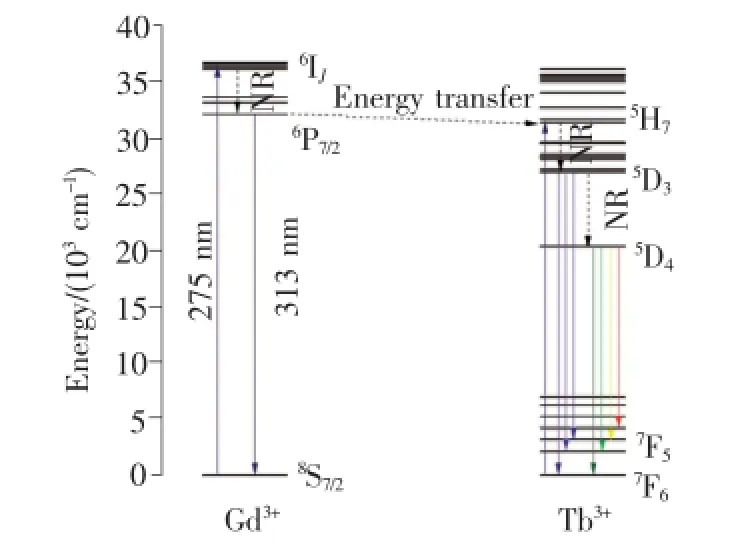
圖6 Gd3+和Tb3+離子的能級結構(λex=275 nm)Fig.6 Schematic energy-level diagram of selected states of Gd3+and Tb3+ions(λex=275 nm)
3.3.2244 nm(Tb3+特征吸收光)激發下的發射光譜
圖7是在Tb3+特征吸收244 nm激發下的Ba1.95-xSiO4:xGd3+,0.05Tb3+(x=0,0.05,0.10,0.15,0.20)的發射光譜。由圖可知,Gd3+的摻入使樣品在381,416,437 nm處(5D3→7FJ(J=6,5,4))的發射減弱,而488,544,584,616 nm處(5D4→7FJ(J=6,5,4,3))的發射增強。分析圖中不同Gd3+摻雜量引起Tb3+的熒光強度變化可知,5%~15%Gd3+的摻入使得摻雜5%的Tb3+在544 nm處的發射增強了59%~128%,而416 nm處的發射強度單調降低。
對單摻0.05Tb3+和雙摻0.1Gd3+、0.05Tb3+樣品在244 nm激發下的Tb3+:5D3→7F5(416 nm)處的熒光衰減進行監測,得到圖8。當離子間相互作用不明顯時,衰減曲線可使用單指數函數擬合。單摻0.05Tb3+樣品的衰減曲線稍微偏離單指數衰減,這是因為樣品中存在Tb3+間交叉馳豫過程[16]。隨著樣品中Gd3+的摻入,衰減曲線偏離程度加大,且Tb3+:5D3能級的熒光壽命從8.50μs減小到7.28 μs,說明Gd3+的摻入增大了Tb3+間交叉馳豫的幾率,使Tb3+:5D3能級布居的電子數減少。
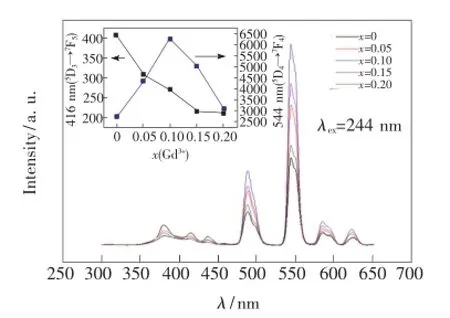
圖7 244 nm Tb3+特征吸收光激發下的Ba1.95-xSiO4:xGd3+,0.05Tb3+(x=0,0.05,0.10,0.15,0.20)的發射光譜Fig.7 Emission spectra of Ba1.95-xSiO4:xGd3+,0.05Tb3+under the excitation of 244 nm(x=0,0.05,0.10,0.15,0.20)
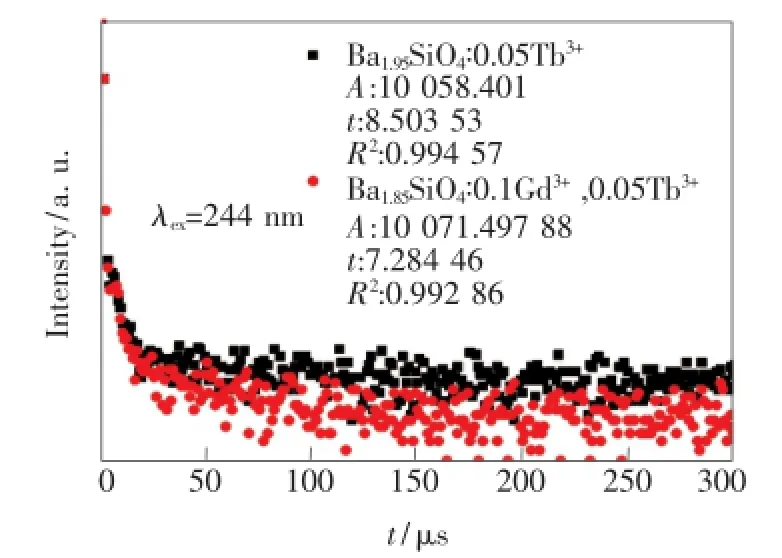
圖8 244 nm激發下的Tb3+:5D3→7F5(416 nm)的發光衰減曲線Fig.8 Decay time of Tb3+:5D3emission(416 nm)under the excitation of 244 nm
在Tb3+發射過程中,5D3→5D4與7F6→7F0由于能級差相近,存在交叉馳豫現象(圖9),使得Tb3+中處于5D3激發態上的電子躍遷到5D4能級,而另一個處于基態的Tb3+(7F6)被激發到7F0能級。結合圖7、8可知,Gd3+的摻入增大了Tb3+間交叉馳豫的幾率,從而使得5D3能級發射減弱,5D4能級發射增強。

圖9 Ba2SiO4晶體中Tb3+離子的交叉馳豫Fig.9 Cross-relaxation process between Tb3+ions in Ba2SiO4
4 結 論
采用高溫固相法合成的Ba2SiO4:Gd3+,Tb3+熒光粉,在275 nm和244 nm激發下均可產生544 nm的Tb3+特征發射光。在275 nm的Gd3+特征吸收光的激發下,檢測到Tb3+的特征發射,通過對比不同Tb3+摻雜量下Gd3+:6P7/2能級的衰減曲線,發現隨著Tb3+摻雜濃度的增加,該能級的熒光壽命由63.80 μs衰減到46.40 μs,表明樣品中存在Gd3+→Tb3+的能量傳遞,傳遞方式為無輻射共振能量傳遞,可歸結為Gd3+的6P7/2能級和Tb3+的5H7能級間的共振轉移。在244 nm的Tb3+特征吸收光的激發下,Gd3+的摻入可增強Tb3+的發射,使得摻雜5%的Tb3+在544 nm處的發射增強了59%~128%,結合熒光衰減曲線得出Gd3+的摻入對Tb3+能級中5D3→5D4與7F6→7F0交叉馳豫有促進作用。
[1]劉春旭,張家驊,呂少哲,等.納米Gd2O3:Eu3+中Judd-Ofelt參數的實驗確定[J].物理學報,2004,53(11):3945-3949. LIU C X,ZHANG J H,Lü S Z,et al..Judd-Ofelt parameters determined experimentally for nanoparticles Gd2O3:Eu3+[J].Acta Phys.Sinica,2004,53(11):3945-3949.(in Chinese)
[2]ZHANG Y,GENG D L,SHANG M M,et al..Single-composition trichromatic white-emitting Ca9MgNa(PO4)7:Ce3+/ Tb3+/Mn2+phosphors-soft chemical synthesis,luminescence,and energy-transfer properties[J].Eur.J.Inorg. Chem.,2013,2013(25):4389-4397.
[3]侯青月,李金凱,段廣彬,等.白光發光二極管用熒光粉的研究進展[J].中國粉體技術,2015,21(5):44-47. HOU Q Y,LI J K,DUAN G B,et al..Progress of research on phosphors for white light emitting diodes[J].China Powder Sci.Technol.,2015,21(5):44-47.(in Chinese)
[4]YE S,XIAO F,PAN Y X,et al..Phosphors in phosphor-converted white light-emitting diodes:recent advances in materials,techniques and properties[J].Mater.Sci.Eng.,2010,71(1):1-34.
[5]曾琦華,張信果,梁宏斌,等.白光LED用熒光粉的研究進展[J].中國稀土學報,2011,29(1):8-17. ZENG Q H,ZHANG X G,LIANG H B,et al..Progress of research on phosphors for white-light emitting diodes[J].J. Chin.Rare Earth Soc.,2011,29(1):8-17.(in Chinese).
[6]LIU Y,LIU G X,WANG J X,et al..Single-component and warm-white-emitting phosphor NaGd(WO4)2:Tm3+,Dy3+,Eu3+:synthesis,luminescence,energy transfer,and tunable color[J].Inorg.Chem.,2014,53(21):11457-11466.
[7]ZHANG X G,ZHOU L Y,PANG Q,et al..Tunable luminescence and Ce3+→Tb3+→Eu3+energy transfer of broadband-excited and narrow line red emitting Y2SiO5:Ce3+,Tb3+,Eu3+phosphor[J].J.Phys.Chem.C,2014,118(14):7591-7598.
[8]謝飛燕,董志月,刁貴強,等.一種新型的白光LED用綠色熒光粉Ca8MgLu(PO4)7:Tb3+[J].發光學報,2015,36(10):1132-1136. XIE F Y,DONG Z Y,DIAO G Q,et al..A novel green phosphor Ca8MgLu(PO4)7:Tb3+for near ultraviolet white lightemitting diodes[J].Chin.J.Lumin.,2015,36(10):1132-1136.(in Chinese)
[9]倪海勇,梁宏斌,王靈利,等.Na3GdSi2O7:Tb3+熒光粉發光特性及Gd3+→Tb3+之間的能量傳遞[J].發光學報,2013,34(8):970-975. NI H Y,LIANG H B,WANG L L,et al..Luminescent properties of phosphor Na3GdSi2O7:Tb3+and Gd3+→Tb3+energy transfer[J].Chin.J.Lumin.,2013,34(8):970-975.(in Chinese)
[10]FERNáNDEZ-CARRIóN A J,óCA?A M,GARC I'A-SEVILLANO J,et al..New single-phase,white-light-emitting phosphors based on δ-Gd2Si2O7for solid-state lighting[J].J.Phys.Chem.C,2014,118(31):18035-18043.
[11]ZENG W,WANG Y H,HAN S C,et al..Enhancement of CdSiO3:Tb3+green long-lasting phosphors by co-doping with Re3+(Re3+=Gd3+,Y3+,La3+)ions[J].J.Lumin.,2014,152:210-213.
[12]SUN X Y,HUANG S M,GU M,et al..Enhanced Tb3+luminescence by non-radiative energy transfer from Gd3+in silicate glass[J].Phys.B,2010,405(2):569-572.
[13]楊志平,楊富,侯春彩,等.新型綠色熒光粉BaLa2ZnO5:Tb3+的合成與發光性質[J].發光學報,2014,35(10):1153-1157. YANG Z P,YANG F,HOU C C,et al..Synthesis and luminescence properties of novel green emitting phosphor BaLa2ZnO5:Tb3+[J].Chin.J.Lumin.,2014,35(10):1153-1157.(in Chinese)
[14]許少鴻.固體發光[M].北京:清華大學出版社,2011. XU S H.Luminescence of Solids[M].Beijing:Tsinghua University Press,2011.(in Chinese)
[15]RAJU G S,PARK J Y,JUNG H C,et al..Gd3+sensitization effect on the luminescence properties of Tb3+activated calcium gadolinium oxyapatite nanophosphors[J].J.Electrochem.Soc.,2011,158(2):J20-J26.
[16]VAN DER ENDE B M,AARTS L,MEIJERINK A.Near-infrared quantum cutting for photovoltaics[J].Adv.Mater.,2009,21(30):3073-3077.

肖林久(1958-),男,遼寧莊河人,博士,教授,2005年于東北大學獲得博士學位,主要從事稀土發光材料、精細化工、催化和反應技術等方面的研究。
E-mail:x109@163.com
Luminescence Properties of Ba2SiO4:Gd3+,Tb3+Under UV Excitation
XIAO Lin-jiu*,GENG Yan-li,XIE Ying,JIANG Xin-dong,CUI Yong-qiang,WANG Shu-dong
(Key Laboratory of Rare-earth Chemistry and Applications of Liaoning Province,Shenyang University of Chemical Technology,Shenyang 110142,China)
*Corresponding Author,E-mail:x109@163.com
A series of Ba2SiO4:Gd3+,Tb3+green phosphors were synthesized by solid state reaction.The influence of the factors such as the amount of ion doping on the luminescence properties was investigated.Their structure and luminescence properties were characterized by X-ray diffraction(XRD)analysis,fluorescence spectrometry(FL),and decay curves.The spectroscopic properties of Ba2SiO4:Gd3+,Tb3+under the excitation of the characteristic absorption wavelength of Gd3+and Tb3+were investigated. The emission of Tb3+was observed under 275 nm(Gd3+:8S7/2→6IJ)excitation.By comparing Gd3+:6P7/2energy decay curve with different Tb3+doping amount,it is found that the level of fluorescence continuously shorten the service life with the increasing of Tb3+concentration,indicating the occurrence of energy transfer from Gd3+to Tb3+with the mode of nonradiative resonance transfer.Under the excitation of 244 nm(Tb3+:4f8→4f75d1),the emission intensity of5D3level decreases,but the5D4level increases with the doping of Gd3+.The emission intensity at 544 nm(5D4→7F5)increases 59%~128%with the doping of Gd3+.Combining with the fluorescence decay curves,it is found that the doping of Gd3+can promote the cross relaxation of5D3→5D4and7F6→7F0in Tb3+.
phosphors;energy transfer;cross relaxation;BaSiO:Gd3+,Tb3+24
O482.31
A
10.3788/fgxb20163706.0644
1000-7032(2016)06-0644-06
2016-02-03;
2016-03-13
遼寧省教育廳科研項目(L2012149,L2015420)資助

