基于N-((3-吡啶基)磺酰基)天冬氨酸的Co(Ⅱ)、Zn(Ⅱ)、Cd(Ⅱ)配位聚合物的合成、磁性和熒光性質
廖蓓玲 李石雄 銀秀菊 賈晶晶 蔣毅民*,
(1河池學院化學與生命工程學院,宜州546300)
(2廣西師范大學化學與藥學學院,桂林541004)
基于N-((3-吡啶基)磺酰基)天冬氨酸的Co(Ⅱ)、Zn(Ⅱ)、Cd(Ⅱ)配位聚合物的合成、磁性和熒光性質
廖蓓玲1,2李石雄1,2銀秀菊1,2賈晶晶2蔣毅民*,2
(1河池學院化學與生命工程學院,宜州546300)
(2廣西師范大學化學與藥學學院,桂林541004)
以N-((3-吡啶基)磺酰基)天冬氨酸(H2L)為配體,采取水熱合成的方法,合成了配位聚合物[M(HL)2]n(M=Co,1;Zn,2;Cd,3)。X射線單晶衍射分析表明,它們的晶體同構。1的磁性分析表明,1的Curie常數為4.66 cm3·mol-1·K,Curie-Weiss常數為-21.23 K;與配體H2L相比,2和3的發射光譜發生了明顯的藍移,可能歸因于配體到金屬的電荷轉移。
配位聚合物;N-((3-吡啶基)磺酰基)天冬氨酸;熒光;磁性
0 Introduction
Amino acids are the basic unit of building blocks of proteins giving a specific protein molecular structure and morphology,and they have biological activity.When amino acids andmetal ions form amino acid complexes by coordination bond,these complexes not only still have biological activity,but also maintain the life macromolecular complex structures and function as an active center.Over the years,the N-acylated amino acid complexes catch much attention of researchers[1-5],and a number of innovative resultswere reported[6-8].N-sulphonylated amino acid refers to the introduction of sulphonyl in the nitrogencontaining carbonic acid ligands.From the viewpoint of biological effects,not only the N-sulphonylatedamino acid contains the peptide bond,but also the carboxyl-terminal is very similar to peptide chain.So, N-sulphonylated amino acid and its derivatives are a very promising ligand[9-11].Studies have shown that when the amino acid changes,the protein molecules will lose the biological activity.At the same time,the fluorescent probes can be applied to the detection of amino acids.Therefore,the study of small molecular fluorescent probe technique and its application for protein detection are very important.In this article we report three new coordination polymers based on N-((3-pyridine)sulfonyl)aspartate(H2L)ligand:[Co(HL)2]n(1);[Zn(HL)2]n(2);[Cd(HL)2]n(3),and their magnetic and fluorescent properties.
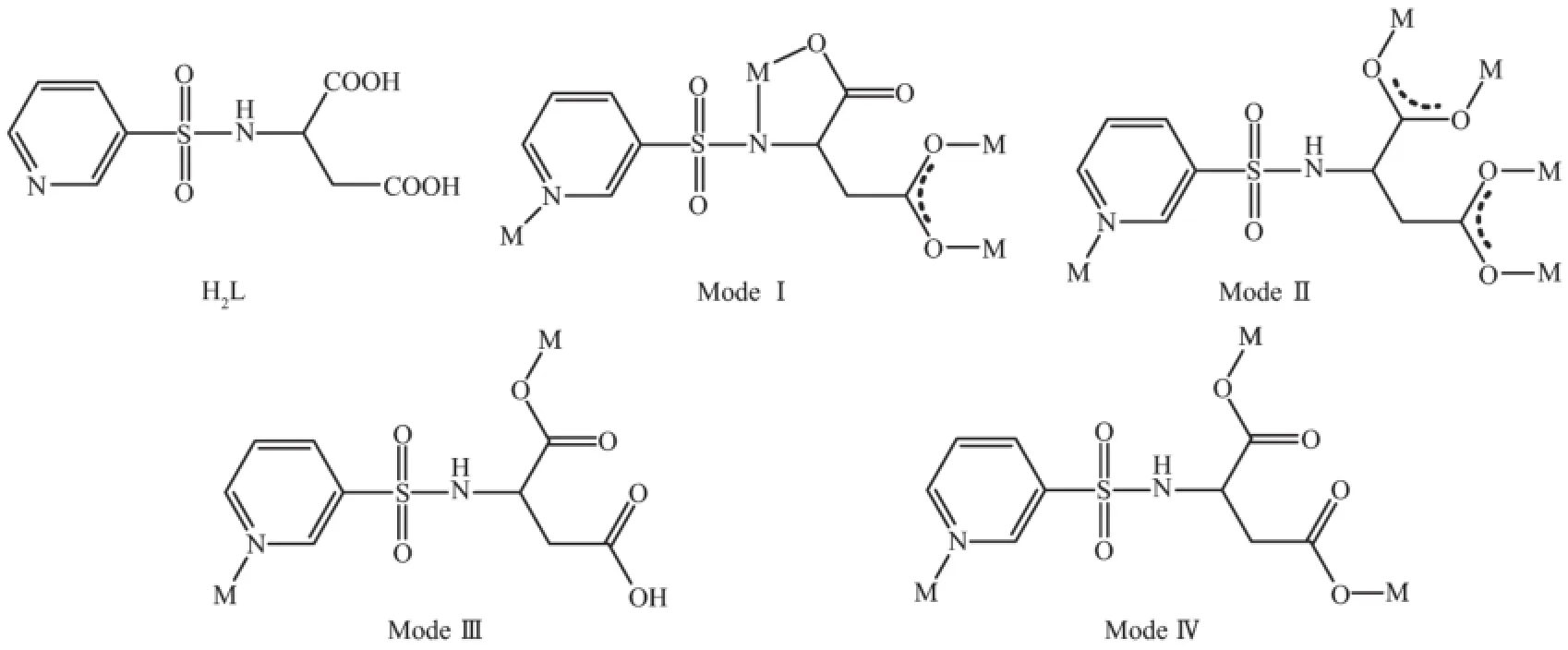
Scheme 1 Ligand H2L and its coordinationmodes
1 Experimental
All solvents,chemicalswere commercial reagents and used without further purification.H2L was synthesized according to the reference[12].Elementalanalyses (carbon,hydrogen and nitrogen)were performed with a Perkin-Elmer 240 elemental analyzer.The crystal structure was determined by an Agilent supernova diffractometer.The magnetic measurements were carried outwith a Quantum Design MPMS-XL7 and a PPMS-9 ACMSmagnetometer.
1.1Synthesis of polym ers
1.1.1Synthesis of[Co(HL)2]n(1)
AmixtureofCo(NO3)2·6H2O(0.058 2 g;0.2mmol), H2L(0.054 8 g,0.2mmol),10mL ofwaterand 10mL of methanol was stirred for 10 min at room temperature, and the pH value was adjusted to about 6 with triethylamine solution.Then the solution was sealed in a 25 mL Teflon-lined stainless steel container and heated at 100℃for 3 days.The mixture was cooled to room temperature at a rate of 10℃·h-1,and red needle crystals of 1 were obtained with yield of 46% (based on Zn).Anal.Calcd.for C18H18CoN4O12S2(%):C, 35.68;H,2.97;N,9.25;S,10.57.Found(%):C,35.60; H,2.87;N,9.26;S,10.50.
1.1.2Synthesis of[Zn(HL)2]n(2)
A mixture of Zn2(OH)2CO3(0.089 9 g,0.4 mmol), H2L(0.054 8 g,0.2mmol)and 20mL distilled H2Owas stirred for10min at room temperature,and the following procedure was similar to synthesis of 1 except that the reaction temperature was 120℃.Colorless needle crystals of 2 were obtained with yield of 50%(based on Zn).Anal.Calcd.for C18H18N4ZnO12S2(%):C,35.30; H,2.94;N,9.15;S,10.46.Found(%):C,35.27;H, 2.96;N,9.25;S,10.51.
1.1.3Synthesis of[Cd(HL)2]n(3)
The procedures were similar to the synthesis of 2 except that the reaction temperature was 130℃,and colorless columnar crystals of 3 were obtained with a yield of 70%(based on Cd),Anal.Calcd.for C18H18Cd N4O12S2(%):C,32.78;H,2.73;N,8.50;S,9.71.Found (%):C,32.76;H,2.63;N,8.54;S,9.65.
1.2Crystallographic data collection and refinement
Diffraction intensities for three polymers were collected on a computer controlled Agilent supernovadiffractometer equipped with graphite-monochromated Mo Kαradiationwith radiation wavelength of0.071 073 nm by using the X-scan technique.Lorentz polarization and absorption corrections were applied.The structure was solved by directmethods using Olex2 program[13]and refined with Olex2 program[14].Anisotropic thermal parameters were assigned to all non-hydrogen atoms. The hydrogen atoms were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter.Crystallographic data for 1~3 are listed in Table1.Selected bond lengths and bond angles for 1~3 are listed in Table2and hydrogen bonds for 1 and 2 are listed in Table3.
CCDC:1445365,1;1426017,2;1426018,3.
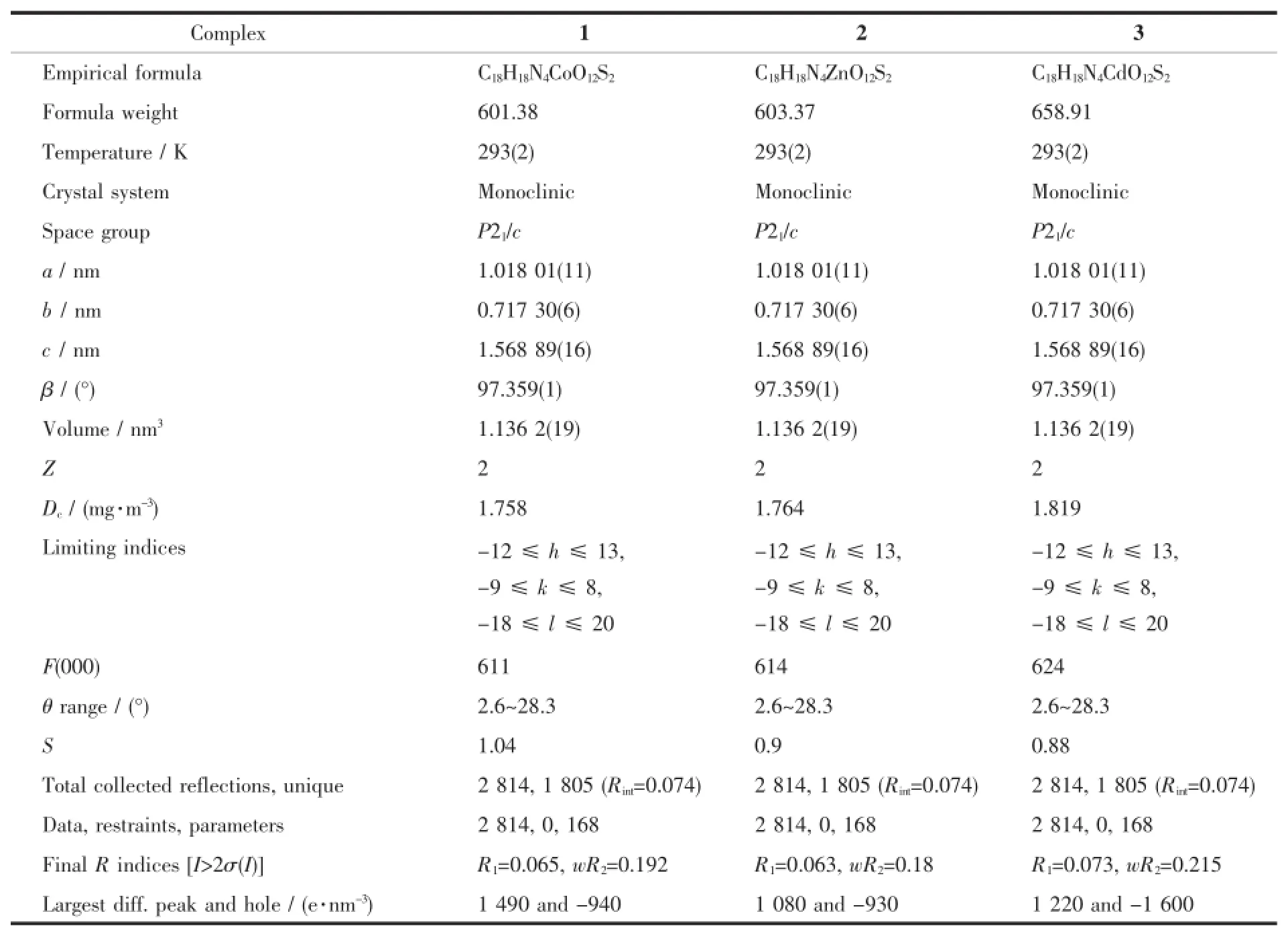
Table1 Crystallographic data and structure refinement parameters for 1~3

Table2 Selected bond lengths(nm)and bond angles(°)for 1~3

Continued Table2
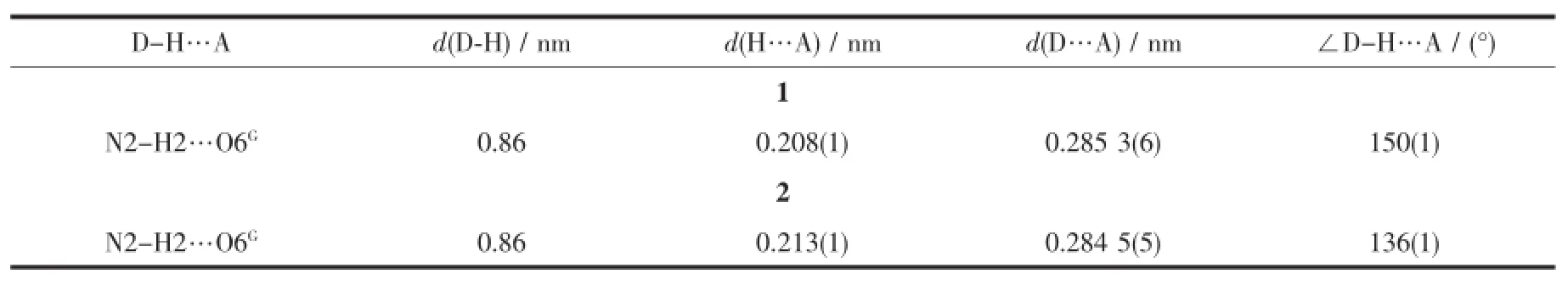
Table3 Hydrogen bond parameters for 1 and 2
2 Results and discussion
2.1Crystal structures of 1~3
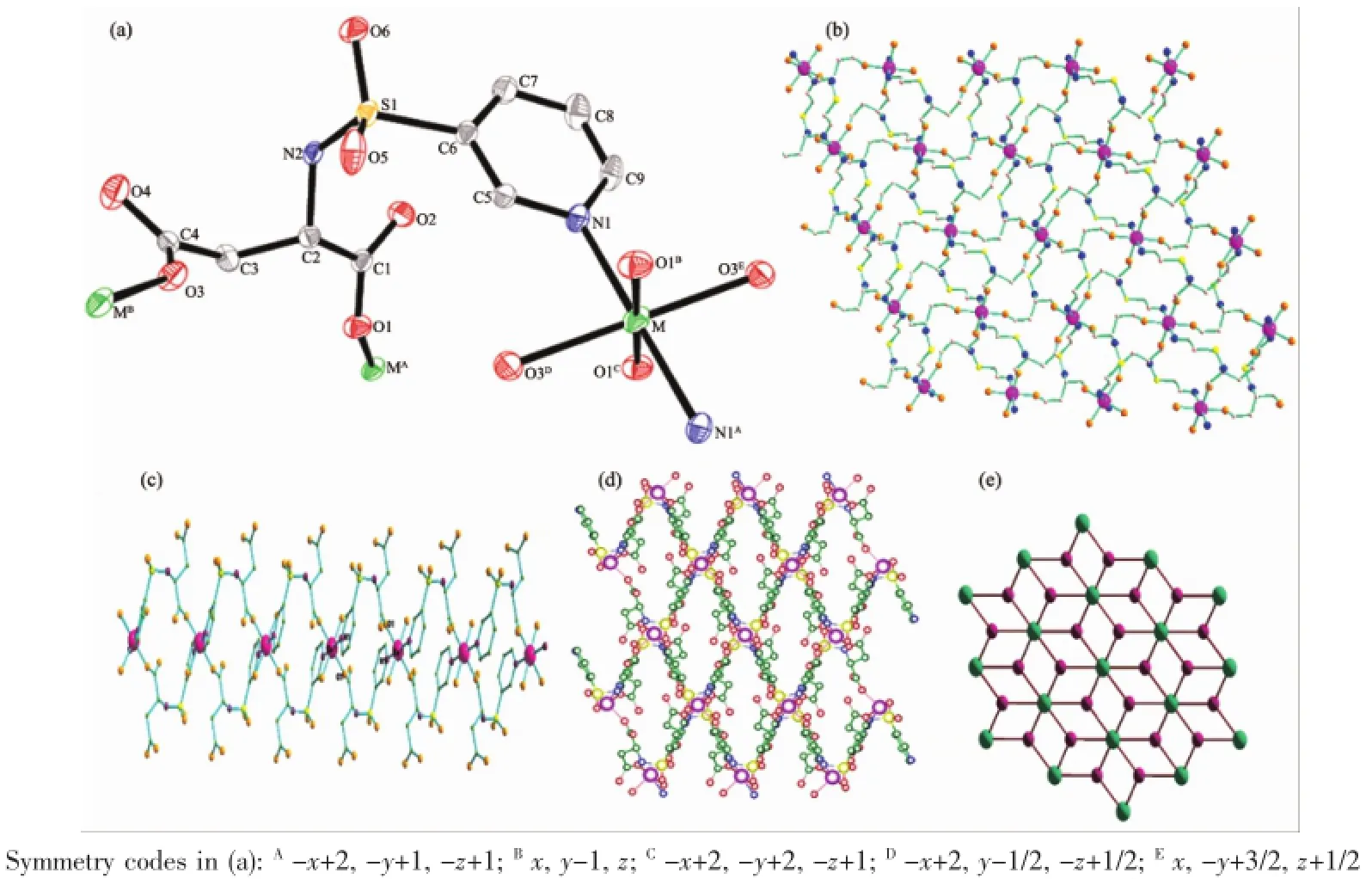
Fig.1(a)Coordination environmentofM(Ⅱ)(M=Co,Zn,Cd)ions in 1~3 with thermal ellipsoids at50%level;(b)1D chain along the a-axis of 1~3;(c)2D network structure of 1~3;(d)2D fishing net structure of 1~3;(e)Topology(43.412)of 1~3
X-ray single-crystal diffraction analysis reveals that1~3 crystallizes inmonoclinic system,space group P21/c.They are allomerism.The coordination environment of M(Ⅱ)in 1~3 is shown in Fig.1a(M=Co,Zn, Cd).The asymmetric unit consists of one M(Ⅱ)ion,twoHL-ligands.The M(Ⅱ)ion in 1~3 is coordinated by two nitrogen atoms(N1,N1A)and four oxygen atoms (O1B,O1C,O3D,O3E)of two different HL-ligands.For complex 1,the Co-O distances fall in the range of 0.207 1(3)~0.228 0(3)nm and the Co-N distance is 0.211 2(4)nm.The bond angles of O1A-Co1-O1 and O4E-Co-O1 are 180.0°and 98.81(13)°,respectively. For complex 2,the Zn-O distances fall in the range of 0.207 1(3)~0.228 3(3)nm and the Zn-N distance is 0.211 2(4)nm.For 3,the Cd-O distances fall in the range of 0.207 4(4)~0.228 6(4)nm and the Cd-N distance is 0.211 1(5)nm.These bond angles and bond distances all fall in the normal ranges[15-18].
The atoms of O1B,O1C,N1,N1Aare on the same plane and occupy the octahedralequatorialplane,while the atoms of O3Dand O3Eoccupy the axial position. In addition,a smooth hand-dimensional double rope structure(Fig.1b)is formed by two atoms(O1,N1) from two ligands connecting two adjacent M(Ⅱ)ions. Meanwhile,the two-dimensional fishing net structure (Fig.1d)is formed parallel to the bc plane by two atoms (O1,O3)from two ligands connecting two adjacency Co(Ⅱ)ions.The topology of 1~3 is(43.412)(Fig.1e).
2.2M agnetic analysis of polymer 1
Polymer1 isaCo(Ⅱ)(d7)complex havingunpaired electrons,so itsmagnetic properties are studied.The magnetic susceptibilities,χmof 1 weremeasured in the 2~300 K temparature range,and shown asχmandχmT versus T plots in Fig.2a and 1/χmversus T in Fig.2b, respectively.As shown in Fig.2a,the molar magnetic susceptibilityχmincreases gradually as the temperature lowers,and increases more rapidly below 25 K,then reaches a maximum value of 0.92 cm3·mol-1at 2 K. Combined with the dccrease in theχmT value when cooling,this result indicates the presence of weak antiferromagnetic interactionsin complex1.The thermal variation of the molar magnetic susceptibility obeys the Curie-Weiss law(χm=C/(T-θ)or 1/χm=(T-θ)/Cm)in the whole process(2~300 K)(Fig.2b).The values of the Curie and Curie-Weiss constants of 1 are Cm=4.66 cm3·mol-1·K andθ=-21.23 K.The negativeθvalue supports the presence of overall antiferromagnetic interactions in complex 1.At 300 K,χmT=4.32 cm3· mol-1·K,and themagneticmoment(μeff)of cobalt(Ⅱ), which is determined by the equationμeff=2.828(χmT)1/2, reaches the peak value of 5.88μB.This value is slightly higher than that expected for an isolated divalent high-spin Co(Ⅱ)system withμeff=3.87μB.
2.3Fluorescence properties of ligand and complexes 2~3
The solid fluorescence spectra of H2L and complexes 2~3 are shown in Fig.3.The ligand has a maximum emission peak at 447 nm with an excitation wavelength of 419 nm(Fig.3a),which could be attributed to the transition ofπ*→πorπ*→n of the ligand.As shown in Fig.3b and Fig.3c,though H2L ligand displays a very weak fluorescence in the solid state at room temperature,its complexes 2~3 all exhibit different intense fluorescence.For complexes 2 and 3,blue-shifted emission peak has occurred with a peak broadening,but the degree of blue-shift is not same.For example,the emission peak(438 nm)ofcomplex 2 occurred with a slight blue shift of 9 nm (Fig.3b,λex=371 nm),while the emission peak of complex 3 has a blue-shiftof 69 nm(Fig.3c,λem=378 nm,λex=329 nm).So,the fluorescence spectra indicate that emission of 2 and 3 show obvious blue shift due to the ligand-to-metal charge-transfer(LMCT)effect, which may be resulted from coordination environments around the metal ion,since photoluminescence behavior is closely associated with the metal center and the ligands[19].
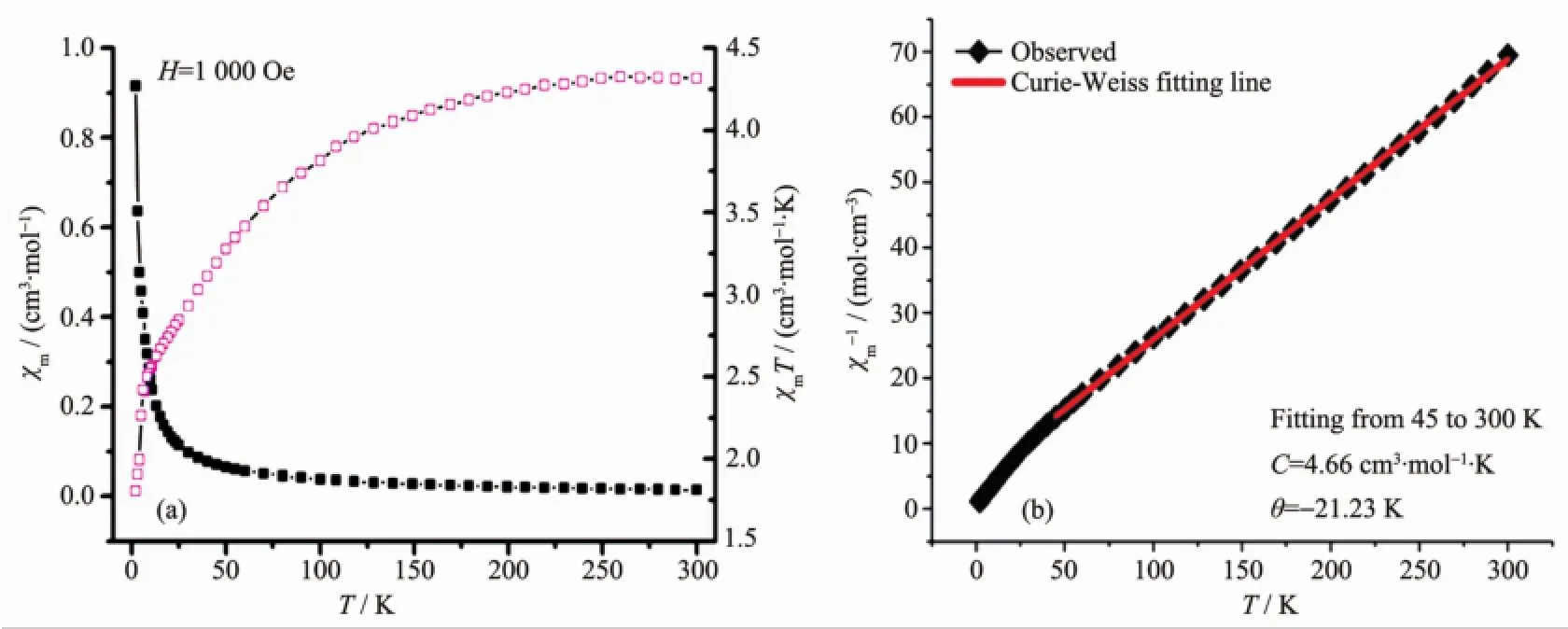
Fig.2(a)χmandχmT vs T plots of polymer 1;(b)1/χmvs T p lot of polymer 1
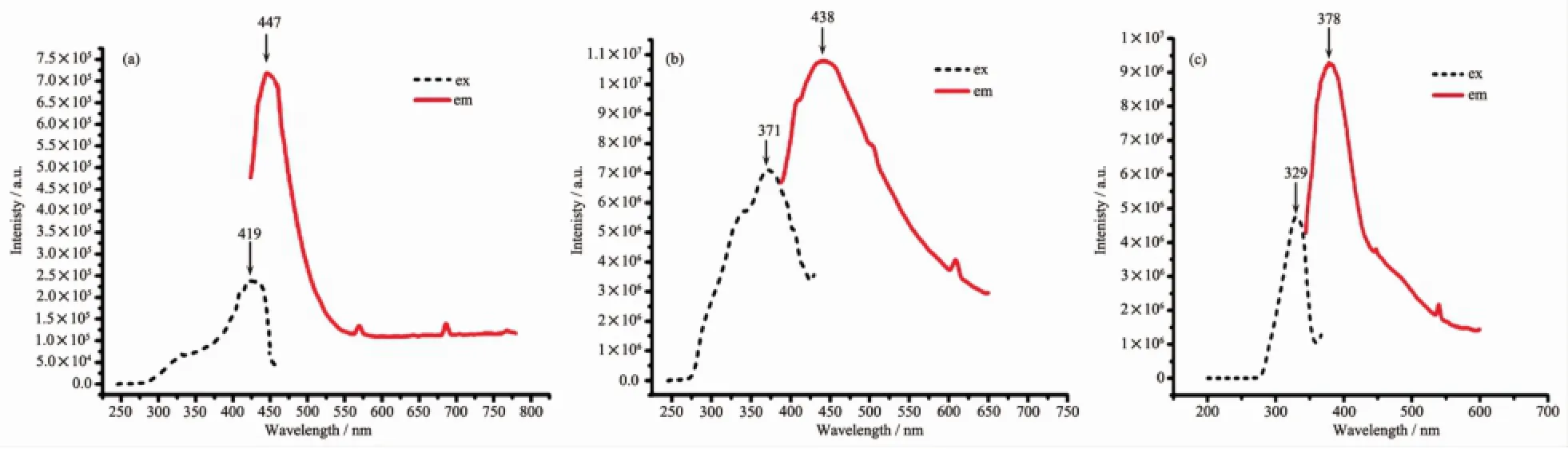
Fig.3 Solid fluorescence spectra of H2L(a),2(b)and 3(c)
3 Conclusions
In summary,three new coordination polymers based on the N-((3-pyridine)sulfonyl)aspartate had been synthesized and characterized.Single-crystal X-ray diffraction study reveals that the complexes 1~3 are isostructural and have 2D network structure with (43.412)topology.Magnetic analysis showed that in complex 1 antiferromagnetic coupling is dominant and the values of the Curie and Curie-Weiss constants are Cm=4.66 cm3·mol-1·K andθ=-21.23 K.The fluorescence spectra indicate that the emissions of 2 and 3 show obvious blue shift due to the ligand-to-metal charge-transfer(LMCT)effect.
References:
[1]Fleischer B,Shachter AM.Inorg.Chem.,1991,30:3763-3769
[2]Withersby M A,Blake A J,Champness N R,et al.J.Am. Chem.Soc.,2000,122:4044-4046
[3]Holliday B J,Mirin C A.Angew.Chem.Int.Ed.,200l,113: 2076-2097
[4]Takashi U,Tetsuya K,Susumu K.Chem.Mater.,2013,25: 3772-3776
[5]Mujahuddin M S,Joel T M,Maravanji S B.Inorg.Chem., 2015,54(13):6063-6065
[6]Kall PO,Grins J,Fahlman,et al.Polyhedron,2001,20:2747 -2753
[7]Brueckner S,Menabue L,TolazziM,etal.Inorg.Chim.Acta, 1993,214:185-191
[8]Ma L F,Wang Y Y,Wang L Y,et al.Eur.J.Inorg.Chem., 2008,5:693-703
[9]Ma L F,Wang L Y,Chen S H.et al.Polyhedron,2009,28: 2494-2502
[10]Cheng M Q,Ma L F,Wang L Y.Chin.J.Chem.,2007,25: 498-502
[11]Li SX,Liao B L,Yang G G,etal.Synth.React.Inorg.Met. -Org.Chem.,2015,45:926-929
[12]Panchaud P,Renaud P.Adv.Synth.Catal.,2004,346:925-928
[13]Dolomanov O V,Bourhis L J,Gildea R J,et al.J.App l. Crystallogr.,2009,42:339-341
[14]Bourhis L J,Dolomanov O V,Gildea R J,et al.Acta Crystallogr.A,2015,A71:59-75
[15]Ghames A,Douadi T,Haffar D.Polyhedron,2006,25:3201-3208
[16]ZHANG Qi-Long(張奇龍),CHEN Ming-Hua(陳明華),RAN Xia(冉霞),et al.Chinese J.Inorg.Chem.(無機化學學報), 2015,31:1387-1392
[17]LIShi-Xiong(李石雄),LIAO Bei-Ling(廖蓓玲),LUO Pei(羅培),et al.Chinese J.Inorg.Chem.(無機化學學報),2015, 31:291-296
[18]GUO Hai-Fu(郭海福),LEIJia-Mei(雷佳眉),MA De-Yun(馬德運).Chinese J.Inorg.Chem.(無機化學學報),2015,31: 2385-2392
[19]Glen G,Briand A D,Smith G S,et al.Inorg.Chem.,2007, 46:8625-8637
Co(Ⅱ),Zn(Ⅱ)and Cd(Ⅱ)Coordination Polymers Based on N-((3-Pyridine)sulfonyl)aspartate:Syntheses,Magnetic and Fluorescence Properties
LIAO Bei-Ling1,2LIShi-Xiong1,2YIN Xiu-Ju1,2JIA Jing-Jing2JIANG Yi-Min*,2
(1College of Chemistry and Biological Engineering,HechiUniversity,Yizhou,Guangxi546300,China)
(2College of Chemistry and Pharmacy,Guangxi Normal University,Guilin,Guangxi 541004,China)
The title coordination polymers of[M(HL)2]n(M=Co,1;Zn,2;Cd,3)based on H2L(H2L=N-((3-pyridine) sulfonyl)aspartate)have been synthesized under hydrothermal conditions.X-ray diffraction analysis of three complexes show that they are isomorphic.The values of the Curie and Curie-Weiss constants of 1 are Cm=4.66 cm3·mol-1·K andθ=-21.23 K.The fluorescence spectra indicate thatemission of 2 and 3 show obvious blue shift due to the ligand-to-metal charge-transfer(LMCT)effect.CCDC:1445365,1;1426017,2;1426018,3.
coordination polymer;N-((3-pyridine)sulfonyl)aspartate;fluorescence;magnetic
O614.81+2;O614.24+1;O614.24+2文獻識別碼:A
1001-4861(2016)07-1255-06
10.11862/CJIC.2016.157
2016-01-05。收修改稿日期:2016-05-15。
廣西自然科學基金(No.2014GXNSFAA11803)、廣西高校科研項目(No.YB2014331)和廣西教育廳基礎研究(No.200807MS090)項目資助。
*通信聯系人。E-mail:790223684@qq.com

