Nanobiomaterials for neural regeneration
Nuan Chen, Lingling Tian, Liumin He, Seeram Ramakrishna, Center for Nanofibers and Nanotechnology, Department of Mechanical Engineering, Faculty of Engineering, National University of Singapore, Singapore, Singapore Department of Biomedical Engineering, College of Life Science and Technology, Jinan University, Guangzhou, Guangdong Province, China3 Guangdong-Hongkong-Macau Institute of CNS Regeneration (GHMICR), Jinan University, Guangzhou, Guangdong Province, China
Nanobiomaterials for neural regeneration
Nuan Chen1, Lingling Tian1, Liumin He2, Seeram Ramakrishna1,3,*
1 Center for Nanofibers and Nanotechnology, Department of Mechanical Engineering, Faculty of Engineering, National University of Singapore, Singapore, Singapore
2 Department of Biomedical Engineering, College of Life Science and Technology, Jinan University, Guangzhou, Guangdong Province, China
3 Guangdong-Hongkong-Macau Institute of CNS Regeneration (GHMICR), Jinan University, Guangzhou, Guangdong Province, China
Seeram Ramakrishna, Ph.D., seeram@nus.edu.sg;
seeram.rk@gmail.com.
orcid:
0000-0001-8479-8686 (Seeram Ramakrishna)
Accepted: 2016-06-13
Diseases and disorders associated with nervous system such as injuries by trauma and neurodegeneration are shown to be one of the most serious problems in medicine, requiring innovative strategies to trigger and enhance the nerve regeneration. Tissue engineering aims to provide a highly biomimetic environment by using a combination of cells, materials and suitable biological cues, by which the lost body part may be regenerated or even fully rebuilt. Electrospinning, being able to produce extracellular matrix (ECM)-like nanostructures with great flexibility in design and choice of materials, have demonstrated their great potential for fabrication of nerve tissue engineered scaffolds. The review here begins with a brief description of the anatomy of native nervous system, which provides basic knowledge and ideas for the design of nerve tissue scaffolds, followed by five main parts in the design of electrospun nerve tissue engineered scaffolds including materials selection, structural design, in vitro bioreactor, functionalization and cellular support. Performances of biomimetic electrospun nanofibrous nerve implant devices are also reviewed. Finally, future directions for advanced electrospun nerve tissue engineered scaffolds are discussed.
nerve regeneration; tissue engineering; contact guidance; electrospun scaffold; nanostructured materials; nanofibers
Introduction
Nervous system injury and degeneration due to traumas, diseases and aging are among the thorniest problems in medicine due to their serious consequence and especially hopeless self-recovery. The functional self-recovery after injury is possible in peripheral nervous system (PNS) when the gap is small. However, this kind of self-healing has high possibility to fail due to some problems such as intrinsically low regenerative potential in aged people. Large injury gap requires autografts, but the challenges such as donor site morbidity, insufficient donor site availability and complex surgical procedures remain. Thus, it is crucial to employ medical devices which are able to trigger and assist the nerve tissue regeneration for nervous system injury therapy. During the past decades, nerve tissue engineered scaffolds have been developed as promising devices for neural regeneration.
本文中我們采用了Schnorr ZKP方案[10],假設(shè)Alice對秘密值 wA做了零知識證明ZKP(wA),可通過如下方式使Bob相信自己擁有這一秘密而不向Bob泄露秘密wA的任何信息。
The key principle of nerve tissue engineering is to provide a favorable environment including biomimetic scaffolds, cells and bioreacting condition in vitro, and further to inspire the body’s own potential to functionally heal previously irreparable tissues rather than directly to implant the artificial tissues (Place et al., 2009). Hence, the ideal scaffold should be the one which can mimic the neuronal cells’ micro-environment in native nerve tissue to the greatest extent. In the nervous system, the main functional unit is neuron and its axon account for a large proportion of the cells in length. Axon can be recognized as a bundle of ultra-small fibers as neurotubules and neurofilaments inside it form the longitudinal skeleton connecting the cell body with the axon terminal. For most of neurons, supporting cells (oligodendrocytes in central nervous system (CNS) and Schwan cells (SCs) in PNS) warp around the axon and form the myelin sheath. These neurons and their supporting cells, together with longitudinally aligned collagen fibers and capillaries, form a number of fascicles-like structures which transmit neural signals from one part to another in the body (Wang et al., 2011). These nanfibrous conduit structures not only provide sufficient support for neuronal survival and function, but also serve as contact guidance for targeted signal transmission. Based on the anatomy of nervous system, there are two basic requirements for the design of the scaffold. Firstly, the structure and materials of scaffold should be similar to those of extracellular matrix (ECM). Besides, specific properties such as conductivity and the nanofibrous conduit structure should be considered due to the unique feature of nerve tissue.
Although the high complexity makes the fabrication of nanofibrous scaffold with the similar functions as those of native microenvironment in vivo a difficult task, the emergence of nanotechnology has significantly increased the possibility to achieve this goal. Electrospinning and electrospraying are techniques which employ electrostatic force to draw charged polymer solution into submicron fibers/particles. Over the past ten years, these techniques have shown their superiorities for the regenerative scaffold fabrication due to the ability to produce ECM-like structures, wide applicability for different materials and high flexibility in functionalities.
Our laboratory has more than 10 years’ experience in fabrication of biomaterial nerve tissue engineered scaffold, and main works are focused on electrospun nanofibrous scaffolds. The schematic of the design of tissue engineered nerve scaffold using electrospinning are shown in Figure 1.
Materials Selection, In Vitro Bio-reactor and Structure Design
Poly(L-lactic acid) (PLLA) fibrous scaffolds with controllable diameter and thickness were fabricated by electrospinning and their satisfactory biocompatibilities have been proved by successful differentiation and neurite outgrowth of neural stem cells (NSCs) cultured on them. Cell differentiation with significantly higher efficiency was shown on fibrous scaffolds at nano-scale than those on micro scale (Yang et al., 2005). Based on blend electrospinning, functionalized composite nanofibrous scaffolds were formed by incorporating polymer which have some specific properties such as cellular affinity and conductivity. With natural polymers like collagen, cell adhesion and proliferation were promoted significantly due to the good hydrophilicity and increased biological recognition sites of the scaffold. Furthermore, neuronal morphologies of human bone marrow derived mesenchymal stem cells (MSCs) were shown on the scaffolds, which indicated the successful differentiation of MSCs and great potential of this composite scaffold for bionanomaterial-cell transplantation therapy (Prabhakaran et al., 2009). In order to mimic the anisotropic conduction of natural nerve tissue, conducting polymer was added into the nanofibrous scaffolds and the effect of electrical stimulation on nerve cells was evaluated. The application of PANi-incorporated nanofibrous scaffolds with the assistance of electrical stimulation was found to highly support the nerve cell proliferation and neurite outgrowth, proving them to be suitable platform for nerve tissue engineering (Ghasemi-Mobarakeh et al., 2009). With appropriate combination of different polymers, the physical properties of the scaffold including mechanical properties, porosity and degradation rate were adjustable. As for the morphology of the nanofibers, both random fibers which have high similarity to ECM and aligned fibers which may serve as guidance cues are obtainable by choosing the collecting device of electrospinning. NSCs cultured on PLLA aligned nanofibrous scaffolds appeared to be bipolar elongated morphology which were parallel to aligned fibers and extended outgrowing neurites with an average length of 100 μm in 2 days, which were desirable for the gap connection after the injuries (Yang et al., 2005).
Functionalization
Based on electrospun nanofibrous scaffolds with appropriate materials and tailorable physical properties, strategies of surface and blend modification were utilized to further improve the properties of the scaffold. Plasma treatment, a simple and cost-effective method of introducing functional group at surface, was employed to improve surface adhesive property and permeability of scaffold. It showed better promotion in SC proliferation than incorporation of collagen, with 17% more cell viability and well cell-scaffold interaction after 8 days of cell culture (Prabhakaran et al., 2008). The binding of biomolecule like laminin at the surface of scaffold was also achieved by covalent coupling, physical absorption and layer-by-layer assembly. The successful attachment of laminin made the electrospun nanofibers more supportive scaffolds for functional recovery of injured nerve tissue (Koh et al., 2008; He et al., 2013). In addition to surface modification, modifications by blending with biological cues were also utilized. Blended electrospinning of laminin at high voltage had not shown adverse effect on its bioactivity and even exhibited better promotion on neurite outgrowth than covalent coupling and physical absorption (Koh et al., 2008). To further preserve the bioactivity of encapsulated protein or drug and prevent the burst release, core-shell nanofibrous scaffolds were fabricated by coaxial electrospinning (Tian et al., 2015) and emulsion electrospinning (Hu et al., 2016). Sustained release of nerve growth factor (NGF) from the core of the scaffold provided a more effective way to stimulate neuronal differentiation than direct addition in cultured medium and a sustained and continuous release for 28 days was obtained (Hu et al., 2016).
Cellular Support and Nerve Implant Device
With well-designed electrospun nanofibrous scaffold, stem cells were seeded on the scaffold and 16-weeks in vivo studies in male Wistar rats were conducted to evaluate the efficiency on nerve regeneration. Results showed that the cell-seeded scaffold showed better performance than that without cell seeding in terms of functional recovery (sensory & motor neuron recovery and vascularization) (Beigi et al., 2014). Novel biomimetic electropun nanofibrous nerve implant devices have also been fabricated with a combination of physical and biochemical cues. The device consisted of bilayered nanofibrous conduit with the longitudinally aligned nanofibers in the lumen and randomly oriented ones on the outer surface and intra-luminal guidance channels made up of bundles of nanofibers, which highly resembled the hierarchical levels of the native peripheral nerve. Functionalized with laminin and nerve growth factor, this device showed its superior properties in nerve defect repair with better performance than the autograft in muscle reinnervation and withdrawal reflex latency tests, which have great potential as an alternative of autograft in the future (Koh et al., 2010).
Conclusion
Although considerable researches have been devoted to the fabrication of nanostructure scaffold for nerve regeneration, the design has yet to be perfected. In the future, attention will be paid on more intimate cell-scaffold interaction throughcontrollable system such as drug-encapsulated nanoparticles and customized 3D scaffolds through combination with 3D printing technique. Besides, scaffolds targeted to CNS repair will be given more attention. It is believed that salamander’s ability to regenerate limbs after amputation will be realized in nerve tissue engineering in the future, which is always a motivation for tissue engineers.
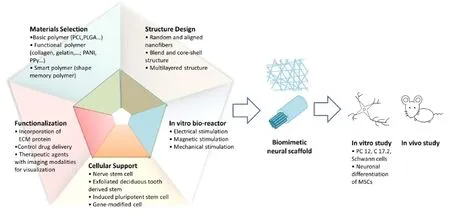
Figure 1 Schematic of the design of tissue engineered nerve scaffold using electrospinning.
Authors contributions: NC and LT were responsible for the conception and design of this review. NC drafted the manuscript. LT and LH assisted in revision. SR critically reviewed the paper and was responsible for fundraising and guided the whole review process. All authors approved the final version of the manuscript.
Conflicts of interest: None declared.
References
Beigi MH, Ghasemi-Mobarakeh L, Prabhakaran MP, Karbalaie K, Azadeh H, Ramakrishna S, Baharvand H, Nasr-Esfahani MH (2014) In vivo integration of poly(epsilon-caprolactone)/gelatin nanofibrous nerve guide seeded with teeth derived stem cells for peripheral nerve regeneration. J Biomed Mater Res A 102:4554-4567.
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S (2009) Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng Part A 15:3605-3619.
He LM, Tang S, Prabhakaran MP, Liao S, Tian LL, Zhang YM, Xue W, Ramakrishna S (2013) Surface modification of PLLA nano-scaffolds with laminin multilayer by LbL assembly for enhancing neurite outgrowth. Macromol Biosci 13:1601-1609.
Hu J, Tian LL, Prabhakaran MP, Ding X, Ramakrishna S (2016) Fabrication of nerve growth factor encapsulated aligned poly(epsilon-caprolactone) nanofibers and their assessment as a potential neural tissue engineering scaffold. Polymers 8:17.
Koh HS, Yong T, Chan CK, Ramakrishna S (2008) Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 29:3574-3582.
Koh HS, Yong T, Teo WE, Chan CK, Puhaindran ME, Tan TC, Lim A, Lim BH, Ramakrishna S (2010) In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J Neural Eng 7:046003.
Place ES, Evans ND, Stevens MM (2009) Complexity in biomaterials for tissue engineering. Nat Mater 8:457-470.
Prabhakaran MP, Venugopal JR, Ramakrishna S (2009) Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials 30:4996-5003.
Prabhakaran MP, Venugopal J, Chan CK, Ramakrishna S (2008) Surface modified electrospun nanofibrous scaffolds for nerve tissue engineering. Nanotechnology 19:8.
Tian LL, Prabhakaran MP, Hu J, Chen ML, Besenbacher F, Ramakrishna S (2015) Coaxial electrospun poly(lactic acid)/silk fibroin nanofibers incorporated with nerve growth factor support the differentiation of neuronal stem cells. Rsc Adv 5:49838-49848.
Wang C, Koh HS, Ramakrishna S, Liao SS (2011) Nerve tissue regeneration. In: Electrospinning for tissue regeneration (Bosworth L and Downes S, eds), pp168-201. England: Woodhead.
Yang F, Murugan R, Wang S, Ramakrishna S (2005) Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 26:2603-2610.
10.4103/1673-5374.191195
*Correspondence to:
- 中國神經(jīng)再生研究(英文版)的其它文章
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats
- Stem Cell Ophthalmology Treatment Study (SCOTS):improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment
- Changes in microtubule-associated protein tau during peripheral nerve injury and regeneration
- Does crossover innervation really affect the clinical outcome? A comparison of outcome between unilateral and bilateral digital nerve repair
- Effects of triptolide on hippocampal microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer's disease