兩個基于半剛性雙甲基苯并咪唑配體的螺旋型鎘配位聚合物
許春鶯 唐四葉 苗少斌 吉保明
(洛陽師范學院化學化工學院,河南功能導向多孔材料重點實驗室,洛陽471934)
兩個基于半剛性雙甲基苯并咪唑配體的螺旋型鎘配位聚合物
許春鶯 唐四葉 苗少斌 吉保明*
(洛陽師范學院化學化工學院,河南功能導向多孔材料重點實驗室,洛陽471934)
以1,4-雙(2-甲基苯并咪唑-1-亞甲基)苯(bmb)為主配體與Cd(NO3)2反應,通過改變輔助雙羧酸配體2個羧基間的連接基團,得到了2個配位聚合物{[Cd(bmb)(tba)]·DMF}n(1)和[Cd(bmb)(ada)]n(2)(H2tda=5-叔丁基間苯二甲酸,H2ada=1,3-金剛烷二乙酸)。結構分析表明聚合物1顯示二維三明治結構,帶有交替的左手和右手螺旋鏈。聚合物2也顯示了二維三明治結構,帶有meso-螺旋和微孔。聚合物1和2都顯示了強的熒光發射和高的熱穩定性。
螺旋;鎘;熒光;熱穩定性
Helical structures,as the foundation of the genetic code,have attracted intense interest in field of metal-organic frameworks(MOFs)not only for their ubiquitous appearance in nature,a typical example being the DNA molecule,but also for their practical implications in multidisciplinary areas,such as biomimetic chemistry,optical devices,asymmetric catalysis chemistry,and structural biology[1-5].Until now,there are many reports about single-,double-,triple-,and even multiple-stranded helices as well as circular and cylindrical helices[6-9].In contrast,the series reports about single-,double-,triple-or more flexures helix are extremely infrequent[10-13].The reason may be that it is quite difficult to control the formation of two or more flexures in single-stranded helix.To get such series of helices,the crucial step is to choose
multifunctional organic ligands containing appropriate coordination sites and spacers with specific positional orientation[14-15].According to our previous studies[16-17], 1,4-bis(2-methylbenzimidazol-1-ylmethyl)benzene(bmb) is an excellent semi-rigid N-heterocyclic ligand with an“arm-spacer-arm”type structural feature,which may generate flexures and favor to form helicalmotifs. Bmb comprises two flexible methylbenzimidazol pendants as arms attached to a rigid aromatic core as a spacer via freely rotatablemethylene(-CH2-)hinges. It is quite different from highly rigid and completely flexible ligands.The rigid components of bmb could take on distinctive conformations to enhance the structural variation,while the restricted flexibility of the ligand would provide limited modes subjected to subtle influences in the assembly of target coordination structures.In view of the characters,bmb has the potential to produceunique n-flexurehelicalmotifs.
On the other hand,it is well known that dicarboxylate organic groups are excellent structural constructors[18].In view of the developmentof synthetic strategy,it will be valuable to introduce the dicarboxylates with different spacers into the meso-helices synthetic process based on N-donor ligand bmb, which can result in greater tunability of the helical features and build much more complicated and fascinating MOFs.
Based on the above considerations,by introducing two dicarboxylate co-ligandswith different spacer into the Cd(II)-bmb synthesis system,fascinating helical coordination polymers,namely{[Cd(bmb) (tba)]·DMF}n(1)and[Cd(bmb)(ada)]n(2)were synthesized under similar hydrothermal conditions.A systematically structural comparison of polymers 1 and 2 indicates that the conformation of bmb plays an important role in the conformation of helix from single-to two-flexures helices.In addition,the thermal stabilities and photoluminescence properties of 1~2 in the solid state have also been investigated.
1 Experimental
1.1 Materials and methods
All chemicals and reagentswere used as received from commercial sources without further purification except for bmb,which was synthesized according to the literature[19].All reactions were carried outunder hydrothermal conditions.Elementalanalyses(C,H,N) were determined with a Elementar Vario ELⅢelemental analyzer;IR spectra were recorded as KBr pellets on a Bruker EQUINOX55 spectrophotometer in the 4 000~400 cm-1region.Powder X-ray diffractions (XRD)were made under a Rigaku D/Max-2500 PCT with Cu Kαradiation(λ=0.154 2 nm),using an operating voltage of 45 kV and an operating current of 40 mA at room temperature in the 2θrange between 5° and 50°.Thermogravimetric analyses(TGA)were carried out on a Perkin-Elmer TAG-7 instrument with a heating rate of 10℃·min-1in air.Fluorescence spectra were performed on a Hitachi F-4500 fluorescence spectrophotometer at room temperature.
1.2 Synthesis of{[Cd(bmb)(tba)]·DM F}n(1)
Amixture of Cd(NO3)·4H2O(61.7 mg,0.2mmol), bmb(0.2 mmol,73.2 mg),H2tba(0.2 mmol,24.4 mg), NaOH(0.4mmol,16 mg),DMF(3 mL)and deionized water(7 mL)was sealed in a sealed Teflon-lined stainless steel vessel(25 mL)at 150℃for 5 days. After being cooled to room temperature,crystals of 1 were collected by filtration.Yield:48%based on Cd. Anal.Calcd.for C39H41CdN5O5(%):C,60.66;H,5.35; N,9.07.Found(%):C,60.71;H,5.40;N,9.10.IR (KBr,cm-1):2 957(s),1 705(w),1 655(m),1 619(m), 1 562(s),1 514(m),1 462(m),1 408(s),1 349(m), 1 265(m),1 163(w),1 017(w),876(m),792(s),743(s), 672(m),618(m),524(m),470(m).
1.3 Synthesis of[Cd(bm b)(ada)]n(2)
The same synthetic method as that for 1 was used except that H2tba was replaced by H2ada(0.2 mmol,50.4 mg).Yield:43%based on Cd.Anal. Calcd.for C38H40N4O4Cd(%):C,62.59;H,5.53;N, 7.68.Found(%):C,64.52;H,5.49;N,7.65.IR(KBr, cm-1):3 438(s),2 907(m),2 842(m),1 617(w),1 546 (s),1 452(m),1 406(s),1 337(m),1 289(m),1 249(m), 1 216(w),1 158(m),975(m),948(m),755(s),665(m), 613(w),549(w),587(w),459(m).
1.4 Crystal structural determ ination
The data of the two polymerswere collected on a
Rigaku Saturn 724 CCD diffractomer(Mo Kα,λ= 0.071 073 nm)at temperatureof(20±1)℃.Absorption correctionswere applied by usingmulti-scan program. The data were corrected for Lorentz and polarization effects.The structures were solved by directmethods and refined with a full-matrix least-squares technique based on F2with the SHELXL-97 crystallographic software package[20].The hydrogen atoms were placed at calculated positions and refined as riding atoms with isotropic displacement parameters.Crystal data and structural refinement parameters for 1~2 are summarized in Table 1.
CCDC:1474502,1;1474503,2.
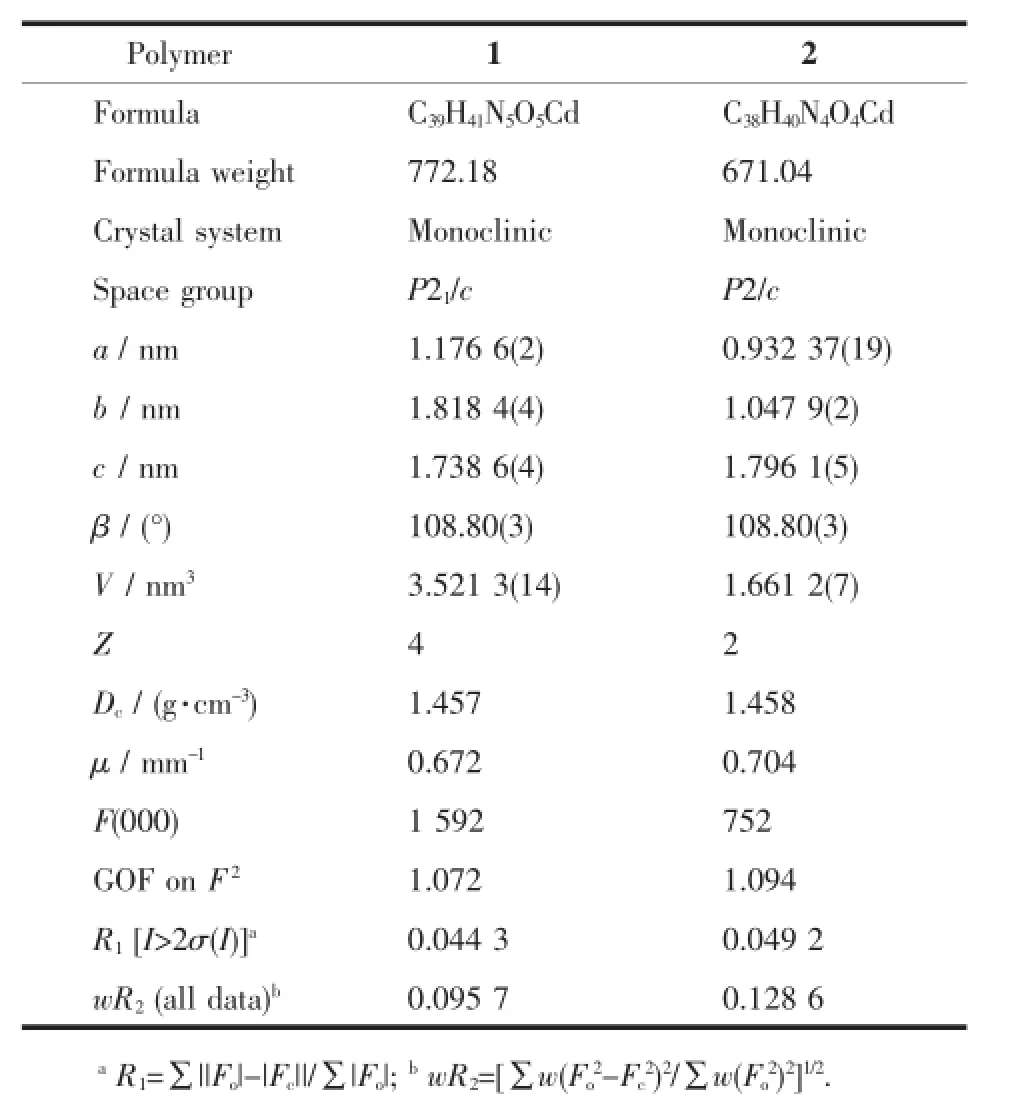
Table 1 Crystal data and structure refinement details for polymers 1~2
2 Results and discussion
2.1 Structure description of{[Cd(bmb)(tba)]· DM F}n(1)
Single X-ray diffraction analysis reveals that 1 features a 2D sandwiche structure.As shown in Fig. 1a,the asymmetric unit contains one crystallographically independent Cd(II)ions,one bmb,one completely deprotonated tba2-and one free DMFmolecule. The center Cd(II)ions are defined in distorted tetragonal pyramid coordination environment supplied by three carboxylate oxygen atoms from two symmetryrelated tba2-anions and two nitrogen atoms from bmb. Both Cd-O and Cd-N bond lengths are fall into the normal range[21-22].
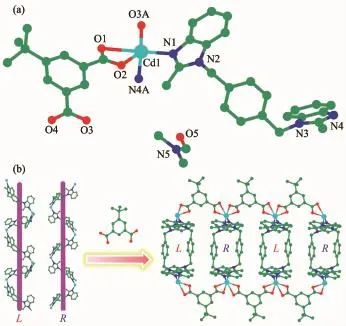
Fig.1(a)Coordination environmentof Cd(II)ion with hydrogen atoms omitted for clarity;(b)2D sandwiche structure of 1 with alternate left-and right-handed helices
Bmb adopts asymmetric cis-conformation with the dihedral angle between twomethylbenzimidazole rings being 33.0(1)°and the Ndonor…N-Csp3…Csp3torsion angles being 102.4(7)°and 114.2(7)°.Bmb link with Cd(II)ions to form the left-and right-handed helices (Fig.1b)with large quadrilateral microchannels.Both the helical pitches are 1.82(0)nm corresponding to the length of c axis.One carboxyl of tba2-adoptsμ1-η1∶η0and the other adoptsμ2-η1∶η1coordination fashions.The tba2-ligands connect the left-and righthanded helices alternately to form a 2D sandwiche architecture.The Cd/bmb helix chains locate in middle as ntermediate sandwichmaterial and the tba2-locate in the top and bottom.
2.2 Structure description of[Cd(bm b)(ada)]n(2) When H2ada with adamantane spacer instead of H2tba is introduced into the synthetic procedure,a similar 2D sandwiche structure with rare meso-helix was obtained.As shown in Fig.2a,the asymmetric unitof 2 contains one crystallographically independent
Cd(II)ions,one bmb and one ada-anion.The coordination environment around the Cd(II)center is best portrayed as a distorted octahedral geometry,and the equatorial plane comprises of three carboxylate oxygen atoms(O1,O2 and O1A)from two symmetryrelated ada2-anions and one nitrogen atoms from bmb (N1A).One carboxylate oxygen atom from ada2-anion (O2A)and one nitrogen atoms from bmb(N1)occupy the apical site.The Cd-O/N bond lengths are in the normal range[21-22].
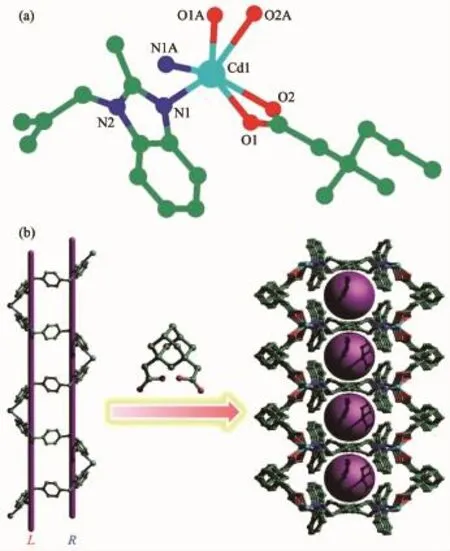
Fig.2(a)Coordination environmentof Cd(II)ion with hydrogen atoms omitted for clarity;(b)2D sandwiche structure of 2 with meso-helix and micropore
Bmb adopts symmetric trans-conformation with the Ndonor…N-Csp3…Csp3torsion angles being 111.0(9)°.
As shown in Fig.2b,Bmb are linked by Cd(II)ions to form a rare meso-helix motif with two flexures in single-stranded helix.The helical pitches are 1.80(0) nm corresponding to the length of c axis.Two carboxyl groups of ada2-adoptμ2-η1∶η1coordination pattern coordinating with Cd(II)as linking.As a result,the Cd/bmb meso-helix chains are linked to form a 2D sandwiche structure.The Cd/bmb meso-helix act as cores and the ada2-locate in the top and bottom as adorn.Interestingly,polymer 2 exhibits a micropore structure.
2.3 Effect of sem i-rigid bis(methylbenzim idazole) ligand
Due to the semi-rigid character,bmb can show stable cis/trans-conformation and symmetric(with the same Ndonor…N-Csp3…Csp3torsion angles of twomethylbenzimidazol arms)/asymmetric(with different Ndonor…N-Csp3…Csp3torsion angles of twomethylbenzimidazol arms)conformations.From the structural descriptions above,the spacer of carboxylate co-ligands have important effects on the spatial structure of bmb, which further effect on the ultimate frameworks.From polymers 1 to 2,the spacer of carboxylate co-ligands change from tert-butyl benzene to adamantane.Both the conformation and symmetry of bmb are changed. So,1 exhibits common helix and 2 shows raremesohelix.Apparently,these analyses above forcefully demonstrate that the change of spacer and the freely conformational bmb play essential roles in determining the architecture of the final products.
2.4 XRD patterns and therm al analyses
In order to confirm the phase purity of polymer 1~2,the PXRD patterns were recorded for 1~2,and they were comparable to the corresponding simulated ones calculated from the single-crystal diffraction data (Fig.3),indicating a pure phase of each bulky sample. Thermal gravimetric analysis(TGA)was used to evaluate the framework stability.As shown in Fig.4,1 shows a weight loss of 9.44%in the range of 217~290℃corresponding to the loss of free DMF molecule (Calcd.9.47%).Such high temperature probably results from the presence of hydrogen-bonding(C-H…O 0.340 8(6)nm)between the DMFand ligand bmb. Then a plateau region follows.The overall framework starts to decompose at about 380℃.At 546℃,a CdO residue of 16.91%(Calcd.16.63%)is obtained. No obvious weight loss is observed for anhydrous polymer 2 until the decomposition of the framework occurs at390℃.At607℃,a CdO residue of 18.24% (Calcd.17.61%)is obtained.
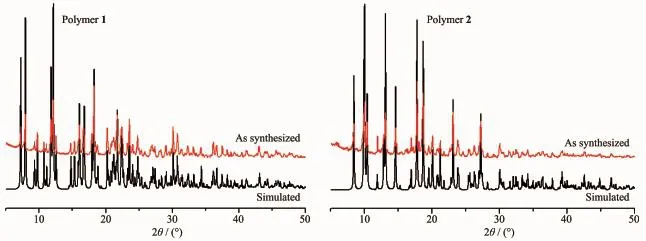
Fig.3 PXRD patterns of polymers 1~2
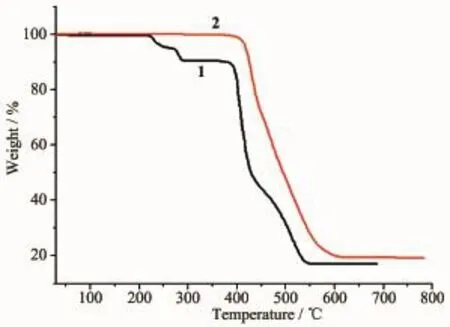
Fig.4 TGA curves of polymers 1~2
2.5 Photolum inescence properties
Coordination polymers with d10metal centers and conjugated organic linkers are promising candidates for photoactive materials with potential applications such as chemical sensors and photochemistry[23-26]. Hence,the solid state photoluminescence properties of Cd(II)polymers 1~2,together with the free bmb ligand and dicarboxylic acid co-ligands were investigated at room temperature(Fig.5)under the same experimental conditions.The free ligands bmb and H2tba show intense emissions bands at309 nm(λex=293 nm)and 330 nm(λex=305 nm),respectively.Thenon-conjugated organic linker H2ada has noemissionsband.Obviously, the fluorescent emissions bands of 1(λem=308 nm, λex=292 nm)can be attributed to the intraligand charge transitions of bmb and H2tba due to their similar emission bands.Polymer 2 exhibits an intense emission at 305 nm(λex=305 nm),which also can be attributed to the intraligand charge transitions of bmb. The small blue-shifted peak of 1 and 2 are probably caused by the coordination of aromatic carboxylic acid to themetal centers.
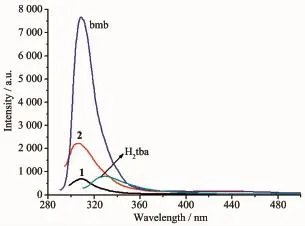
Fig.5 Solid-state photoluminescent spectra of polymers1~2 and free ligands
3 Conclusions
In summary,two fluorescent polymers with different dicarboxylic acid co-ligands have been obtained under the same experimental conditions.The investigation indicates that the spacers of dicarboxylic acid co-ligands have important influences in the conformation of bmb,which lead to diverse helices from common single-helix to meso-helix.
[1]Fujikawa T,Segawa Y,Itami K.J.Am.Chem.Soc.,2016, 138(10):3587-3595
[2]Jiang JC,Zhao Y B,Yaghi O M.J.Am.Chem.Soc.,2016, 138(10):3255-3265
[3]Yoon M,SrirambalajiR,Kim K.Chem.Rev.,2012,112:1196 -1231
[4]Xu Z X,Ma Y L,Xiao Y,et al.Cryst.Growth Des.,2013, 13:5487-5498
[5]Ma S,Simmons JM,Sun D,et al.Inorg.Chem.,2009,48: 5263-5268
[6]Zhang G,Yao S Y,Guo D W,et al.Cryst.Growth Des., 2010,10:2355-2361
[7]Ye B H,Tong M L,Chen X M.Coord.Chem.Rev.,2005, 249:545-550
[8]Ma Y,Han Z,He Y,et al.Chem.Commun.,2007:4107-4109
[9]Hu Y,LiG,Liu X,et al.CrystEngComm,2008,10:888-893
[10]Plasseraud L,Maid H,Hampel F,et al.Chem.Eur.J., 2001,7:4007-4010
[11]Xiao D R,Li Y G,Wang E B,et al.Inorg.Chem.,2007,46: 4158-4166
[12]Qi Y,Luo F,Batten S R,et al.Cryst.Growth Des.,2008,8: 2806-2813
[13]Jin JC,Wang Y Y,Liu P,et al.Cryst.Growth Des.,2010, 10:2029-2032
[14]Xu C,Guo Q,Wang X,et al.Cryst.Growth Des.,2011,11 (5):1869-1879
[15]Xu C,Wang X,Guo Q,et al.Synth.React.Inorg.Met.-Org. Chem.,2012,43(5):741-745
[16]Xu C,Li L,Wang Y,et al.Cryst.Growth Des.,2011,11(10): 4667-4675
[17]Xu C,Wang X,Ding D,et al.Inorg.Chem.Commun.,2011, 14(9):1410-1413
[18]LIJin-Ping(李金萍),FAN Jian-Zhong(范建中),WANG Duo -Zhi(王多志).Chinese J.Inorg.Chem.(無機化學學報), 2016,32(5):753-761
[19]Aakero?CB,Desper J,Leonard B,etal.Cryst.Growth Des., 2005,5:865-873
[20]Sheldrick G M.SHELXL-97,Program for the Solution of Crystal Structures,University of G?ttingen,Germany,1997.
[21]Fernández B,Seco JM,Cepeda J,et al.CrystEngComm, 2015,17:7636-7645
[22]Reger D L,Pascui A E,Smith M D,et al.Inorg.Chem., 2012,51:11820-11836
[23]Fang Q R,Zhu G S,Xue M,et al.Inorg.Chem.,2006,45: 3582-3587
[24]Allendorf M D,Bauer C A,Bhaktaa R K,et al.Chem.Soc. Rev.,2009,38:1330-1343
[25]Yao X Q,Zhang M D,Hu J S,et al.Cryst.Growth Des., 2011,11:3039-3044
[26]Lan A,Li K,Wu H,et al.Angew.Chem.Int.Ed.,2009,48: 2334-2339
Two Helical Cadm ium Coordination Polymers Based on Sem i-rigid Bis(methylbenzim idazole)Ligand
XU Chun-Ying TANG Si-Ye MIAO Shao-Bin JIBao-Ming*
(Henan Key Laboratory of Function-Oriented PorousMaterials,College of Chemistry and Chemical Engineering, Luoyang Normal University,Luoyang,Henan 471934,China)
By varying the spacers between two carboxyl of dicarboxylic acid co-ligands,the reaction between ligand bmb((bmb=1,4-bis(2-methylbenzimidazol-1-ylmethyl)benzene)and Cd(NO3)2gave two coordination polymers,{[Cd(bmb)(tba)]·DMF}n(1)and[Cd(bmb)(ada)]n(2)(H2tba=5-tert-butylisophthalic acid,H2ada=1,3-adamantanediacetic acid).Structural analyses reveal that polymer 1 shows a 2D sandwiche structure with alternate left-and right-handed helices.Polymer 2 also exhibits a 2D sandwiche structure but with meso-helix and micropore.In addition,polymers 1 and 2 show intense fluorescence emissions and high thermostability. CCDC:1474502,1;1474503,2.
helix;cadmium;photoluminescence;thermostability
O614.24+2
A
1001-4861(2016)10-1825-06
10.11862/CJIC.2016.231
2016-04-17。收修改稿日期:2016-09-20。
國家自然科學基金(No.21372112,21476102)資助項目。
*通信聯系人。E-mail:lyhxxjbming@126.com