玉米光敏色素A1與A2在各種光處理下的轉(zhuǎn)錄表達特性
楊宗舉閆 蕾宋梅芳蘇 亮孟凡華李紅丹1,白建榮郭 林,*楊建平,*
1中國農(nóng)業(yè)科學院研究生院, 北京 100081;2中國農(nóng)業(yè)科學院作物科學研究所, 北京 100081;3山西大學生物工程學院, 山西太原030006;4北京市輻射中心, 北京 100875;5山西省農(nóng)業(yè)科學院作物科學研究所, 山西太原 030031
玉米光敏色素A1與A2在各種光處理下的轉(zhuǎn)錄表達特性
楊宗舉1,2,**閆 蕾2,3,**宋梅芳2,4蘇 亮2孟凡華2李紅丹1,2白建榮5郭 林2,*楊建平2,*
1中國農(nóng)業(yè)科學院研究生院, 北京 100081;2中國農(nóng)業(yè)科學院作物科學研究所, 北京 100081;3山西大學生物工程學院, 山西太原030006;4北京市輻射中心, 北京 100875;5山西省農(nóng)業(yè)科學院作物科學研究所, 山西太原 030031
光敏色素是一類紅光/遠紅光受體, 它們在植物體內(nèi)有非活性形式的紅光吸收型(Pr)和活性形式遠紅光吸收型(Pfr) 2種狀態(tài), 通常其活性形式負責調(diào)控植物的種子萌發(fā)、株高、開花時間和避蔭性等生長發(fā)育過程。在禾本科中, 光敏色素只有 PHYA、PHYB和 PHYC三個基因亞家族, 古四倍體化造成的玉米光敏色素基因有 6個成員, 即PHYA1、PHYA2、PHYB1、PHYB2、PHYC1和PHYC2。光敏色素A參與抑制下胚軸的伸長、促進張開子葉和花青素的積累、阻斷持續(xù)遠紅光條件下的變綠。為了評價ZmPHYA1和ZmPHYA2對光的響應(yīng)能力及其功能差異, 本研究采用實時定量PCR技術(shù)分析玉米自交系B73和Mo17中ZmPHYA1和ZmPHYA2對不同光照處理響應(yīng)的表達模式。結(jié)果表明玉米光敏色素A主要在葉片和花絲中表達, 并且ZmPHYA1轉(zhuǎn)錄豐度是ZmPHYA2的2~8倍; 玉米自交系B73 和Mo17中胚軸在黑暗、遠紅光和藍光條件下較紅光和白光下更長。ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄水平在持續(xù)遠紅光和藍光條件下均較高; 并且均較迅速響應(yīng)黑暗到遠紅光和藍光光質(zhì)轉(zhuǎn)換, 但是前者的豐度顯著高于后者,ZmPHYA1在遠紅光下更重要, 而ZmPHYA2在藍光下更重要。ZmPHYA1和ZmPHYA2同樣響應(yīng)于黑暗到紅光和白光的轉(zhuǎn)換, 并且ZmPHYA1和ZmPHYA2表達模式基本一致。ZmPHYA1和ZmPHYA2的表達均能響應(yīng)長日照和短日照處理, 但是ZmPHYA1轉(zhuǎn)錄豐度高于ZmPHYA2的2~5倍。以上結(jié)果表明, ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄能有效地響應(yīng)各種光處理, 可能ZmPHYA1在作物改良上比ZmPHYA2更有效。本研究為進一步了解ZmPHYA1和ZmPHYA2基因功能以及評價二者的光反應(yīng)能力提供了理論基礎(chǔ)。
玉米; 光敏色素; 光信號轉(zhuǎn)導; 表達分析; 光處理
植物生長發(fā)育過程會受到來自環(huán)境的生物及非生物因素影響[1], 其中光作為重要的非生物環(huán)境因子, 對植物整個生命過程起至關(guān)重要的作用[2]。光不僅是植物光合作用的能量來源, 而且作為環(huán)境信號參與調(diào)控種子萌發(fā)、幼苗去黃化、葉片展開、下胚軸伸長、向光性、氣孔開關(guān)、葉綠體移動、避蔭性、節(jié)律、開花等生長和發(fā)育過程[2-6]。植物的光受體系統(tǒng)負責感知光的強度、波長、節(jié)律和方向[6-11], 高等植物至少存在 3類光受體系統(tǒng), 以滿足它們對不同波長信號(紅光/遠紅光、藍光/紫外光A, 以及紫外光B)的吸收[12], 其中光敏色素是紅光/遠紅光(600~750 mm)的受體[13]。模式植物擬南芥具有 5個光敏色素基因(PHYA-PHYE)[14], 它們在植物生長和發(fā)育過程中明確分工, 又功能冗余[2]。光敏色素A (phyA)和光敏色素 B (phyB)均參與萌發(fā)的調(diào)節(jié), 但前者主要是負責極低輻照反應(yīng)(very-low-fluence response, VLFR,0.1~1.0 μmol m-2s-1), 而后者主要負責低輻照反應(yīng)(low-fluence response, LFR, 1~1000 μmol m-2s-1)[15-17]。光敏色素A屬于光不穩(wěn)定類型[18], 在黑暗和遠紅光條件下轉(zhuǎn)錄和蛋白豐度較高, 而在紅光和白光下蛋白質(zhì)穩(wěn)定性極差, 轉(zhuǎn)錄水平也很低[19]。擬南芥的光敏色素 A不但參與遠紅光信號途徑, 而且介導紅光和藍光信號轉(zhuǎn)導[20]。研究表明, phyA在植物整個生命過程中起重要作用[21]。黑暗條件下豐度最高的光受體 phyA對萌發(fā)種子光形態(tài)建成的開啟起主要作用; 露出土壤后的幼苗通過 phyA感知周圍因遮蓋導致的弱輻照, 啟動極低輻照反應(yīng)來完成最初的去黃化反應(yīng)[22]。phyA在黑暗條件下是穩(wěn)定的紅光吸收型(Pr), 受光激活后變?yōu)闃O易被降解的遠紅光吸收型(Pfr)[23-25]。phyA突變體在短日照處理條件下開花延遲、葉片增多, 表明phyA參與感受光周期的變化[26]。在長日照條件下, phyA 通過增加CONSTANS (CO)蛋白的穩(wěn)定性來提高FLOWERING LOCUS T (FT)的表達, 進而加速開花[27]。phyA不僅在促進種子萌發(fā)和幼苗去黃化過程發(fā)揮重要作用[28],而且通過增強 phyB的作用來抑制植物的避蔭性反應(yīng)[29]。馬鈴薯的 phyA通過促進體內(nèi)花青素合成和蔗糖磷酸合成酶活性, 來增強幼苗早期對周圍環(huán)境感知的能力[30]。水稻的 phyA在長日照條件下延遲開花, 卻在短日照條件下促進開花[31]。
通過修飾作物的光敏色素途徑可以改良株高、增強光反應(yīng)能力和提高產(chǎn)量。在水稻中過量表達擬南芥PHYA基因, 盡管沒有導致轉(zhuǎn)基因植株節(jié)數(shù)的變化, 但是節(jié)間卻明顯縮短, 導致成株高度顯著降低[32]。在馬鈴薯中轉(zhuǎn)入擬南芥 PHYB基因, 轉(zhuǎn)基因植株在高密度栽培條件(20株 m-2)下, 節(jié)間和葉柄縮短而株高明顯降低, 葉綠素含量高而光合作用增強, 單株塊莖產(chǎn)量得到顯著提高[33-35]。在煙草中過量表達燕麥PHYA基因可以增加葉面積指數(shù)[36]。最新的研究表明, 在長日照條件下小麥 PHYC的缺失使生物鐘及光周期基因表達發(fā)生改變, 因而開花延遲[37]。
迄今, 人們對于雙子葉植物模式植物擬南芥的光敏色素研究較為深入, 但對于單子葉植物光敏色素的研究還鮮有報道。在禾本科植物中, 光敏色素基因只存在3個亞家族: PHYA、PHYB和PHYC[40-41]。玉米的古四倍體化過程導致其產(chǎn)生光敏色素A有2個拷貝, ZmPHYA1和ZmPHYA2, 它們之間是否存在功能分化, 以及二者之間的相互關(guān)系, 一直引起我們的關(guān)注。本研究以玉米自交系B73和Mo17為材料, 測量不同光質(zhì)條件下玉米中胚軸長度; 通過實時定量PCR技術(shù)研究不同光照處理下ZmPHYA1和ZmPHYA2的表達差異, 了解它們的表達模式, 為評價二者的光反應(yīng)能力及在玉米改良中的價值提供科學依據(jù)。
1 材料與方法
1.1 試驗材料及樣品處理
1.1.1 試驗材料 選用玉米自交系B73和Mo17(本實驗室保存)。
1.1.2 器官特異性表達樣品的準備 將自交系B73的種子在北京地區(qū)4月下旬播種于花盆中自然條件下生長60 d, 分別取成株的根、莖、葉、葉枕、葉鞘、花絲、花柄、雄花、苞葉和幼穗。
1.1.3 各種持續(xù)光質(zhì)處理 將玉米種子消毒殺菌后, 平放在濕潤濾紙的培養(yǎng)皿中28℃催芽3 d, 挑選萌動一致的種子播于裝有培養(yǎng)土塑料小盆(長寬高均為8.5 cm)中, 每小盆播9粒, 所有玉米幼苗均生長在22℃。分別放黑暗(Dk)、遠紅光(FR, 0.5 μmol m-2s-1)、紅光(R, 22.3 μmol m-2s-1)、藍光(B, 13 μmol m-2s-1)和白光(WL, 17 μmol m-2s-1)培養(yǎng)箱中生長13 d。
1.1.4 黑暗轉(zhuǎn)換各種光質(zhì) 將幼苗自黑暗中生長13 d, 分別轉(zhuǎn)入以上各種光質(zhì)條件下, 0.25、0.5、1、2、4、8、12和24 h后, 分別取幼苗地上部為試材。
1.1.5 長日照和短日照處理 玉米幼苗在22℃長日(LD, 16 h光照/8 h黑暗)或短日(SD, 8 h光照/16 h黑暗)條件下中生長13 d, 每隔2 h取一次樣。
1.2 RNA提取及cDNA合成
用 TRIzol (Invitrogen, USA)法提取各種處理的玉米幼苗總RNA, 經(jīng)DNase I (RNase-free, TaKaRa大連公司)處理后作為模板, Oligo-dT18為引物, 利用RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific公司)反轉(zhuǎn)錄成cDNA備用。
1.3 玉米實時熒光定量RT-PCR (qRT-PCR)分析
以不同處理的B73玉米的cDNA第1鏈為模板, 玉米 Tubulin基因為內(nèi)參, 測定 ZmPHYA1和ZmPHYA2的相對表達量。ZmPHYA1和ZmPHYA2 與Tubulin的序列分別來源于PLAZA網(wǎng)站和NCBI網(wǎng)站, 利用Primer Premier 5.0軟件設(shè)計熒光定量PCR引物(表1)。熒光定量 PCR儀為 Roche 480 (Roche, 瑞士), PCR程序為95℃預變性30 s, 再進行以下循環(huán): 95℃變性5 s, 60℃退火20 s, 72℃延伸10 s, 共進行50個循環(huán); 然后60~95℃繪制溶解曲線。定量PCR試劑為SYBR Premix Ex Taq II (TaKaRa大連公司), 按照商家使用說明操作。采用 2-ΔΔCT的方法計算結(jié)果[42]。經(jīng) 3次獨立的生物學重復, 并依此計算標準差。采用SAS9.2軟件對數(shù)據(jù)進行方差及相關(guān)分析, 用 Duncan’s新復極差法進行多重比較, 并采用Microsoft Excel 2010軟件繪圖。
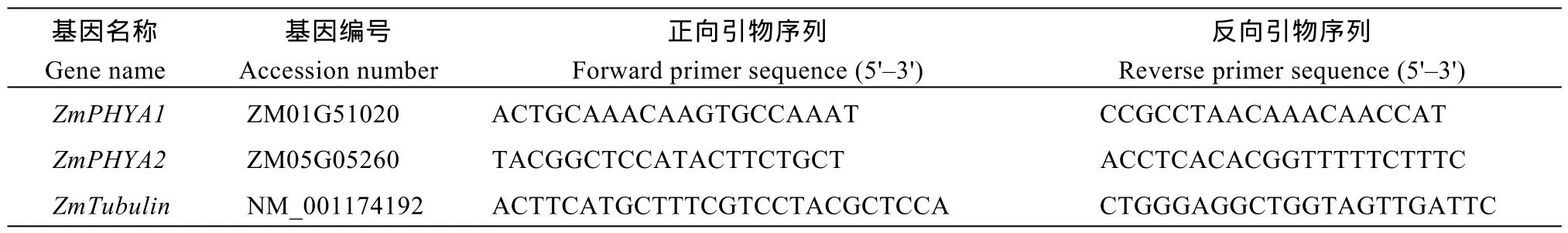
表1 qRT-PCR所用引物Table1 Primers for qRT-PCR
2 結(jié)果與分析
2.1 ZmPHYA1和ZmPHYA2器官特異性表達分析
玉米光敏色素A基因在根、莖、葉、葉枕、葉鞘、花絲、花柄、雄花、苞葉和幼穗中均有表達, 尤其在葉片和花絲中的表達量較高, 其中ZmPHYA1在葉片和花絲中分別為在根中的6.5倍和10.9倍, 推測葉片和花絲是玉米光敏色素A主要起作用的部位。從圖1可知, 根、莖、葉、葉枕、葉鞘、花絲、花柄和苞葉中ZmPHYA1的轉(zhuǎn)錄豐度約為ZmPHYA2的4~10倍,雄花和幼穗中ZmPHYA1的轉(zhuǎn)錄豐度約為ZmPHYA2 的1.5倍和3.0倍。ZmPHYA1在各器官中的表達豐度均明顯高于ZmPHYA2, 暗示ZmPHYA1可能比ZmPHYA2有更重要的作用。
2.2 玉米中胚軸伸長響應(yīng)不同光質(zhì)處理
采用玉米中胚軸作為衡量其光形態(tài)建成程度的指標, 為了解它的伸長與光質(zhì)的關(guān)系, 測量了在不同光質(zhì)持續(xù)照射條件下玉米自交系B73和Mo17的中胚軸長度。在黑暗條件下因黃化反應(yīng), 中胚軸明顯長于其他光質(zhì)處理(圖2)。遠紅光、紅光、藍光和白光均可以抑制玉米中胚軸伸長, 并且紅光和白光條件下對中胚軸的抑制作用較遠紅光和藍光更為明顯。可見, 玉米中胚軸伸長是響應(yīng)各種光質(zhì)處理的。
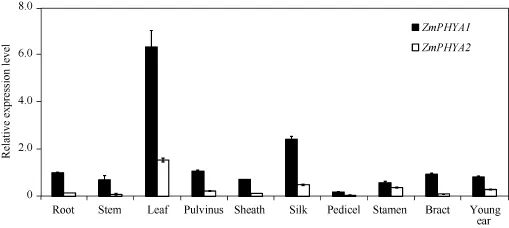
圖1 ZmPHYA1和ZmPHYA2器官特異性表達的定量RT-PCR分析Fig.1 qRT-PCR assay of ZmPHYA1和ZmPHYA2 expression in different organs of maize
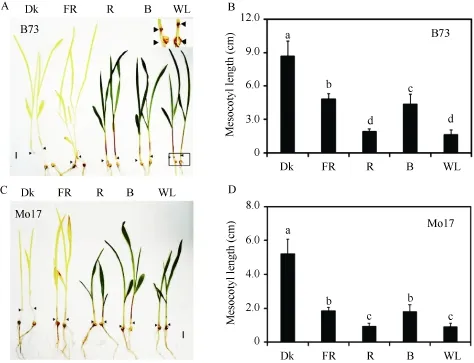
圖2 各種持續(xù)光質(zhì)條件下B73和Mo17中胚軸長度Fig.2 Mesocotyl lengths of B73 and Mo17 under different continuous light conditions
2.3 ZmPHYA1和ZmPHYA2對持續(xù)不同光質(zhì)處理的響應(yīng)
圖3-A顯示ZmPHYA1在紅光和白光下的轉(zhuǎn)錄豐度很低, 僅為自身黑暗中的 30%和 40%; 遠紅光條件下, ZmPHYA1的轉(zhuǎn)錄豐度最高, 約為自身黑暗中的1.4倍; 而藍光下的表達豐度與黑暗條件下相似。ZmPHYA2在紅光和白光中表達豐度也非常低(圖3-B), 在藍光下的轉(zhuǎn)錄豐度約為自身黑暗中的1.3倍;而遠紅光下 ZmPHYA2的轉(zhuǎn)錄豐度僅為其在黑暗中的 80%。持續(xù)光條件下的轉(zhuǎn)錄表達分析結(jié)果顯示,ZmPHYA1和ZmPHYA2均受到紅光和白光的強烈抑制, 這與擬南芥的PHYA基因結(jié)果類似, 可能與它們編碼的蛋白質(zhì)在光下不穩(wěn)定有關(guān)。另外從二者在遠紅光和藍光下的轉(zhuǎn)錄豐度推測, 2個基因在遠紅光和藍光中均具有作用; ZmPHYA1在遠紅光下更重要,而ZmPHYA2在藍光下更重要。
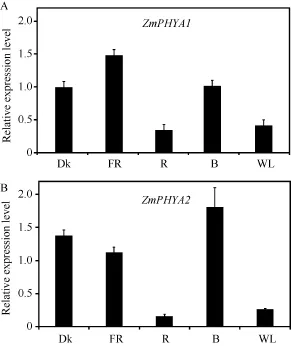
圖3 各種持續(xù)光質(zhì)條件下ZmPHYA1和ZmPHYA2轉(zhuǎn)錄表達的定量RT-PCR分析Fig.3 qRT-PCR assay of ZmPHYA1 and ZmPHYA2 expression under different continuous light conditions
2.4 ZmPHYA1和ZmPHYA2對黑暗到不同光質(zhì)轉(zhuǎn)換的響應(yīng)
我們將黑暗中生長13 d的B73幼苗分別轉(zhuǎn)入到遠紅光、藍光、紅光和白光下0、0.25、0.5、1、2、4、8、12和24 h, 來進一步探究ZmPHYA1和ZmPHYA2轉(zhuǎn)錄豐度對不同光質(zhì)轉(zhuǎn)換的響應(yīng)。ZmPHYA1的轉(zhuǎn)錄能迅速響應(yīng)黑暗到遠紅光的轉(zhuǎn)換, 0.25 h內(nèi)其轉(zhuǎn)錄水平上升到自身黑暗中的1.9倍, 0.5 h內(nèi)繼續(xù)上升到自身黑暗中的4.5倍, 之后迅速上升并在1 h達到峰值(黑暗中的18倍); 但在2 h、4 h和8 h分別下降到峰值的70%、60%和6%, 其較低的轉(zhuǎn)錄水平一直維持到12 h; 在遠紅光處理24 h后, 其轉(zhuǎn)錄水平又回升到自身黑暗中的3.6倍(圖4-A)。ZmPHYA2在黑暗到遠紅光的轉(zhuǎn)換最初0.5 h之內(nèi)與ZmPHYA1的轉(zhuǎn)錄水平相當, 屬于較平緩的上升, 但在1 h上升到僅為黑暗中ZmPHYA1的3.4倍的峰值; 隨后緩慢下降, 8 h時達到超低值(黑暗中ZmPHYA1的2%), 并且其轉(zhuǎn)錄在24 h內(nèi)均維持較低水平(黑暗中ZmPHYA1的2%~10%)。由此可見, ZmPHYA1和ZmPHYA2均能迅速響應(yīng)遠紅光;ZmPHYA2的轉(zhuǎn)錄在24 h內(nèi)只有一個峰值, 上升和下降均較緩慢; 而ZmPHYA1的轉(zhuǎn)錄不但迅速響應(yīng)黑暗到遠紅光的轉(zhuǎn)換, 并且峰值達到了自身黑暗中的18倍。這些結(jié)果暗示ZmPHYA2在持續(xù)遠紅光中起重要作用, 而ZmPHYA1在對遠紅光光質(zhì)轉(zhuǎn)換的響應(yīng)中更重要。
ZmPHYA1和ZmPHYA2轉(zhuǎn)錄豐度能迅速響應(yīng)黑暗到藍光的轉(zhuǎn)換(圖4-B)。在0.25 h時ZmPHYA1急劇上升至自身黑暗中的15倍, 之后繼續(xù)飆升, 在0.5 h時達到峰值(約為自身黑暗中的 56倍); 隨后其轉(zhuǎn)錄水平迅速下降, 在 2 h時已基本恢復至自身黑暗的水平; 在4 h出現(xiàn)第2個峰值(自身黑暗中的5倍,最大峰值的 9%); 隨后其轉(zhuǎn)錄豐度穩(wěn)定在相對較低的水平(自身黑暗中的44.6%~71.7%)。同ZmPHYA1類似 ZmPHYA2峰值也出現(xiàn)在 0.5 h (約為黑暗中ZmPHYA1的10倍); 隨后迅速下降, 在1 h后穩(wěn)定在黑暗中ZmPHYA1的6.7%~23.7%的水平。可以看出,二者均能迅速響應(yīng)黑暗到藍光的轉(zhuǎn)換, 且光轉(zhuǎn)換早期表達豐度上升快; ZmPHYA2的轉(zhuǎn)錄豐度在24 h內(nèi)只有一個峰值; 而ZmPHYA1出現(xiàn)了兩個峰值, 并且峰值極大(自身黑暗中的56倍)。以上結(jié)果表明ZmPHYA1 和 ZmPHYA2在藍光信號途徑中起重要作用, 而ZmPHYA1在對藍光光質(zhì)轉(zhuǎn)換的響應(yīng)更為重要。
ZmPHYA1和ZmPHYA2對黑暗到紅光轉(zhuǎn)換的轉(zhuǎn)錄表達模式基本一致(圖4-C)。在轉(zhuǎn)入紅光 0.25 h,ZmPHYA1的轉(zhuǎn)錄水平未發(fā)生變化; 在0.5 h達到第1個峰(黑暗中自身的 3.4倍), 隨后迅速下降, 在 2 h恢復至自身黑暗起點時的水平, 而在4 h略有上升;在 8~12 h期間其轉(zhuǎn)錄豐度為自身黑暗的 35%左右;之后迅速上升, 在24 h已上升至轉(zhuǎn)入紅光24 h內(nèi)的峰值(自身黑暗的4.5倍)。ZmPHYA2的轉(zhuǎn)錄模式整體與 ZmPHYA1一致, 稍有不同的是在剛轉(zhuǎn)入紅光0.25 h內(nèi), ZmPHYA2的轉(zhuǎn)錄豐度先下降至ZmPHYA1黑暗的 31%, 之后在 0.5 h上升至第 1個高于此時ZmPHYA1的峰值; 以后其轉(zhuǎn)錄豐度均低于ZmPHYA1, 在24 h, ZmPHYA2僅恢復至自身黑暗時的水平。由此看來, ZmPHYA1和ZmPHYA2在黑暗到紅光轉(zhuǎn)換的轉(zhuǎn)錄模式基本一致, 但從表達豐度上看,ZmPHYA1在前期作用更重要。
從圖4-D可以看出, ZmPHYA1和ZmPHYA2由黑暗到白光轉(zhuǎn)換的轉(zhuǎn)錄模式基本一致, 二者均在0.5 h時達到各自唯一的峰值, 此時 ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄豐度分別達到黑暗中ZmPHYA1的35和10倍, 之后迅速下降至低于黑暗水平并保持穩(wěn)定。以上結(jié)果表明, ZmPHYA1和ZmPHYA2均迅速響應(yīng)黑暗到白光的轉(zhuǎn)換。
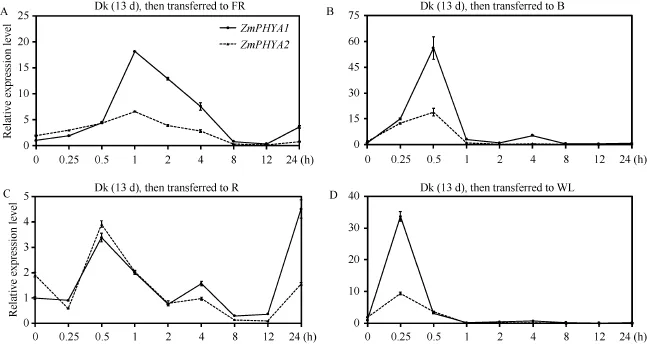
圖4 黑暗到不同光質(zhì)轉(zhuǎn)換條件下ZmPHYA1和ZmPHYA2轉(zhuǎn)錄表達的定量RT-PCR分析Fig.4 qRT-PCR assays of ZmPHYA1 and ZmPHYA2 expressions during transition from the dark to different light conditions
2.5 ZmPHYA1和ZmPHYA2在長日照和短日照處理下的響應(yīng)
長日照處理條件下, ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄表達模式一致(圖5-A), 但是整體上 ZmPHYA2轉(zhuǎn)錄豐度僅為ZmPHYA1的22%~51%。在光照和黑暗階段各出現(xiàn)一個峰值。在光照階段峰值出現(xiàn)在8 h,此時ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄豐度分別是各自在黑暗階段結(jié)束時的2.4倍和4.6倍。黑暗階段的峰值出現(xiàn)在進入黑暗后 4 h, 此時 ZmPHYA1和 ZmPHYA2的轉(zhuǎn)錄豐度分別是各自在黑暗階段結(jié)束時的 7倍和 5倍。在白光到黑暗轉(zhuǎn)換時 ZmPHYA1 和ZmPHYA2都能迅速上升至較高水平。
ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄表達模式對短日照處理的響應(yīng)亦相似(圖5-B), ZmPHYA2轉(zhuǎn)錄豐度僅為ZmPHYA1的21%~53%。在光照階段, 二者轉(zhuǎn)錄豐度基本保持穩(wěn)定狀態(tài)。在轉(zhuǎn)入黑暗后二者開始波動性上升, 在轉(zhuǎn)入黑暗10 h均出現(xiàn)第1個峰值, 此時ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄豐度分別是各自在黑暗階段結(jié)束時的6倍和9倍。隨后迅速下降, 2 h后達到最低(恢復至各自在黑暗階段結(jié)束時水平)。在轉(zhuǎn)入黑暗14 h達到第2個峰值(分別是各自在黑暗階段結(jié)束時的 9倍和 5倍)。可見, ZmPHYA1和ZmPHYA2對長日照和短日照處理響應(yīng)的表達模式極其一致, 前者轉(zhuǎn)錄豐度總體上是后者的2~5倍。
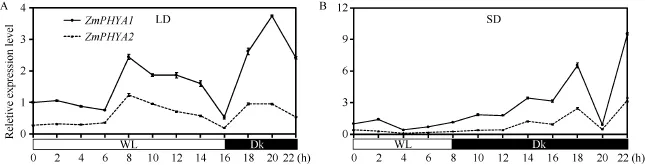
圖5 長日照和短日照件下ZmPHYA1和ZmPHYA2轉(zhuǎn)錄表達的定量RT-PCR分析Fig.5 qRT-PCR assays of ZmPHYA1 and ZmPHYA2 expression under long day and short day conditions
3 討論
本研究表明, ZmPHYA1和ZmPHYA2在玉米的葉片以及花絲中表達量較高, ZmPHYA1表達量約是ZmPHYA2的2~8倍, 且ZmPHYA1在各個器官中的表達豐度均顯著高于ZmPHYA2 (圖1)。玉米中胚軸伸長是響應(yīng)不同光質(zhì)處理的, 紅光和白光下中胚軸較遠紅光和藍光下更短(圖2)。ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄表達均響應(yīng)遠紅光、藍光、紅光和白光處理(圖3), 在黑暗到遠紅光、藍光、紅光或白光轉(zhuǎn)換下 ZmPHYA1的轉(zhuǎn)錄豐度均高于 ZmPHYA2(圖4)。另外, 二者在長日照和短日照處理下,ZmPHYA1轉(zhuǎn)錄豐度總體上是ZmPHYA2的2~5倍(圖5), 推測ZmPHYA1和ZmPHYA2在響應(yīng)光周期上可能是不同的, 也暗示著二者功能可能存在差異。
水稻、玉米、小麥、高粱、谷子等是世界范圍的糧食作物, 人類對它們已經(jīng)進行了 5000~10 000年的人工選擇。作物的開花期和株高, 以及產(chǎn)量均與其栽培季節(jié)、緯度、海拔相適應(yīng), 因此這些性狀都受到特定光照(紅光/遠紅光)的調(diào)節(jié)[43]。光是影響玉米生長發(fā)育的重要因素, 減少光照時間會造成玉米早花[44]。玉米在1.4~2.0億年前發(fā)生的異源四倍體化造成玉米中光敏色素基因為雙拷貝[45-49]。ZmPHYB1與 ZmPHYB2功能上的差異主要表現(xiàn)在phyB1抑制紅光下的中胚軸伸長, 而 phyB2主要負責光周期介導的開花調(diào)控[50]。我們的研究表明,ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄豐度對黑暗到各種光質(zhì)的轉(zhuǎn)換, 以及長日照和短日照處理的響應(yīng)存在差異, 這是否反映二者在功能上也存在差異, 還待進一步研究。
光受體過量表達的轉(zhuǎn)基因植株節(jié)間縮短和植株矮化, 暗示修飾光信號轉(zhuǎn)導途徑同樣能有效地改良株高和株型。在雙子葉植物番茄中過量表達單子葉燕麥的 PHYA基因, 導致植株顯著降低[51]。在煙草中轉(zhuǎn)化水稻類型 I光敏色素(PHYA)后, 轉(zhuǎn)基因植株表現(xiàn)下胚軸變短, 并且在伸長期生長速率減緩, 造成下胚軸變短的原因是表皮細胞變短而不是細胞數(shù)量的減少[52]。水稻phyA參與抑制幼苗中胚軸伸長[53]。玉米elm1突變體表現(xiàn)幼苗中胚軸變長、成株株高增加[54]。單子葉植物玉米中胚軸(mesocotyl)在功能上類似于雙子葉植物的下胚軸, 可作為光形態(tài)建成的指標[54]。本研究表明玉米中胚軸伸長是響應(yīng)光質(zhì)處理的, 這種響應(yīng)是否與光敏色素活性相關(guān)還需要進一步探究。
在擬南芥中, 長日照條件下CRY2與phyA通過與生物鐘基因相互作用可以加速開花[55]。短日照植物水稻phyB或phyC突變體在長日照條件下均表現(xiàn)早花, 而phyA突變體卻對開花時間沒有影響, 但是phyA突變體在phyB或phyC突變體背景下卻出現(xiàn)早花現(xiàn)象[56]。研究表明phyA參與光周期調(diào)節(jié)[26], 在長日照條件下, 水稻的 phyA通過促進開花抑制因子GRAIN NUMBER及降低開花誘導物 EARLY HEADING DATE 1的活性來導致開花推遲[56]。由此可見, 光信號轉(zhuǎn)導途徑與植物的開花期誘導緊密相關(guān), 通過修飾光信號轉(zhuǎn)導途徑改良植物開花期性狀是行之有效的方法[10]。本研究還表明, ZmPHYA1和ZmPHYA2轉(zhuǎn)錄豐度均強烈地響應(yīng)在長日照和短日照處理, 玉米光敏色素是否參與其開花調(diào)控, 以及分子機制值得進一步探討。
4 結(jié)論
ZmPHYA1和ZmPHYA2主要在葉片和花絲中表達; 玉米中胚軸伸長是響應(yīng)不同光質(zhì)處理的, 遠紅光和藍光下中胚軸較紅光和白光下更長; ZmPHYA1 和ZmPHYA2轉(zhuǎn)錄豐度較強地響應(yīng)遠紅光和藍光, 并呈現(xiàn)不同轉(zhuǎn)錄表達模式, ZmPHYA1在遠紅光下更重要, 而 ZmPHYA2在藍光下更重要。ZmPHYA1和ZmPHYA2的轉(zhuǎn)錄能有效地響應(yīng)各種光處理, 可能ZmPHYA1在作物改良上比ZmPHYA2更有效。
References
[1] Fankhauser C, Chory J.Light control of plant development.Annu Rev Cell Dev Biol, 1997, 13: 203-229
[2] Deng X W, Quail P H.Signalling in light-controlled development.Semin Cell Dev Biol, 1999, 10: 121-129
[3] Casal J J, Candia A N, Sellaro R.Light perception and signalling by phytochrome A.J Exp Bot, 2014, 65: 2835-2845
[4] Wang H Y, Deng X W.Dissecting the phytochrome A-dependent signaling network in higher plants.Trends Plant Sci, 2003, 8:172-178
[5] Jiao Y, Lau O, Deng X.Light-regulated transcriptional networks in higher plants.Nat Rev Genet, 2007, 8: 217-230
[6] Li J, Li G, Wang H, Deng X W.Phytochrome Signaling Mechanisms.Arabidopsis Book, 2011, 9: e0149
[7] Quail P H.Phytochrome photosensory signalling networks.Nat Rev Mol Cell Biol, 2002, 3: 85-93
[8] Bae G, Choi G.Decoding of light signals by plant phytochromes and their interacting proteins.Annu Rev Plant Biol, 2008, 59:281-311
[9] Quail P H.Phytochrome photosensory signalling networks.Nat Rev Mol Cell Biol, 2002, 3: 85-93
[10] 詹克慧, 李志勇, 侯佩, 習雨琳, 肖陽, 孟凡華, 楊建平.利用修飾光敏色素信號途徑進行品種改良的可行性.中國農(nóng)業(yè)科學, 2012, 45: 3249-3255 Zhan K H, Li Z Y, Hou P, Xi Y L, Xiao Y, Meng F H, Yang J P.A new strategy for crop improvement through modification of phytochrome signaling pathways.Sci Agric Sin, 2012, 45:3249-3255 (in Chinese with English abstract)
[11] Boylan M T, Quil P H.Oat phytochrome is biologically active in transgenic tomatoes.Plant Cell, 1989, 1: 765-773
[12] Gyula P, Sch?fer E, Nagy F.Light perception and signalling in higher plants.Curr Opin Plant Biol, 2003, 6: 446-452
[13] Quail P H.Photosensory perception and signalling in plant cells:new paradigms? Curr Opin Plant Biol, 2002, 14: 180-188
[14] Sharrock R A, Clack T, Goosey L.Differential activities of the Arabidopsis PHYB/D/E phytochromes in complementing PHYB mutant phenotypes.Plant Mol Biol Rep, 2003, 52: 135-142
[15] Reed J W, Nagatani A, Elich T D, Fagan M, Chory J.Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development.Plant Physiol, 1994, 104:1139-1149
[16] Botto J F, Sanchez R A, Whitelam G C, Casal J J.Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis.Plant Physiol,1996, 110: 439-444
[17] Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M,F(xiàn)uruya M.Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana.Proc Natl Acad Sci USA, 1996, 93: 8129-8133
[18] Furuya M.Molecular properties and biogenesis of phytochrome I and II.Adv Biophys, 1989, 25: 133-167
[19] Quail P H.An emerging molecular map of the phytochromes.Plant Cell Environ, 1997, 20: 657-665
[20] Franklin K A, Whitelam G C.Phytochrome a function in red light sensing.Plant Signal Behav, 2007, 2: 383-385
[21] Whitelam G C, Devlin P F.Roles of different phytochromes in Arabidopsis photomorphogenesis.Plant Cell Environ, 1997, 20:752-758
[22] Casal J J, Luccioni L G, Oliverio K A, Boccalandro H E.Light,phytochrome signalling and photomorphogenesis in Arabidopsis.Photochem Photobiol Sci, 2003, 2: 625-636
[23] Clough R C, Vierstra R D.Phytochrome degradation.Plant Cell Environ, 1997, 20: 713-721
[24] Nagy F, Sch?fer E.Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants.Annu Rev Plant Biol, 53: 329-355
[25] Sharrock R A, Clack T.Patterns of expression and normalized levels of the five Arabidopsis phytochromes.Plant Physiol, 2002,130: 442-456
[26] Johnson E, Bradley M, Harberd N P, Whitelam G C.Photoresponses of light-grown phyA mutants of Arabidopsis (phytochrome A is required for the perception of daylength extensions).Plant Physiol, 1994, 105: 141-149
[27] Yanovsky M J, Kay S A.Molecular basis of seasonal time measurement in Arabidopsis.Nature, 2002, 419: 308-312
[28] Yanovsky M J, Casal J J, Whitelam G C.Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies.Plant Cell Environ, 1995, 18:788-794
[29] Casal J J.Phytochrome A enhances the promotion of hypocotyl growth caused by reductions in levels of phytochrome B in its far-red-light-absorbing form in light-grown Arabidopsis thaliana.Plant Physiol, 1996, 112: 965-973
[30] Yanovsky M J, Alconada-Magliano T M, Mazzella M A, Gatz C, Thomas B, Casal J J.Phytochrome A affects stem growth,anthocyanin synthesis, sucrose-phosphate-synthase activity and neighbour detection in sunlight-grown potato.Planta,1998, 205: 235-241
[31] Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T,Yano M, Nishimura M, Miyao A, Hirochika H, Shinomura T.Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice.Plant Cell,2005, 17: 3311-3325
[32] Garg A K, Sawers R J H, Wang H, Kim J K, Walker J M, Brutnell T P, Wu R J.Light-regulated overexpression of an Arabidopsis phytochrome A gene in rice alters plant architecture and increases grain yield.Planta, 2006, 223: 627-636
[33] Thiele A, Herold M, Lenk I, Quail P H, Gatz C.Heterologous expression of Arabidopsis phytochrome B in transgenic potato influences photosynthetic performance and tuber development.Plant Physiol, 1999, 120: 73-82
[34] Heyer A G, Mozley D, Landschutze V, Thomas B, Gatz C.Function of phytochrome A in potato plants as revealed through the study of transgenic plants.Plant Physiol, 1995, 109: 53-61
[35] Boccalandro H E, Ploschuk E L, Yanovsky M J, Sánchez R A,Gatz C, Casal J J.Increased phytochrome B alleviates density effects on tuber yield of field potato crops.Plant Physiol, 2003,133: 1539-1546
[36] Robson P R, McCormac A C, Irvine A S, Smith H.Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene.Nat Biotechnol, 1996, 14: 995-998
[37] Chen A, Li C X, Hu W, Lau M Y, Lin H Q, Rockwell N C, Martin S S, Jernstedt J A, Lagarias J C, Dubcovsky J, Lagarias J C.PHYTOCHROME C plays a major role in the acceleration of wheat flowering under long-day photoperiod.Proc Natl Acad Sci USA, 2014, 111: 10037-10044
[38] Mathews S, Sharrock R A.The phytochrome gene family in grasses (Poaceae): a phylogeny and evidence that grasses have a subset of the loci found in dicot angiosperms.Mol Biol Evol,1996, 13: 1141-1150
[39] Mathews S, Sharrock R A.Phytochrome gene diversity.Plant Cell Environ, 1997, 20: 666-671
[40] Liu X B, Zhang X Y, Wang Y X, Sui Y Y, Zhang S L, Herbert S J,Ding G.Soil degradation: a problem threatening the sustainable development of agriculture in Northeast China.Plant Soil Environ, 2010, 56: 87-97
[41] Gao Y, Jiang W, Dai Y, Xiao N, Zhang C, Li H, Lu Y, Wu M, Tao X, Deng D, Chen J.A maize phytochrome interacting factor 3 improves drought and salt stress tolerance in rice.Plant Mol Biol,2015, 87: 413-428
[42] Rajeevan M S, Ranamukhaarachi D G, Vernon S D, Unger E R.Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies.Methods, 2001, 25: 443-451
[43] Sheehan M J, Farmer P R, Brutnell T P.Structure and expression of maize phytochrome family homeologs.Genetics, 2004, 167:1395-1405
[44] Markelz N H, Costich D E, Brutnell T P.Photomorphogenic responses in maize seedling development.Plant Physiol, 2004,133: 1578-1591
[45] Basu D, Dehesh K, Schneider-Poetsch H J, Harrington S E,McCouch S R, Quail P H.Rice PHYC gene: structure, expression,map position and evolution.Plant Mol Biol, 2000, 44: 27-42
[46] Childs K L, Miller F R, Cordonnier-Pratt M M, Pratt L H, Morgan P W, Mullet J E.The sorghum photoperiod sensitivity gene,Ma3, encodes a phytochrome B.Plant Physiol, 1997, 113:611-619
[47] Gaut B S.Patterns of chromosomal duplication in maize and their implications for comparative maps of the grasses.Genome Res,2001, 11: 55-66
[48] Gaut B S, Doebley J F.DNA sequence evidence for the segmental allotetraploid origin of maize.Proc Natl Acad Sci USA, 1997,94: 6809-6814
[49] Wilson W A, Harrington S E, Woodman W L, Lee M, Sorrells M E, McCouch S R.Inferences on the genome structure of progenitor maize through comparative analysis of rice, maize and the domesticated panicoids.Genetics, 1999, 153: 453-473
[50] Sheehan M J, Kennedy L M, Costich D E, Lee M, Sorrells M E,McCouch S R.Subfunctionalization of PhyB1 and PhyB2 in the control of seedling and mature plant traits in maize.Plant J, 2007,49: 338-353
[51] Boylan M T, Quail P H.Oat phytochrome is biologically active in transgenic tomatoes.Plant Cell, 1989, 1: 765-773
[52] Nagatani A, Kay S A, Deak M, Chua N H, Furuya M.Rice type I phytochrome regulates hypocotyl elongation in transgenic tobacco seedlings.Proc Natl Acad Sci USA, 1991, 88: 5207-5211
[53] Takano M, Kanegae H, Shinomura T, Miyao A, Hirochika H, Furuya, M.Isolation and characterization of rice phytochrome A mutants.Plant Cell, 2001, 13: 521-534
[54] Sawers R J H, Linley P J, Farmer P R, Hanley N P, Costich D E,Terry M J, Brutnell T P.Elongated mesocotyl1, a phytochromedeficient mutant of maize.Plant Physiol, 2002, 130: 155-163
[55] Halliday K J, Salter M G, Thingnaes E, Whitelam G C.Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT.Plant J, 2003, 33:875-885
[56] Asami O, Hironori I, Kyoko I K, Takano M, Izawa T.Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice.Plant Physiol, 2011, 157: 1128-1137
Transcription Characteristics of ZmPHYA1 and ZmPHYA2 under Different Light Treatments in Maize
YANG Zong-Ju1,2,**, YAN Lei2,3,**, SONG Mei-Fang2,4, SU Liang2, MENG Fan-Hua2, LI Hong-Dan1,2, BAI Jian-Rong5, GUO Lin2,*, and YANG Jian-Ping2,*1Graduate School of Chinese Academy of Agricultural Sciences, Beijing 100081, China;2Institute of Crop Science, Chinese Academy of Agricultural Sciences, Beijing 100081, China;3College of Biology Engineering, Shanxi University, Taiyuan 030006, China;4Beijing Radiation Center, Beijing 100875, China;5Institute of Crop Science, Shanxi Academy of Agricultural Sciences, Taiyuan 030031, China
Plant phytochromes are a family of red/far-red light photoreceptors, which have two forms in plant: inactive red light absorbing form (Pr) and active far-red light absorbing form (Pfr).During plant growth and developmental processes, phytochromes playpivotal roles in regulations of seed germination, plant height, flowering time, and shade-avoidance.In the grasses, three subfamilies are present: PHYA, PHYB and PHYC.In maize, an ancient genome duplication has increased the family member to six: PHYA1,PHYA2, PHYB1, PHYB2, PHYC1, and PHYC2.Phytochrome A facilitates the inhibition of hypocotyl elongation, opening of the apical hook, expansion of cotyledons, accumulation of anthocyanin and blocking of greening by continuous FR (FRc) light.In order to evaluate the light response capability and difference of transcription abundance between ZmPHYA1 and ZmPHYA2, we employed quantitative real-time PCR (qRT-PCR) assay to investigate the expression patterns of ZmPHYA1 and ZmPHYA2 in the inbred line B73 and Mo17 with different light treatments.The results indicated that both ZmPHYA1 and ZmPHYA2 had a high expression level in leaf and silk, and the transcription abundance of ZmPHYA1 was 2-8 times higher than that of ZmPHYA2.Inbred lines of both B73 and Mo17 possessed longer mesocotyls in dark, far-red and blue light conditions than in red or white light conditions.Both ZmPHYA1 and ZmPHYA2 had a high expression level in far-red and blue lights and rapidly responded to dark-to-far-red and dark-to-blue transitions.ZmPHYA1 was more important under far-red light, so was ZmPHYA2 in blue light.Both of the genes could rapidly respond to transitions from dark to red or white light with similar expression pattern.The both genes also respond to long-day or short-day treatments,however the transcription abundance of ZmPHYA1 was 2-5 times higher than that of ZmPHYA2 during the treatments.All the results suggested that the transcription of both ZmPHYA1 and ZmPHYA2 could rapidly responded to different light treatments; ZmPHYA1 might be more effective than ZmPHYA2 in crop improvement.Our results provide a theoretical basis for the function study and evaluation of light response ability for both ZmPHYA1 and ZmPHYA2.
Maize; Phytochrome; Light signaling transduction; Expression analysis; Light treatment
10.3724/SP.J.1006.2016.01462
本研究由國家重點研發(fā)計劃試點專項(SQ2016ZY03002918), 國家轉(zhuǎn)基因生物新品種培育重大專項(2016ZX08010002-003-002), 北京市自然科學基金(重點)項目(6151002)和中國農(nóng)業(yè)科學院科技創(chuàng)新工程項目資助。
This study was supported by the National Research and Development Program (SQ2016ZY03002918), the Genetically Modified Organisms Breeding Major Projects of China (2016ZX08010002-003-002), the Key Project of Beijing Natural Science Foundation (6151002) and the Agricultural Science and Technology Innovation Program of CAAS.
(Corresponding authors): 郭林, E-mail: guolin@caas.cn, Tel: 010-82105851; 楊建平, E-mail: yangjianping02@caas.cn, Tel/Fax:010-82105859
**同等貢獻(Contributed equally to this work)
聯(lián)系方式: 楊宗舉, E-mail: zongjuyang@163.com; 閆蕾, E-mail: yanlei2723@126.com, Tel: 010-82105851
Received(): 2016-02-06; Accepted(接受日期): 2016-05-09; Published online(網(wǎng)絡(luò)出版日期): 2016-06-06.
URL: http://www.cnki.net/kcms/detail/11.1809.S.20160606.0855.004.html

