一種基于乙二胺-丁二醇體系的新穎的二氧化碳捕集利用方法
沙 峰 郭 波 張 飛 趙天翔 李 強(qiáng) 張建斌
(內(nèi)蒙古工業(yè)大學(xué)化工學(xué)院,呼和浩特010051)
一種基于乙二胺-丁二醇體系的新穎的二氧化碳捕集利用方法
沙峰郭波張飛趙天翔李強(qiáng)張建斌*
(內(nèi)蒙古工業(yè)大學(xué)化工學(xué)院,呼和浩特010051)
在溫和條件下,發(fā)展了一種以丁二醇-乙二胺體系新穎、高效地固定CO2的方法。在此方法中,CO2被快速激活并轉(zhuǎn)化為一種固態(tài)的CO2儲(chǔ)集材料(CO2SM),通過(guò)XPS、XRD、FTIR和13C NMR等技術(shù)表征證實(shí)為烷基碳酸胺。基于TGA結(jié)果,CO2SM的水溶液可以與Ca(OH)2和Ba(OH)2反應(yīng)制備CaCO3和BaCO3微粒,還可用于循環(huán)吸收和解吸CO2的過(guò)程。此外,丁二醇-乙二胺水溶液在20℃下吸收CO2并在98.6℃下解吸CO2,沒有明顯的溶液損失。因此,丁二醇-乙二胺體系提供了一種綠色、高效、低成本的二氧化碳捕集利用方法。
CO2捕獲、儲(chǔ)集和利用;CO2儲(chǔ)集材料;CaCO3和BaCO3微粒;吸收和解吸循環(huán)
0 Introduction
Carbon dioxide(CO2)is a major anthropogenic greenhouse gas(GHG)in the atmosphere[1].Due to international efforts to decrease greenhouse gases,the reduction of CO2has been extensively studied using electrochemical and photochemical reactions[2-3]; however,the results obtained are still not satisfactory.Nowadays,the ways to reduce CO2mainly have CCS (CO2capture and storage/sequestration)and CCU(CO2capture and ctilization)[4-6].Although CCS has helped to reduce continuing damage to the environment by CO2,ithas significant disadvantages that it resulted in waste of C1 resources.In comparison,CCU offers some distinctadvantages because itnot only consumes CO2,but uses CO2to afford an environmentally friendly C1 feedstock and to produce value-added chemicals.To maximally use CO2,CO2had to be chemically activated because carbon atom in CO2is very stable[8].Recently,it was reported that CO2could be converted to carbamate or carbamic ester[9]with amines,including monoethanolamine[10-11],diethanolamine[12],triethanolamine[13],N-methyldiethanolamine[14], and diglycolamine[15].However,these processes have have the major drawbacks,such as the corrosive nature and volatility of the amines,their occasional decomposition and the high energy cost of their regeneration.Recently,Jeesop et al.[16]found that the diazabicyclo[5.4.0]-undec-7-ene(DBU),which contains amidogen,could be combined with alcohols to fix CO2.Nevertheless,these systems are still expensive and are not able to attract significant industrial attention.
Our previous studies[17-20]showed that ethylene glycol(EG)and its derivatives compounds presented native hydrogen bonding sites and the potential absorption properties for acid gases.Meanwhile,this work showed that EDA could react readily with CO2with an absorption ability of about 0.46 mol CO2per mol EDA,which was in good agreement with Parks work[21].However,the volatility of EDA significantly weakened its CO2absorption ability.Recently, Sengwa[22]had reported the hydrogen bonding interaction and hydrogen bonded structures in aminealcohol mixed solvents might decrease the loss of amines.Moreover,our previous work[23]also indicated that BDO had multiple hydrogen bonding sites and could form hydrogen bond with EDA to reduce amine loss.Therefore,as a fixing agent of EDA,BDO,which has favorable properties such as low vapor pressure, low toxicity,and low melting temperature,was added into EDA to form a mixed system.On the one hand, this could reduce the loss of EDA and improve the absorption performance of CO2when CO2was exposed into the binary system.On the other hand,this could form the novel solid CO2-storage material(CO2SM) when CO2was brief introduced into the mixed solution.At the same time,CO2SM was took advantage of CO2SM to prepare CaCO3and BaCO3microparticles.In addition,the absorption and desorption capability of the binary mixed solution was also investigated.As a result,the combination of EDA+ BDO seemed to provide a green CCU approach featur ing high efficiency and low cost.
1 Experimental
1.1M aterials
The analytical grade EDA and BDO were purchased from Tianjin Reagent Company.They are used after drying over 0.4 nm molecular sieves and decompression filtration before measurements,and they were degassed by ultrasound just before the experiment.Doubly distilled water with a conductivity lower than 0.1ms·cm-1(25℃)was used.Compressed CO2(99.999%,V/V)was purchased from the Standard Things Center(China).All the materialswere used as received.

Fig.1 Reaction processes of the system EDA+BDO with CO2at various time
1.2Agile CO2Fixation into the CO2SM
CO2was bubbled into the system BDO+EDA under mild condition.In this process,an interesting phenomenon was found that the system BDO+EDA became turbid at t=20 min and changed into solid powder at t=110 min.And then,the solid powder was washed three times with ethanol and dried under vacuum at 60℃for 3 h,which was stored at room temperature and named as the CO2SM(Fig.1).
The solid CO2SM was characterized by X-ray photoelectron spectroscopy(XPS),X-ray diffraction (XRD),Fourier transform infrared reflectance(FTIR) and13C NMR spectroscopic techniques.XPS data were obtained with a KRATOS Axis ultra X-ray photoelectron spectrometer with a monochromatized Al KαX-ray(hν=1 486.6 eV)operated at 150 W. XRD patterns were collected on a powder X-ray diffractometer(Siemens D/max-RB)with Cu Kα(λ= 0.154 06 nm)radiation and scanning rate of 0.05°·s-1atworking voltage of 40 kV and working current of 40 mA.FTIR spectra of CO2SM were taken as 1% dispersion in KBr powder using a Nexus 670 FTIR spectrometer with a resolution of 1 cm-1in the range from 4 000~400 cm-1,and a base line correction were made for the spectra thatwere recorded in air at room temperature.13C NMR spectra was recorded on a Bruker ARX-400 nuclear magnetic resonance spectrometer equipped with a 4 mm standard bore CP/ MAS probe head.In addition,for monitoring the reaction real-time,the liquid13C NMR of mixing system was measured as follows:(Ⅰ)pure EDA and EDA in DMSO-d6;(Ⅱ)the system BDO+EDA(molar ratio 1∶1);(Ⅲ)CO2bubbled into the system BDO+EDA system 15 min,30 min,and 45 min,respectively;and(Ⅱ)the liquid13C NMR spectra of the system CO2+ BDO+EDA were notmeasured after 45 min,and only the solid CO2SM wasmeasured by the solid13C NMR spectra.
At the same time,thermogravimetry analysis (TGA,Q50 V20.6 Build 31)was employed tomeasure the weight change of CO2SM.In the thermal stability experiments,the sample was heated up to 400℃with a rate of 10℃·min-1in the nitrogen atmosphere.
1.3Absorp tion and desorp tion p rocesses
Absorption-desorption cycles of the aqueous BDO+EDA system with CO2were carried out at an absorption temperature of 20℃and a desorption temperature of 98.6℃at ambient pressure.The absorption process was conducted for 15 min and desorption process was run for 90 min.All reactions were carried out in a 100 mL gas-washing bottle,and all measurements of mass were performed on an electronic balance with an accuracy of 0.1 mg (Sartorius BS224S).The desorption experiments were carried out after the absorption.The CO2-saturated absorption system was heated in a pre-heated oil bath with condensation and stirring.And then a follow-up absorption cycle wasmarched after the system cooled.
2 Results and discussion
2.1Study on CO2absorption process
Firstly,the loading capacity of CO2,which was expressed as molar ratio of CO2(absorbed)/EDA(starting),in pure BDO and pure EDA were studied at 400 mL· min-1of CO2,respectively.The result showed that the pure BDO only had little-to-no CO2binding capacity (about 0.003 15 mol of CO2per mole BDO)through physical absorption and the pure EDA had strong CO2capability of approximately 0.467 mol CO2per mol EDA.However,in the system BDO+EDA,it showed an unimaginable capability to capture CO2(0.709 mol per mol),indicating BDO participated in the reactionof CO2with EDA.In the process,the varieties in weight,electrical conductivity and temperature of the system BDO+EDA were recorded with the bubbling CO2(Fig.2).
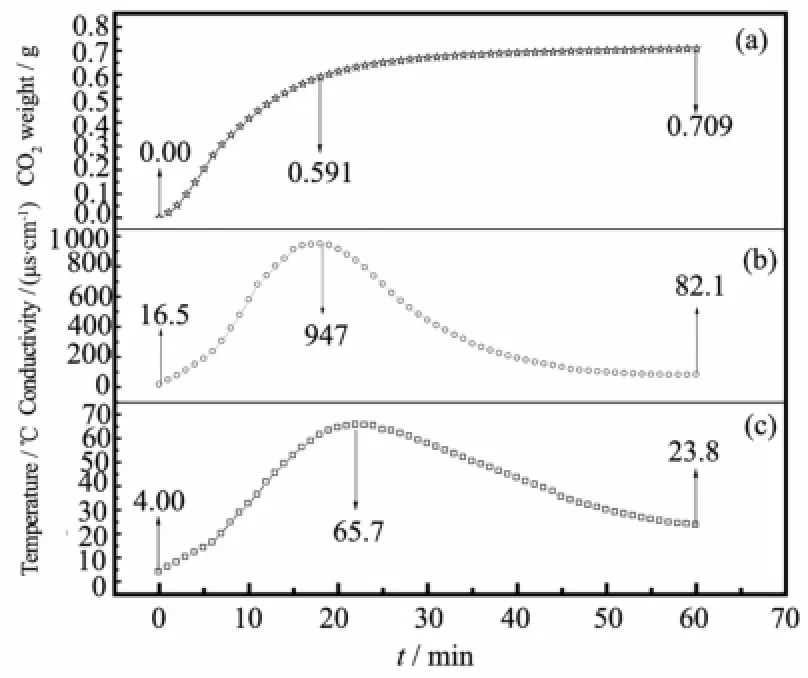
Fig.2 Variation ofweight(a),electrical conductivity(b), and temperature(c)changes for the system EDA+ BDO at CO2flow rate of 400m L·m in-1
As shown in Fig.2(a),the loading capability of CO2rapidly increased before 20 min,and then slowly increased with the lengthening absorption time. Moreover,the solution became turbidity after 20 min. Before turbidity,the system CO2+BDO+EDA promoted mass transfer and intensified intermolecular collision. After turbidity,solid granules preventedmass transfer, slowed down intermolecular collision and decreased saturation time.Therefore,the concentration of solid granules increased with the increasing reaction time, and the solution finally changed into solid.Compared with the previously-reported loading performance of CO2in various aqueous alkanolamines[24],including MEA(0.462 8mol CO2permol amine),DEA(0.236 0 mol CO2permol amine)and TEA(0.194 4 mol CO2per mol amine),the novel system BDO+EDA with much higher CO2loading capacity because BDO,as a reactant,participated in the reaction between CO2and EDA.
From Fig.2(b),the conductivity of the system BDO+EDA kept on increasing from 16.49μS·cm-1to 947μS·cm-1rapidly within the first 19 min,which indicated that the molecules violentlymotioned among BDO,EDA,and CO2to form ionic compound.With the increase of reaction time,the conductivity values decreased from 947μS·cm-1to 82.1μS·cm-1within another 41 min,which was mainly due to the gradually increased viscosity of this system,the generation of white solid granules,and the decreased ion movement.Simultaneously,the conductivity change also demonstrated the dramatic change in charge between the low ionic strength solution and the high ionic strength solution caused the increasing viscosity of the system BDO+EDA.This also proved that the system firstly translated into ionic compound when CO2was exposed in solution,and then precipitated in the form of solid precipitation.
As shown in Fig.2(c),the change trend of temperature was in good agreement with that of conductivity.Obviously,CO2capture process was exothermal,in which the temperature quickly increased from 4.0 to 65.7℃within 22 min,and then slowly decreased by 23.8℃with the increasing absorption time.According to the temperature monitoring,the dramatical change of temperature suggested that the BDO+EDA system capture CO2through chemical reaction rather than physical adsorption only and the change trend of temperaturecould disclose the extent of capturing process.
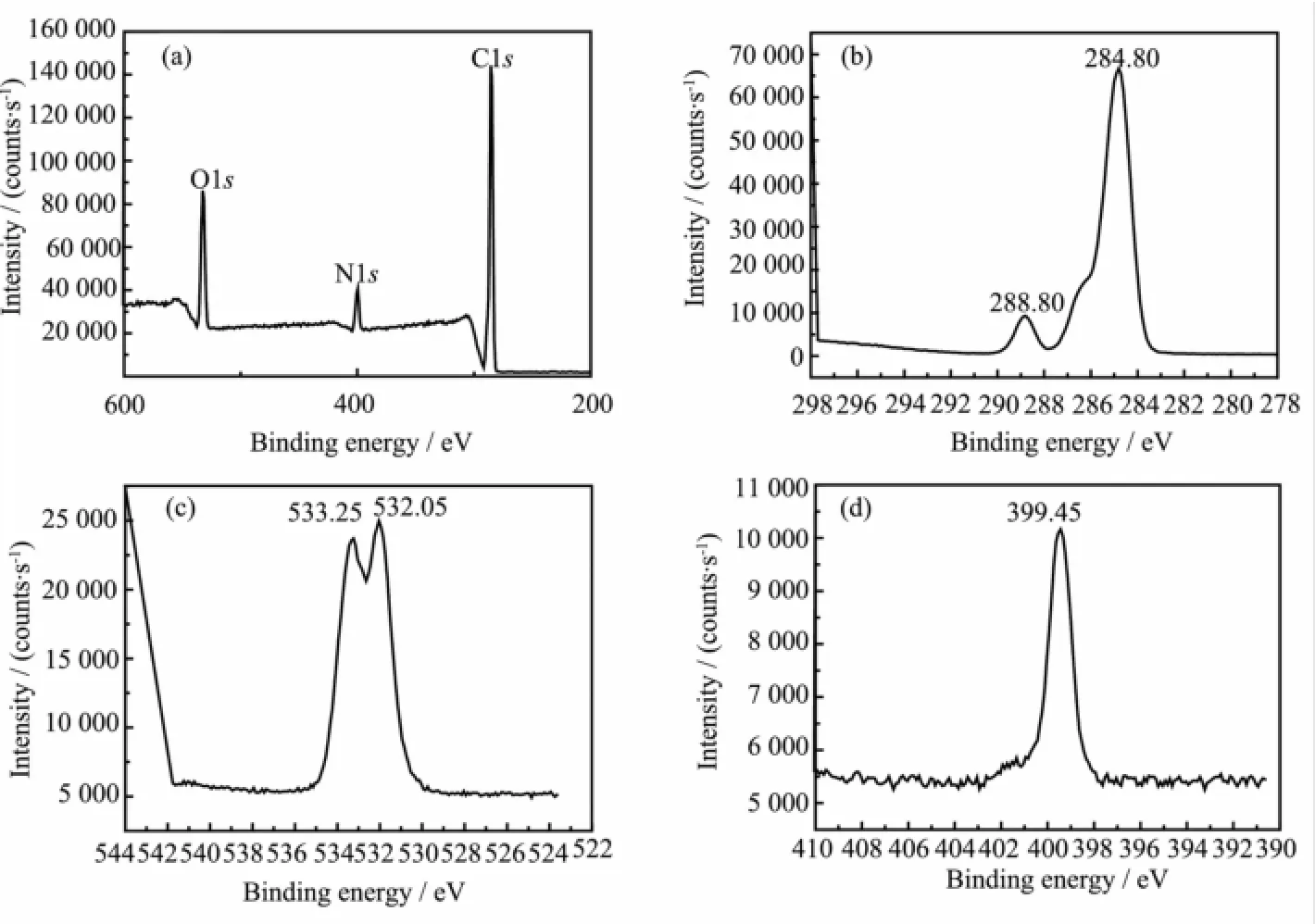
Fig.3 XPS spectra of CO2SM:(a)the total element,(b)oxygen element,(c)carbon and(d)nitrogen element
2.2Agile CO2fixation into the solid CO2SM
In this work,a series of experiments were designed to absorb CO2by pure BDO,pure EDA,and BDO+EDA system,respectively.In surprise,no solid products were formed in pure BDO or pure EDA. However,the white solid precipitate was formed after CO2was bubbled into the system BDO+EDA(molar ratio 1∶1)under mild condition for approximately 110 min.And then,the white solid precipitate,named as the CO2SM,was thoroughly washed three times with ethanol,dried under vacuum at60℃for 3 h.And the properties of CO2SM were characterized with XPS, XRD,FTIR and13C NMR techniques.
XPS spectra of CO2SM weremeasured and shown in Fig.3.The peak at288.80 eV was due to the carbon atomsperturbed by three oxygen bonds[25],and the peak at 284.80 eV was due to single carbon oxygen bond (Fig.3(a)and(b))[26].At the same time,two peaks at 532.05 and 533.25 eV were due to O1s(Fig.3(c))in carbonate-like structures[27-28].In addition,the peak at 399.45 eV,which was due to N1s(Fig.3(d)),and the peak at 284.80 eV(Fig.3(b))showed NH3+existed[29]. Based on above result,it was found that the CO2SM contained the groupsofNH3+and CO32-.
The CO2SM had a structure with 17.50°,21.48°, 22.40°,22.60°,29.24°,and 35.42°(Fig.4(a)),which was similar to ethlenediamine carbamate(-NH-COO-) characteristic diffraction peaks(JCPDS 34-1993).The FTIR peaks at 3 307 and 2 189 cm-1,which could be assigned to stretching vibration mode of N-H and characteristic absorption peak of-NH3+of CO2SM, suggested that the CO2SM wasa primary amine salt[30-31]. Two intense absorption peaks at1 574 and 1 483 cm-1were due to asymmetric and symmetric stretchingmode of-CO2-[32],respectively.In addition,the characteristic absorption peak at1 373 cm-1denoted carbonate(CO32-) rather than bicarbonate because the typical peaks of bicarbonateappeared at1 360 and 835 cm-1(Fig.4(b)). Thus,the obtained solid productswas likely composed ofalkylacrbonate salt[33-35].
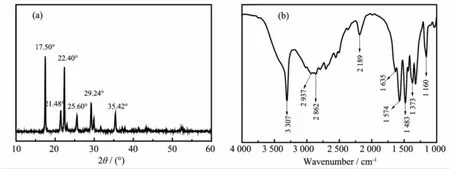
Fig.4 XRD pattern(a)and FTIR(b)of CO2SM
The13C NMR spectra of EDA in DMSO-d6were firstly tested and the result showed that a lathy singlet appeared at 45.594 ppm[36],which was attached to carbon of EDA,and heptet of carbon in DMSO-d6appeared at 39.952(Fig.5a).And the system BDO+ EDA(molar ratio 1∶1)presented three singlets at 60.923,44.574 and 29.580 in the13C NMR spectrum (Fig.5b),in which the chemical shift(45.594)of carbon in EDA shifted towards high-field(44.574) because of the formation of hydrogen bond between BDO and EDA[23],and other new peaks at 60.923 and 29.580 were assigned to carbon in BDO.At the same time,a new peak appeared and stabilized at 163.965 after CO2was introduced into the system BDO+EDA for 15,30 and 45 min,respectively;and the characterization peak of solid CO2SM was observed at 164.664(Fig.5c~f).And this indicated not only the CO2SM was immediately formed and steadily existed after CO2was introduced into the system BDO+EDA, but the CO2SM was alkycarbonate salt rather than bicarbonate because bicarbonate was often found at about 160 in13C NMR[37-43].Therefore,the13C NMR analysis further illustrated that the system BDO+EDA could agilely activate CO2to form the alkylcarbonatesalt.Therefore,a possible formation mechanism (Scheme 1)of the CO2SM was proposed based on above analytic results.Similarly,Jessop and his coworkers[16,44]also reported an innovative class of CO2BOL with an alcohol and an amidine or guanidine superbase,which led to the formation of amidine or guanidine alkylcarbonate salts after CO2capturing.
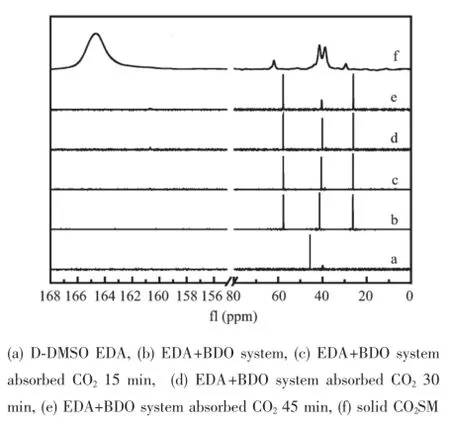
Fig.5 13CNMR spectra of EDA+BDO system absorbed CO2and formed solid CO2SM
TGA-DSC of the CO2SM was firstly investigated and shown in Fig.6.The TGA-DSC results showed that the CO2SM began to decompose at approximately 50℃,losing up to 8%of itsmass from 50 to 75℃. Then,the decomposition of CO2SM was accelerated at about 103℃with massive release of CO2and the evaporation of EDA.As it could be seen,this decomposed step in the temperature range of 75 to 112℃decreased 90.5%.And at about 112℃,a large exothermic peak was due to thermal volatilization of EDA,and the release of CO2,which indicated the CO2SM completely decomposed.In addition,TGA result also indicated that the CO2SM was stable before 50℃and released CO2after heating.
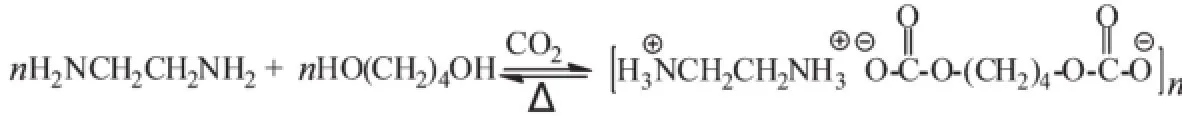
Scheme 1 Formation of CO2SM from CO2,EDA and BDO
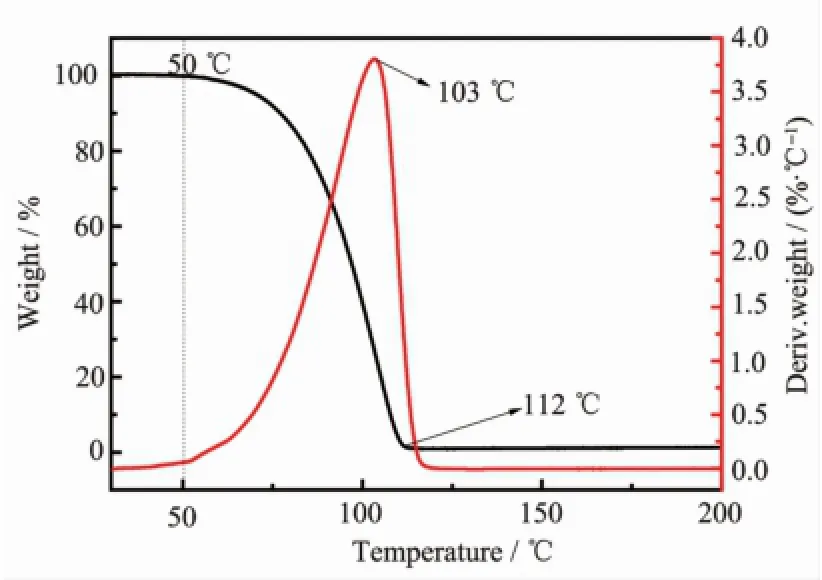
Fig.6 TGA curve for CO2SM under nitrogen ata temperature scan rate of 10℃·min-1
Based on TGA-DSC result,CO2SM could release CO2at 112℃.Therefore,a potential application of CO2SM for preparing CaCO3and BaCO3was developed.And thus,0.05 g CO2SM was added into Ca(OH)2saturated limpid solution,which hydrothermal reaction 2 h at 110℃in bake oven,and the dumbbell-like CaCO3micro-particles were obtained (Fig.7a).The XRD pattern and FTIR spectrum of the dumbbell-like CaCO3suggested that the polymorph of dumbbell-like CaCO3wasmost stable calcite(Fig.S1). Spindle-like BaCO3micro-particles could be obtained after the reaction,which 5 g CO2SM was added into 0.01 mol·L-1Ba(OH)2solution,then hydrothermal reaction at110℃oven for 2 h(Fig.7b).
Meanwhile,the XRD pattern and FTIR spectrum of the Spindle-like BaCO3indicated that BaCO3samples could be indexed as typical orthorhombic phase(Fig.S2).In this process,CO2released from CO2SM by heating reacted with Ca(OH)2and Ba(OH)2solution to generate amorphous CaCO3and BaCO3, respectively.The released EDA and rested BDO, which acted as a dispersant,make amorphous CaCO3, BaCO3to rearrange to form a regular,orderly dumbbell-like CaCO3and spindle-like BaCO3microparticles.As for the specific formation process of CaCO3,BaCO3will be detailedly reported in the future work.
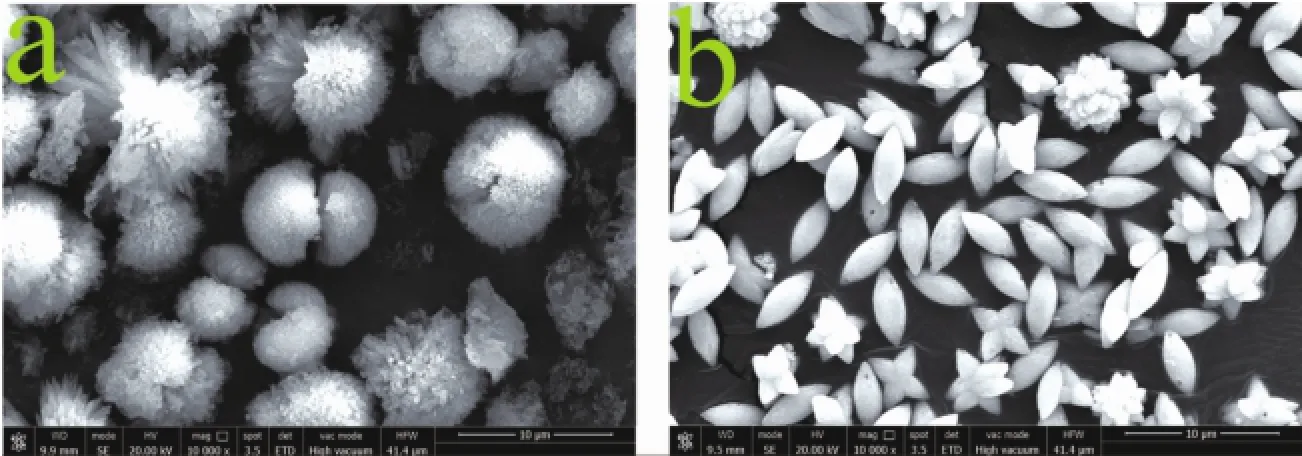
Fig.7(a)SEM image of dumbbell-like CaCO3;and(b)SEM image of spindle-like BaCO3
2.3Absorption-desorption cycles
Firstly,the experiments of CO2absorption with the aqueous BDO+EDA system were conducted at 20.0℃under atmospheric pressure and the changes in sample weightwere recorded by using an electronic balance.Secondly,the desorption of CO2from the loaded solution,prepared as described above,were carried out at 98.6℃with stirring until CO2stopped being released from the solution,which lasted for 90 min.Finally,the continuous cycles of simultaneous CO2absorption-desorption were carried out and the resultswere shown in Fig.8.
As shown in Fig.8,the aqueous BDO+EDA solution could be recycled at least for five continuous absorption-desorption cycles without any important loss of CO2capturing and releasing capability.Whats more,the regeneration temperature was lower than the traditional MEA system in the range from 100 to 140℃[39].Itmightbe due tomuch lower thermostability of the formed alkylcarbonate salts.Moreover,the maximum CO2loading capacity could reach 1.540 mol CO2permol EDA in the absorption-desorption cycles. Overall,all the above results indicate that the aqueous BDO+EDA(molar ratio 1∶1)system provide a high loading capacity,stable absorption-desorption cycles and low energy requirement processes for the application in industry.In short,all results showed that such a process not only restrained the volatilization of EDA,butalso fulfilled the capture,low energy loss and utilization ofCO2.
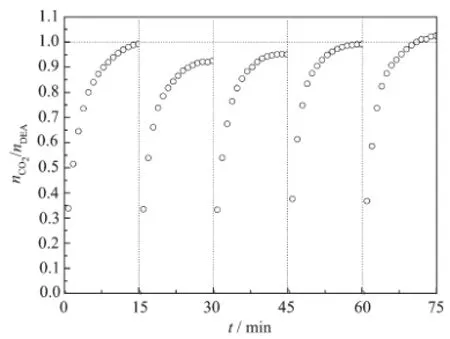
Fig.8 Reversible CO2capture and release by 6%(w/w) aqueous EDA/BDO system
3 Conclusions
In conclusion,an innovative CCU approach featuring cost-effectiveness,high capturing capacity and acceptable regeneration energy for industrial processes was developed.Particularly,it was found that CO2gas can be bubbled into the EDA-BDO system under mild condition to generate the novel solid CO2SM,which was extensively confirmed as an alkylcarbonate salt.Especially,the aqueous EDA+ BDO system is superior CO2storage system,exhibiting remarkable CO2capturing and releasing capability aftermultiple cycles.In addition,the aqueous CO2SM solution could be employed to prepare CaCO3and BaCO3micro-particles.
Acknow ledgments:This work was supported by the National Natural Science Foundation of China(Grant No. 21166017),the Inner Mongolia Science and Technology Key Projects,the Program for Grassland Excellent Talents of Inner Mongolia Autonomous Region,Program for New Century Excellent Talents in University(Grant No.NCET-12-1017),and training plan of academ ic backbone in youth of Inner Mongolia University of Technology.
Supporting information isavailableathttp://www.wjhxxb.cn
References:
[1]Raupach M R,Marland G,Ciais P,et al.Proc.Natl.Acad. Sci.U.S.A.,2007,104:10288-10293
[2]Roberts JL,Sawyer D T.J.Electroanal.Chem.,1965,9:1-7
[3]Grant J L,Goswami K,Spreer L O,et al.J.Chem.Soc., Dalton Trans.,1987:2105-2109
[4]Yu M K,Curcic I,Gabriel J.ChemSusChem,2008,1:893-899
[5]Kazuya G,Katsunori Y,Takayuki H.Appl.Energy,2013, 111:710-720
[6]Wang Q,Luo J,Zhong Z,et al.Energy Environ.Sci.,2011, 4:42-55
[7]Yang Z Z,He L N,Gao J,etal.Energy Environ.Sci.,2012, 5:6602-6639
[8]Takeda Y,Okumura S,Tone S,et al.Org.Lett.,2012,14: 4874-4877
[9]Rochelle G T.Science,2009,325:1652-1654
[10]Kim Y E,Lim J A,Jeong S K,et al.Bull.Korean Chem. Soc.,2013,34:783-787
[11]Zoannou K S,Sapaford D J,Griffiths A J.Int.J.Greenhouse Gas Control.,2013,17:423-430
[12]Button JK,Cubbins K E.Fluid Phase Equilib.,1999,159: 175-181
[13]La-Rubia M D,Garcia-Abuin A,Gomez-Diaz D,et al. Chem.Eng.Process.,2010,49:852-858
[14]Vahidi M,Zoghi A T,Moshtari B M,et al.J.Chem.Eng. Data,2013,58:1963-1968
[15]Valtz A,Coquelet C,Richhon D.Thermochim.Acta, 2006,443:245-250
[16]Heldebrant D J,Yonker C R,Jessopc P G,et al.Energy Environ.Sci.,2008,1:487-493
[17]Zhang J B,Han F,Wei X H,et al.Ind.Eng.Chem.Res., 2010,49:2025-2030
[18]Zhang J B,Li Q,Guo Z H,et al.Ind.Eng.Chem.Res., 2011,50:674-679
[19]LiQ,Zhang JB,Li L H,etal.J.Phys.Chem.B,2013,117: 5633-5646
[20]Zhang JB,Zhang P Y,Han F,et al.Ind.Eng.Chem.Res., 2009,48:1287-1291
[21]Kim M,Park JW.Chem.Commun.,2010,46:2507-2509
[22]Klimaszewski K,Borun A,Sengwa R J.J.Chem.Eng.Data, 2012,57:3164-3170
[23]Sha F,Zhao T X,Guo B,et al.J.Mol.Liq.,2015,208:373-379
[24]MaheswariU,Palanivelu K.J.CO2Util.,2014,6:45-52
[25]Kanamura K,Shiraishi S,Takezawa H,et al.Chem.Mater., 1997,9:1797-1804
[26]Baltrusaitis J,Usher C R,Grassian V H.Phys.Chem.Chem. Phys.,2007,9:3011-3024
[27]Kelemen SR,Freund H.Energy Fuels,1988,2:111-118
[28]Yang JT,Hu G X.RSCAdv.,2012,2:11410-11418
[29]Desimoni E,Casella G I,Cataldi TR I,et al.Surf.Interface Anal.,1992,18:623-630
[30]Heldebrant D J,Koech P K,Ang M T C,et al.Green Chem.,2010,10:713-721
[31]Wang H B,Jessop PG,Liu G J.ACSMacro Lett.,2012,1: 944-948
[32]Blasucci V,Dilek C,Huttenhower H,etal.Chem.Commun., 2009:116-118
[33]Jackson P,Pobinson K,Puxty G,et al.Enenry Procedia, 2009,1:985-994
[34]Heldebrant D J,Jessop P G,Thomas C A.J.Org.Chem., 2005,70:335-5338
[35]Ion A,Doorslaer C V,Parvulescu V,et al.Green Chem., 2008,10:111-116
[36]Wang H L,Kao H M,Digar M,et al.Macromolecules, 2001,34:529-537
[37]Mani F,Peruzzini M,Stoppioni P.Green Chem.,2006,8: 995-1000
[38]Fowler C I,Jessop PG,Michael F.Macromolecules,2012, 45:2955-2962
[39]Dibenedetto A,Aresta M,Fragale C,et al.Green Chem., 2002,4:439-443
[40]Barzagli F,Mani F,Peruzzini M.Energy Environ.Sci., 2009,2:322-330
[41]Garcia A A,Gomez D D,Navaza J M,et al.Energy Procedia,2012,42:1242-1249
[42]Jessop P G,Phan L,Carrier A,et al.Green Chem.,2010, 12:809-814
[43]Phan L,Andreatta JR,Horvey L K,et al.J.Org.Chem., 2008,73:127-132
[44]Jessop P G,Heldebrant D J,Li X W,et al.Nature,2005, 436:1102
A CO2Capture and Utilization Approach Using the System 1,4-Butanediol and 1,2-Ethanediam ine
SHA Feng GUO Bo ZHANG Fei ZHAO Tian-Xiang LIQiang ZHANG Jian-Bin*
(College of Chem ical Engineering,Inner Mongolia University of Technology,Huhhot 010051,China)
A novel and high effective way to fix CO2agilely through the system 1,4-butanediol(BDO)+1,2-ethylenediamine(EDA)undermild condition was developed.In this process,the bubbling CO2could be activated efficiently and directly converted into a novel solid CO2-storage material(CO2SM),which was extensively confirmed as an alkylcarbonate salt using XPS,XRD,FTIR and13C NMR analyses.As a material,the aqueous CO2SM solution could be used to prepare controllablemorphologies CaCO3and BaCO3micro-particles by reacting with Ca(OH)2and Ba(OH)2according to thermogravimetry analysis(TGA)result.Additionally,the aqueous BDO+ EDA system could be recycled multiple times without any important loss of CO2capturing and releasing capability at absorption temperature of 20℃and desorption temperature of 98.6℃.As a result,the combination of BDO+EDA seems to provide a green CO2capture and utilization(CCU)approach featuring high efficiency and low cost.
CO2capture,storage and utilization;CO2-storagematerial;CaCO3and BaCO3micro-particles;absorption-desorption cycle
O611.4
A
1001-4861(2016)07-1207-08
10.11862/CJIC.2016.167
2015-10-08。收修改稿日期:2016-03-21。
國(guó)家自然科學(xué)基金(No.21166017)、內(nèi)蒙古自治區(qū)自然基金杰出青年培育基金(No.2016JQ02)、教育部新世紀(jì)優(yōu)秀人才項(xiàng)目(No.NCET-12-1017)、草原英才項(xiàng)目和內(nèi)蒙科委攻關(guān)項(xiàng)目資助。
*通信聯(lián)系人。E-mail:tadzhang@pku.edu.cn;會(huì)員登記號(hào):S06N9134M1005。
 無(wú)機(jī)化學(xué)學(xué)報(bào)2016年7期
無(wú)機(jī)化學(xué)學(xué)報(bào)2016年7期
- 無(wú)機(jī)化學(xué)學(xué)報(bào)的其它文章
- 在四氯合鋅離子誘導(dǎo)下反式六元瓜環(huán)與堿金屬離子構(gòu)筑的配位超分子自組裝體
- Meso位單取代卟啉及其配合物的合成、表征和光譜性質(zhì)
- 模板劑緩釋法合成分子篩大單晶
- 由氫鍵連接的三個(gè)金屬-有機(jī)網(wǎng)狀化合物的合成、結(jié)構(gòu)及半導(dǎo)體性質(zhì)
- 兩個(gè)四唑配合物的原位合成、多樣化配位模式和強(qiáng)熒光性質(zhì)
- 基于3,5-二((4′-羧基芐基)氧)苯甲酸和4′-(4-吡啶基)-2,2′∶6′,2″-三聯(lián)吡啶為混合配體的兩個(gè)配合物的水熱合成與晶體結(jié)構(gòu)
