Recovery of an injured corticospinal tract by subcortical peri-lesional reorganization in a patient with intracerebral hemorrhage
Recovery of an injured corticospinal tract by subcortical peri-lesional reorganization in a patient with intracerebral hemorrhage
The corticospinal tract (CST) is a neural tract responsible for motor function in the human brain. It is mainly related to hand movements (Jang, 2014). Therefore, recovery of an injured CST contributes to good recovery in stroke patients and a thorough knowledge of the recovery mechanism regarding an injured CST is required for successful brain rehabilitation.
Diffusion tensor tractography (DTT) has a unique advantage in demonstrating the recovery mechanisms of an injured CST by three-dimensional reconstruction and estimation of the CST (Mori et al., 1999). The recovery mechanisms of an injured CST following intracerebral hemorrhage (ICH) have been demonstrated in several follow-up DTT studies (Yeo and Jang, 2012, 2013). Most of these studies focused on the changes at the cortex level (Yeo and Jang, 2012, 2013), and little is known about the recovery mechanisms at the subcortical peri-lesional level.
In this study, we reported a case who showed recovery of an injured CST by subcortical peri-lesional reorganization following ICH, as shown on follow-up DTT.
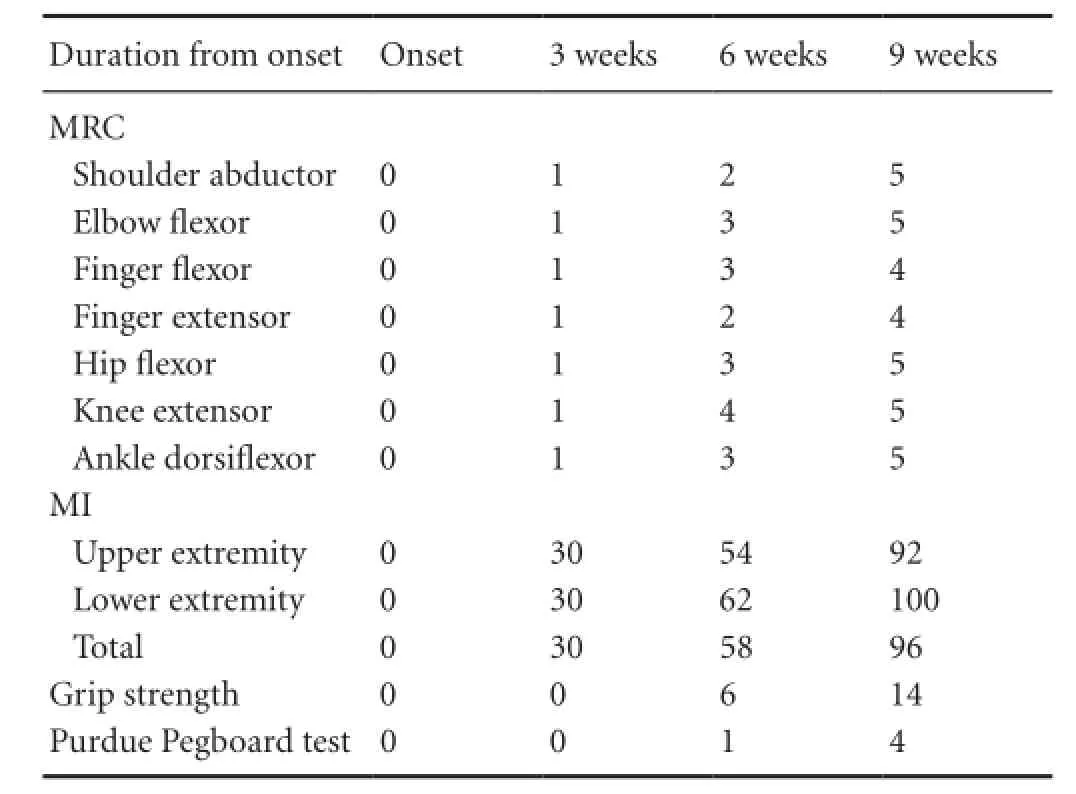
Tabte 1 Motor function change of the patient
At the time of the first DTI scan (3 weeks after onset) before rehabilitation, the patient showed severe weakness of the right extremities (MI: 30). For 6 weeks from 3 to 9 weeks after onset, he received comprehensive rehabilitative therapy including neurotrophic drugs, movement therapy, occupational therapy, and neuromuscular electrical stimulation of the affected finger extensors and ankle dorsiflexors (20 minutes once, twice a day, 6 days a week) (Scheidtmann et al., 2001). Weakness of his right extremities improved from the MI score of 30 points at 3 weeks to 58 points at 6 weeks and 96 points at 9 weeks. In addition, although, at 3 weeks after onset, the grip strength and Purdue pegboard score were uncheckable, these were improved to 6 kg and 1 pin (6 weeks after onset) and 14 kg and 4 pins (9 weeks after onset), respectively. As a result, he could use the right hand for some fine motor activities and walk independently even on stairs at 9 weeks after onset. This study was approved by the review board of Yeungnam University, Republic of Korea.
DTI data were acquired three times (3, 6, and 9 weeks after onset). Seventy contiguous slices (acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192 matrix, field of view (FOV) = 240 × 240 mm2, repetition time (TR) = 10,398 ms, echo time (TE) = 72 ms, SENSE factor = 2, EPI factor = 59 and b = 1,000 s/mm2, number of excitations = 1, thickness of 2.5 mm) were acquired. FACT algorithm was used for fiber tracking. CSTs were reconstructed using fibers passing through different levels of the upper pons on the axial image of the color map (fractional anisotropy threshold of > 0.15 and a direction threshold of <27°) (Kunimatsu et al., 2004). On 3-week DTT, the left CST was discontinued at the posterior margin of the hematoma lesion at the left corona radiata. By contrast, on 6-week DTT, the left CST was connected to the left primary motor cortex and this connection to the primary motor cortex had thickened on 9-week DTT. At the hematoma lesion in the left corona radiata, the passage site of the left CST expanded anteriorly through the white matter between the lateral ventricle and the hematoma lesion on 6- and 9-week DTT, sequentially (Figure 1B).
Discussion
In this patient, we followed up the changes in motor weakness and the injured left CST and found that the injured left CST was recovered by subcortical peri-lesional reorganization, based on the following results. First, the patient showed complete weakness of the affected extremities at ICH onset. Motor function of the affected extremities recovered slowly to a subnormal state until 9 weeks after onset. This mode of progression indicates that this motor recovery could be ascribed to brain plasticity, and not to resolution of local factors such as edema (Witte, 1998). Second, although the left CST was discontinued at the ICH lesion level on 3-week DTT, the integrity of the discontinued left CST was recovered to the primary motor cortex, the main original area of the CST, on 6- and 9-week DTTs. By the way, the left CST fibers expanded anteriorly through the spared subcortical white matter area between the lateral ventricle and hematoma lesion on 6- and 9-week DTTs, sequentially.
Many DTT studies have reported the recovery of an injured CST in patients with ICH (Yeo and Jang, 2012, 2013). Yeo and Jang (2012) have reported the recovery process of an injured CST after ICH. Jang et al. (2007) demonstrated the recovery process of an injured CST in a patient with ICH (the corona radiata and basal ganglia) using DTT and reported that the origin of the injured CST had changed from the posterior parietal cortex to the primary somatosensory cortex and primary motor cortex, sequentially along with motor recovery during 16 months after onset. Yeo and Jang (2012) reported that the origin of the affected CST had changed from the premotor cortex to the primary motor cortex concurrent with motor recovery in a patient with ICH (the fronto-parietal cortex) during 5 months after onset. To the best of our knowledge, this is the first study to demonstrate the recovery of an injured CST, which is achieved through peri-lesional reorganization at the subcortical level.

Figure 1 Brain MR images and results of diffusion tensor tractography (DTT) in the fornical crura of a patient with intracerebral hemorrhage.
In conclusion, the recovery process of an injured CST by subcortical peri-lesional reorganization was demonstrated in a patient with ICH. Our results have important implications for brain rehabilitation in stroke patients because they suggest a recovery mechanism of an injured CST following ICH. However, this study is limited to a case report. Therefore, in-depth studies involving a larger number of patients and various lesion locations should be encouraged.
This work was supported by the National Research Foundation (NRF) of Korea Grant funded by the Korean Government (MSIP), No. 2015R1A2A2A01004073.
Sung Ho Jang, Woo Hyuk Jang*
Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daemyungdong, Namku, Daegu, Republic of Korea
*Correspondence to: Woo Hyuk Jang, Ph.D., wlqtksek@hanmail.net.
Jang SH (2014) The corticospinal tract from the viewpoint of brain rehabilitation. J Rehabil Med 46:193-199.
Jang SH, Kim SH, Cho SH, Choi BY, Cho YW (2007) Demonstration of motor recovery process in a patient with intracerebral hemorrhage. NeuroRehabilitation 22:141-145.
Kim YT, Kang SY, Kim HS, Shin BS (1994) Hand strength and dextricity evaluation with age. J Korean Acad Rehabil Med 18:780-788.
Kunimatsu A, Aoki S, Masutani Y, Abe O, Hayashi N, Mori H, Masumoto T, Ohtomo K (2004) The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci 3:11-17.
Mori S, Crain BJ, Chacko VP, van Zijl PC (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265-269.
Scheidtmann K, Fries W, Muller F, Koenig E (2001) Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 358:787-790. Witte OW (1998) Lesion-induced plasticity as a potential mechanism for recovery and rehabilitative training. Curr Opin Neurol 11:655-662.
Yeo SS, Jang SH (2012) A change in injured corticospinal tract originating from the premotor cortex to the primary motor cortex in a patient with intracerebral hemorrhage. Neural Regen Res 7:939-942.
Yeo SS, Jang SH (2013) Recovery of an injured corticospinal tract and an injured corticoreticular pathway in a patient with intracerebral hemorrhage. NeuroRehabilitation 32:305-309.
A 33-year-old, right-handed male patient
external ventricular drainage of the ICH in the left putamen. After 3 weeks since the onset, the injured CST began to recover. Brain MRI showed a leukomalactic lesion in the left corona radiata and basal ganglia (Figure 1A). The standardized Motricity Index (MI), Medical Research Council (MRC), grip strength, and Purdue Pegboard test were used to determine the motor function of the affected extremities (Kim et al., 1994). At the onset of ICH, he showed complete weakness (MI: 0 out of 100) (Table 1).
2016-06-20
orcid: 0000-0002-7012-4565 (Woo Hyuk Jang)
10.4103/1673-5374.187066
How to cite this article: Jang SH, Jang WH (2016) Recovery of an injured corticospinal tract by subcortical peri-lesional reorganization in a patient with intracerebral hemorrhage. Neural Regen Res 11(7):1191-1192.
- 中國神經再生研究(英文版)的其它文章
- Dose response and time course of manganeseenhanced magnetic resonance imaging for visual pathway tracing in vivo
- Low-power laser therapy for carpal tunnel syndrome: effective optical power
- Extracellular matrix from human umbilical cordderived mesenchymal stem cells as a scaffold for peripheral nerve regeneration
- Differential temporal expression of matrix metalloproteinases following sciatic nerve crush
- Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells
- Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease