三種2-羥基-1-萘醛酰腙的晶體結構、熱分解及其與CT-DNA的相互作用
趙順省 李蘭蘭 劉向榮 丁作成 楊再文
(西安科技大學化學與化工學院,西安 710054)
三種2-羥基-1-萘醛酰腙的晶體結構、熱分解及其與CT-DNA的相互作用
趙順省 李蘭蘭 劉向榮*丁作成 楊再文
(西安科技大學化學與化工學院,西安 710054)
合成了 2-羥基萘醛苯乙酰腙(1)、2-羥基萘醛-4-羥基苯乙酰腙(2)和 2-羥基萘醛-2-甲氧基苯乙酰腙(3)三種新型酰腙化合物,通過單晶 X 射線衍射(XRD)、元素分析和傅里葉變換紅外(FTIR)光譜對化合物結構進行了表征。單晶X射線衍射結果表明,化合物 2 和 3 結晶為單斜晶系,空間群C2/c。而化合物1 結晶為正交晶系,空間 群 為 Pbca。熱重(TG)分析結果 表 明,化合物 1、2、3 分 子骨架熱分 解 的溫度分別 為 318.23、319.04、323.01 °C,對應的熱分解過程表觀活化能分別為 115.90、145.18、129.38 kJ·mol-1。微量熱研究表明,三種酰腙及其前驅體酰肼與小牛胸腺脫氧核糖核酸(CT-DNA)相互作用均為吸熱作用,但作用時間(1.00-50.0 min)和反應熵變(0.47-15.50 kJ·mol-1)有較大的差異。化合物 1 和 2 與 CT-DNA 的反應焓變均大于其前驅體酰肼 a 和 b與CT-DNA的反應焓變,而化合物3 與 CT-DNA 的反應焓變卻小于其前驅體酰肼 c與CT-DNA的反應焓變。
酰腙;晶體結構;熱重;微量熱;DNA作用
1 Introduction
Acyl hydrazones have drawn considerable interest due to their bioactive properties1,including uses as antitrypanasomals2and tools for molecular biology3.Acyl hydrazones also have been used as the basis for switches,sensors,and other materials4,5.Introduction of hydroxyl,methoxyl or carboxyl groups to the acyl hydrazone molecules enhance the affinity between receptors because of the participation of oxygen atom in the formation of hydrogen bond in the organism,thus make the hydrazones have the excellent biological activities and varieties of coordination modes,and have been found to have unique properties6-16.
In this paper,2-hydroxy-1-naphthyl group has been introduced to acyl hydrazone and thus three novel acyl hydrazones were synthesized by using phenyl acetic acid methyl ester,(4-hydroxyphenyl)acetic acid methyl ester,2-methoxy-benzoic acid methyl ester,and hydrazine hydrate as starting materials.The structures of the hydrazones were characterized by elemental analyses and single crystal X-ray diffraction(XRD).Their thermal decomposition behaviors were studied by thermogravimetry(TG). The kinetic parameters of thermal decomposition processes were calculated according to Kissinger and Ozawa equations and their interactions with CT-DNA were determined by microcalorimetry.
2 Experimental
2.1 Materials and instruments
All chemicals were analytical reagent grade and used as purchased without further purification.CT-DNA was purchased from Sigma-Aldrich.
Elemental analyses were determined on PE-2400-II elemental analyzer(PerkinElmer Inc.,USA).Crystal structures were determined on a BRUKER SMART APEX II CCD diffractometer (Bruker Corporation,Germany).Thermal decomposition processes were measured on a METTLER TOLEDO TG-DSC1 HT thermogravimetric analyzer(Mettler Toledo,Swiss).Interactions of the hydrazones with CT-DNA were performed with Setaram C80 microcalorimeter(Setaram instrument,France).
2.2 Syntheses
2.2.1 Hydrazides
General procedure:A solution of methyl phenylacetate(1.00 mmol)in methanol(10 mL)was added slowly to 80%hydrazine hydrate(2.00 mmol).The mixture was heated to reflux for 2 h to give a colorless and transparent solution.The white solid phenylacetyl hydrazide(hydrazide a),which was precipitated from the solution after being cooled to room temperature,was filtered, washed with cold ethanol and dried.The white needle crystal was obtained by recrystallization from ethanol.4-Hydroxy-phenylacetic acid hydrazide(hydrazide b)and 2-methoxy-benzoic acid hydrazide(hydrazide c)were synthesized as the same method to that of hydrazide a using different ester as starting materials.
2.2.2 N′-((2-hydroxynaphthalen-1-yl)methylene)-2-
phenylacetohydrazide(1)
The synthetic routes for the hydrazones are shown in Scheme 1.A solution of 2-hydroxy-1-naphthalene formaldehyde(1.00 mmol)in ethanol(10 mL)was added to a solution of hydrazide a (1.00 mmol)in ethanol(10 mL),and the mixture was heated and stirred at 80 °C for 2 h.The solution was filtered after being cooled down to room temperature and allowed to stand for about two days.Several bright yellow striation-shaped single-crystals suitable for X-ray crystallographic analysis were obtained.Yield: 233.6 mg,76.7%.Anal.calcd.(%)for C19H16N2O2:C,75.00;H, 5.26;N,9.21.Found(%):C,74.96;H,5.76;N,9.02.Fourier transform infrared(FT-IR)spectroscopy(KBr,cm-1):3285(w,―OH),1642(s,―C=O),1607(m,C=N).Electrospray ionizationmass spectrometry(ESI-MS)(CH3CH2OH)m/z:304.3(100%)[M]+.
2.2.3 N′-((2-hydroxynaphthalen-1-yl)methylene)-2-(4-hydroxyphenyl)acetohydrazide(2)
Synthesis procedure of compound 2 was similar to that of compound 1 except(4-hydroxyphenyl)-acetic acid hydrazide (hydrazide b)was used.A pale-pink block crystal of compound 2 was obtained after three days.Yield:237.2 mg,70.1%.Anal. calcd.(%)for C19H18N2O4:C,67.46;H,5.33;N,8.28.Found(%): C,68.32;H,5.16;N,8.37.FT-IR(KBr,cm-1):3283(w,―OH), 1647(s,―C=O),1608(m,―C=N).ESI-MS(CH3CH2OH) m/z:338.3(100%)[M]+.

Table 1 Crystallographic data for compounds 1,2,and 3
2.2.4 N′-((2-hydroxynaphthalen-1-yl)methylene)-2-methoxybenzohydrazide(3)
Synthesis procedure of compound 3 was similar to that of compound 1 except that 2-methoxy-benzoic acid hydrazide(hydrazide c)was used.A yellow grain-shaped crystal of compound 3 was obtained after three days.Yield:266.7 mg,79.3%.Anal.calcd.for C19H16N2O4(%):C,67.86;H,4.76;N,8.33.Found(%):C,67.37; H,4.66;N,8.21.FT-IR(KBr,cm-1):3282(w,―OH),1643(s,―C=O),1605(m,―C=N).ESI-MS(CH3CH2OH)m/z:336.4 (100%)[M]+.
3 Results and discussion
3.1 Crystal structures
The perspective view and packing diagrams of compounds 1, 2,and 3 are represented in Figs.1,3,5 and 2,4,6,respectively. Crystallographic data and hydrogen bonds for these compounds are shown in Tables 1 and 2.The selected bond lengths and bond angles are summarized in Table 3.

Table 2 Intermolecular hydrogen bonds of compounds 1,2,and 3

Table 3 Selected bond lengths and bond angles for compounds 1,2,and 3
Fig.1 shows the ORTEPview of the title compound 1 consisting of phenyl and naphthalene rings which are linked by keto hydrazogroup(―NH―N=).In the crystal,because of the p-π conjugate effect,the C(8)―O(1)bond(0.1216(3)nm)is shorter than the typical C―O bond(0.1430 nm)12and normal C(19)―O(2)bond (0.1346(3)nm),indicates that it is typical double bond and exists as keto-form.Due to the conjugation between benzene rings and C(8)―O(1),the bond lengths of C(1)=C(6)(0.1364(4)nm)and N(1)=N(2)(0.1368(2))nm are slightly longer than that of typical C=C bond(0.1340 nm)17and N=N bond(0.1240 nm)18respectively,and the bond length of C(6)―C(7)(0.1503(3)nm)is slightly shorter than typical C―C bond(0.1540 nm)19.The torsion angles of C(6)―C(7)―C(8)―O(1)and C(9)―C(10)―C(19)―O(2)are 28.5(4)°and -5.7(3)°,which indicate that the phenyl and naphthalene rings have obvious deviation.Intramolecular hydrogen bond of O(2)―H(2)…N(2)induced the co-planarity of hydroxyl substituted naphthalene ring and imine group,while the intermolecular hydrogen bonds N(1)―H(1)…O(1)(symmetry code:-x+1/2,y - 1/2,z)lead to a one-dimensional molecular line as shown in Fig.2.
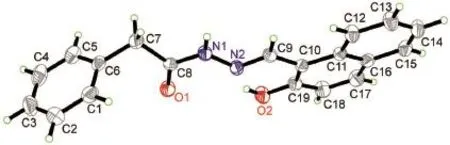
Fig.1 Perspective view of crystal structure of compound 1

Fig.2 Hydrogen bonded interactions in compound 1
The characters of molecular structures of compounds 2 and 3 are similar to that of compound 1.It can be seen from Table 2,the typical intramolecular hydrogen bond between the hydroxyl group on the naphthalene ring and the imino N-atom could be observed both in the crystal structures of compounds 2 and 3,and also induced the co-planarity of hydroxyl substituted naphthalene ring and imine group.Besides that,in the crystal of compound 2,intermolecular hydrogen bonds of N(2) ―H(2) …O(4)(water, symmetry code:x,y - 1,z),O(3)―H(3)…O(2)(symmetry code:-x+1/2,y - 1/2,-z+3/2),and O(4)―H(4B)…O(3)(symmetry code:-x+1/2,y+1/2,-z+3/2)were observed and lead to a twodimensional network as shown in Fig.4.There is no intermolecular hydrogen bond observed in the crystal of compound 3,as detailed in Table 2.
The difference is that in the crystals of compounds 1 and 2,the phenyl ring and the naphthalene ring are obviously nonplanar, while phenyl ring and the naphthalene ring are almost coplanar with little deviation in the crystal of compound 3.
In the crystal structures of the hydrazones,no direct π - π interactions are observed among all aromatic rings in the unit cell.
3.2 Thermal stabilities
Thermogravimetric(TG)analysis and derivative thermogravimetric analysis(DTG)curves of the hydrazones measured at the heating rate of 5 °C·min-1are shown in Fig.7 and Fig.8,respectively.
In compound 1,the first step is connected with the loss of all of the groups except the oxygen atom in keto-form in thermal decomposition process.The temperature of endothermic peak is 319.04 °C on the DTG curve.The mass loss of this stage is 94.64%,which coincides with the calculated value(95.41%).
For the compound of compound 3,the calculated weight loss of hydrazo-phenyl-naphthalene ring group(85.63%)is in agreement with experimental weight loss(87.55%).The temperature of endothermic peak is 318.23 °C.

Fig.3 Perspective view of crystal structure of compound 2

Fig.4 Hydrogen bonded interactions in crystal structure of compound 2
The differences of TG-DTG curves among compounds 1,2,and 3 show that the decomposition process of compound 2 can be divided into two stages,the first stage shows a weight loss (5.38%)at 140-150 °C,corresponding to one endothermic peak (145.02 °C)which might be caused by the decomposition of solvent.The second stage is the further decomposition and the temperature of the peak is 323.01 °C,the weight loss of this stage is 66.67%,which coincides with the calculated value(66.87%)of losing hydrazo-naphthalene ring group from compound 2.Reaction temperature was very low at the first stage in compound 2, indicating that lattice water and solvent molecule were very easy to lose.Compounds 1 and 3 decompose completely and have no residues but compound 2 does not.It can be seen from Fig.8, decomposition temperature of all compounds are above 300 °C, showing the thermal stabilities of the three hydrazones.
Kissinger and Ozawa equations are adopted to calculate the kinetic parameters of thermal decomposition processes for compounds 1,2,and 3 at different heating rates.Kissinger and Ozawa20equations are presented in the following: Kissinger equation,


Fig.5 Molecular structure of compound 3

Fig.6 Packing diagram of the crystal structure of compound 3
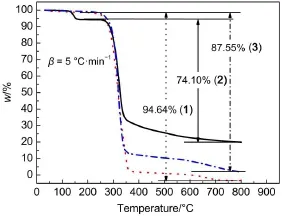
Fig.7 TG curves of compounds 1,2,and 3

Ozawa equation, where β is the heating rate,Tpthe maximum temperature of endothermic peak,Eathe apparent activation energy,A the pre-exponential factor,G(α)is the integral of reaction modal,α is the degree of conversion,and R the gas constant.
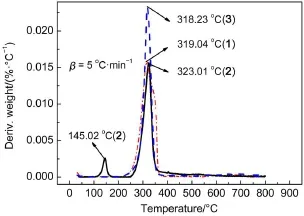
Fig.8 DTG curves of compounds 1,2,and 3
The kinetic parameters calculated from the thermal decompositions are listed in Table 4.The apparent activation energies calculated by Kissinger equation were 115.90,145.18,129.38 kJ· mol-1for compounds 1,2,and 3,respectively,while those calculated by equation were 119.78,149.06,132.55 kJ·mol-1,respectively,which indicate that the order of the apparent activation energy values are as follows:2 > 3 > 1,and compound 2 is more stable than the other two compounds.
3.3 The interactions with CT-DNA
Microcalorimetry is often used to study the biological activities of the compounds by measuring the heat flow of the interaction between the compound and bioactivator11-25.The interactions of hydrazides a,b,c,acyl hydrazones 1,2,3 and 2-hydroxy-1-naphthalene formaldehyde with CT-DNAhave been investigated.
CT-DNA solution(2.00 mL)was added to upper layer of the reference cell and sample cell in the microcalorimeter,then a solution of compound 1(5 mg)in Tris-HCl buffer solution(2.00 mL)was added to lower layer of the sample cell,While Tris-HCl buffer solution(2.00 mL)was added to the lower layer of the reference cell.Heating changes of the interaction between compound 1 and CT-DNAwere detected and recorded at 25 °C.

Table 4 Kinetic parameters of thermal decomposition for compounds 1,2 and 3 at different heating rates

Fig.9 Thermogenic curves for CT-DNA interaction with 2-hydroxy-1-naphthalene formaldehyde and hydrazines(a),(b),and(c)at 25 °C

Fig.10 Thermogenic curves for CT-DNA interaction with 1.000-5.000 mg compound 1 at 25 °C

Fig.11 Thermogenic curves for CT-DNA interaction with 1.000-5.000 mg compound 2 at 25 °C
The measurements of the interaction of the other compounds with CT-DNA are similar to that of compound 1.Fig.9 displays heat flow curves of the interaction of hydrazides a,b,c and 2-hydroxy-1-naphthalene formaldehyde with CT-DNA,respectively. The heat flow curves of the interaction of compounds 1,2 and 3 with CT-DNAat various dosage(1.000-5.000 mg)are shown in Figs.10-12.It can be seen from Figs.9-12,the endothermic peak width(3.1 - 32.0 min)indicates that the interaction time of hydrazide b and CT-DNAis longer than that of the other compounds.While acyl hydrazone 2 spends the least time to finish the interaction with CT-DNA.

Fig.12 Thermogenic curves for CT-DNA interaction with 1.000-5.000 mg compound 3 at 25 °C

Fig.13 Thermogenic curves for CT-DNA interaction with 2.000 mg compounds 1,2,and 3 at 25 °C
It can be seen from the Fig.10,the width of endothermic peak (1.10-6.57 min)indicates that when the dosage is 4.000 mg,the interaction time of compound 1 with CT-DNA is shortest.Besides, it could be calculated according to the peak areas of the heat flow, as shown in Fig.10,enthalpy change of compound 1 is greater than others when the dosage is 2.000 mg.Comparing with that of compound 1,we can see from the Figs.11 - 12,the shortest interaction time of these compounds with CT-DNA are observed respectively when compound 2(3.000 mg)and compound 3 (4.000 mg)are used.And also,the greatest interaction enthalpy changes of compounds 2 and 3 with CT-DNA are determined with the dosage of 1.000 mg and 2.000 mg.As shown in Fig.13,the order of enthalpy changes of compounds 1 - 3,which are calculated from the peak areas,is as follows: ΔH(1) > ΔH(2) >ΔH(3) > 0.It indicates that all of the compounds should interact with the CT-DNA and present endothermic reaction.Comparing with those of the interaction between corresponding hydrazides with CT-DNA,the enthalpy changes of the interaction between compounds 1 and 2 with CT-DNA are increased by introducing 2-hydroxy-1-naphthalene formaldehyde into the hydrazides except that of compound 3,which shows that the biological activities of compounds 1,2 are higher than those of hydrazides a,b,while the biological activities of compound 3 are a little lower than that of hydrazides c(Table 5).
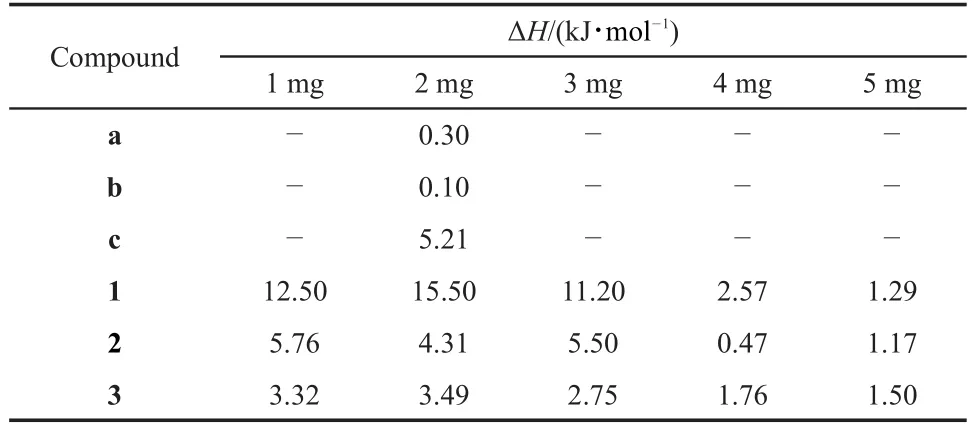
Table 5 Calculated enthalpy changes of interaction with CT-DNA of the acyl hydrazones
4 Conclusions
The single crystals of three novel compounds 1-3 have been obtained.Compound 1 crystallizes in orthorhombic,space group Pbca while compounds 2 and 3 both crystallize in the monoclinic crystal lattice,space group C2/c.
The TG-DTG curves indicate that thermal decomposition processes of compounds 1 and 3 have one stage,but compound 2 can be divided into two stages,one of which could be assigned to the emission of the solvate of water.The temperatures of main endothermic peaks for three compounds are all more than 300 °C. The calculated apparent activation energies of main decomposition stage for three compounds are in the following order:E(1) >E(2)> E(3).
The thermogenic curves for interactions of compounds 1- 3 with CT-DNA show reaction times are between 15-22 min,the reaction processes are all endothermic and the order of enthalpy changes are:ΔH(1) > ΔH(2) > ΔH(3).The enthalpy changes of compounds 1-3 reacting with CT-DNA are all more than that of corresponding hydrazides(a),(b)and(c),showing the three new compounds possess better biological activities and may have potential application on antibiosis or DNA cleavage.
Supporting Information:Crystal and structure refinement data for compounds 1,2,and 3 are summarized in CCDC 917435, 919202,and 919200,respectively.These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif,or by emailing data_request@ccdc.cam.ac.uk,or by contacting The Cambridge Crystallographic Data Center,12 Union Road, Cambridge,CB2 1EZ,UK;Fax:+44-1223-336033.
(1)Rollas,S.;Kucukguzel,S.G.Molecules2007,12,1910. doi:10.3390/12081910
(2)Baliani,A.;Bueno,G.J.;Stewart,M.L.;Yardley,V.;Brun,R.; Barrett,M.P.;Gilbert,I.H.J.Med.Chem.2005,48,5570. doi:10.1021/jm050177+
(3)Webber,M.A.;Coldham,N.G.Measuring the Activity of Active Efflux in Gram-Negative Bacteria.InAntibiotic Resistance Protocols,2nd ed.;Gillespie,S.H.,McHugh,T.D.Eds.;Humana Press:Totowa,NJ,2010;pp 173-180. doi:10.1007/978-1-60327-279-7_13
(4)Su,X.;Aprahamian,I.Chem.Soc.Rev.2014,43,1963. doi:10.1039/C3CS60385G
(5)McKinnon,D.D.;Domaille,D.W.;Cha,J.N.;Anseth,K.N.Chem.Mater.2014,26,2382.doi:10.1021/cm5007789
(6)Ren,J.W.;Liu,X.R.;Yang,Z.W.;Zhao,S.S.Thermochim. Acta2014,82,17.doi:10.1016/j.tca.2014.01.022
(7)Liu,J.H.;Wu,X.Y.;Zhang,Q.Z.;He,X.;Yang,W.B.;Lu,C. Z.Chin.J.Inorg.Chem.2006,22,1028.[劉九輝,吳小園,張全爭,何 翔,楊文斌,盧燦忠.無機化學學報,2006,22,1028.]
(8)Wang,S.X.;Fu,Y.F.;Fan,Z.J.;Mi,N.;Zhang,H.K.;Song, H.B.;Nataliya,P.B.;Vasiliy,A.B.Chin.J.Struct.Chem.2011,30,235.
(9)Fan,N.Y.;Gao,S.;Huo,L.H.;Zhao,J.G.;Wang,H.S.;Xi,S. Q.J.Anal.Chem.2005,33,751.doi:10.1007/s10809-005-0175-x
(10)Li,G.Q.;Zhou,Y.Z.;Xiao,L.M.;Zhu,H.J.;Tu,S.J.Chin.J. Inorg.Chem.2008,24,1023.[李桂琴,周蔭莊,肖嶺梅,朱惠菊,屠淑潔.無機化學學報,2008,24,1023.]
(11)Patel,M.N.;Bhatt,B.S.;Dosi,P.A.J.Therm.Anal.Calorim.2012,107,55.doi:10.1007/s10973-011-1554-1
(12)Yamaguchi,T.;Yamamoto,Y.;Kinoshita,D.;Akiba,K.;Zhang, Y.;Reed,C.A.;Hashizume,D.;Iwasaki,F.J.Am.Chem.Soc.2008,130,6894.doi:10.1021/ja710423d
(13)Yuan,X.R.;Shang,Z.H.;Li,R.Y.;Liu,Y.H.;Chen,X.X.; Zhang,H.L.;Xiu,Y.Acta Phys.-Chim.Sin.2009,25,1785.[原現瑞,尚振華,李潤巖,劉英華,陳曉霞,張慧麗,俢 勇.物理化學學報,2009,25,1785.]doi:10.3866/PKU.WHXB20090833
(14)Wu,W.T.;Yang,R.;Hu,T.;Peng,K.;He,S.Y.;Hu,R.Z.Acta Phys.-Chim.Sin.2004,20,1144.[武望婷,楊 銳,胡 亭,彭科,何水樣,胡榮祖.物理化學學報,2004,20,1144.] doi: 10.3866/PKU.WHXB20040917
(15) Chen, F. Y.; Cao,W. K.; He, S. Y.;Wang, B. H.; Zhang, Y. M.Acta Phys. -Chim. Sin.2 0 0 6 ,2 2, 2 8 0 . [陳鳳英,曹文凱,何水樣,王保懷,張有民.物理化學學報,2 0 0 6 ,2 2, 2 8 0 . ] doi: 10.1016/S1872-1508(06)60003-X
(16)He, S. Y.; Cao,W. K.; Hu, T.; Zhao, J. S.; Zhang,W. P.; Xue, G.L.; Hu, R. Z.Acta Phys. -Chim. Sin.2 0 0 2 ,1 8, 8 6 5 . [何水樣,曹文凱,胡 亭,趙建設,張維平,薛崗林,胡榮祖.物理化學學報,2 0 0 2 ,1 8, 8 6 5 ] doi: 10.3866/PKU.WHXB20021001
(17)Bartell, L. S.; Roth, E. A.; Hollowell, C. D.; Kuchitsu, K.;Young, J. E.J. Chem. Phys.1 9 6 5 ,4 2, 2 6 8 3 . doi: 10.1063/1.1703223
(18)Brown, L. D.; Ibers, J. A.J. Am. Chem. Soc.1 9 7 6 ,9 8, 1 5 9 7 . doi: 10.1021/ja00422a062
(19)Wang, S. X.; Huang, J.; Fan, Z. J.;Wang, H.; Fu, Y. F.; Mi, N.;Zhang, Z. C.; Song, H. B.J. Chem. Crystallogr.2 0 1 1 ,4 1, 1 3 4 8 . doi: 10.1007/s10870-011-0101-z
(20)Khan, M. R.; Omoloso, A. D.Fitoterapia2 0 0 2 ,7 3, 3 2 7 . doi: 10.1016/S0367-326X(02)00076-X
(21)Wads?, I.J . Therm. Anal. Calorim.2 0 0 1 ,6 4, 7 5 . doi: 10.1023/A:1011576710913
(22)Li, X.; Liu, Y.; Jun,W.; Liang, H. G.; Qu, S. S.Thermochim.Acta2 0 0 2 ,3 87 , 5 7 . doi: 10.1016/S0040-6031(01)00825-5
(23)McGulnness, M. S.; Barisas, B. G.Environ. Sci. Technol.1 9 9 1 ,2 5, 1 0 9 2 . doi: 10.1021/es00018a012
(24)Wang, Y. L.; Zhao, F. Q.; Ji, Y. P.; Yi, J. H.; An, T.; Liu,W. X.Chin. Chem. Lett.2 0 1 4 ,2 5, 9 0 2 . doi: 10.1016/j.cclet.2014.01.011
(25)Yi, P. G.; Shang, Z. C.; Yu, Q. S.Chin. J. Inorg. Chem.2 0 0 1 ,1 7, 7 7 . [易平貴,商志才,俞慶森 .無機化學學報,2 0 0 1 ,1 7, 7 7 . ]
Crystal Structure,Thermal Decomposition and Interaction with CT-DNA of Three 2-Hydroxy-1-naphthaldehyde Acyl Hydrazones
ZHAO Shun-Sheng LI Lan-Lan LIU Xiang-Rong*DING Zuo-Cheng YANG Zai-Wen
(College of Chemistry and Chemical Engineering,Xi′an University of Science and Technology,Xi′an 710054,P.R.China)
Three acyl hydrazones N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide(1), N′-((2-hydroxynaphthalen-1-yl)methylene)-2-(4-hydroxyphenyl)acetohydrazide hydrate(2),and N′-((2-hydroxynaphthalen-1-yl)methylene)-2-(2-methoxyphenyl)acetohydrazide hydrate(3)were synthesized and then characterized by elemental analysis and single-crystal X-ray diffraction.The crystallographic data indicated that both compounds 2 and 3 crystallized in the monoclinic crystal lattice,space group C2/c,while compound 1 crystallized in the orthorhombic space group Pbca.The thermal decomposition processes of the three hydrazones were studied by thermogravimetry.The thermal decomposition temperatures of compounds 1,2, and 3 were 318.23,319.04,and 323.01 °C,respectively.Meanwhile,the apparent activation energies for thermal decomposition for compounds 1,2,and 3 were 115.90,145.18,and 129.38 kJ·mol-1,respectively, calculated according to the Kissinger and Ozawa equations.The interactions of compounds 1-3 with calf thymus(CT)-DNA were evaluated by microcalorimetry.The results indicated these interactions were homogenous endothermic processes with non-identical interaction time(1.00 - 50.0 min)and interactionenthalpies(0.47-15.50 kJ·mol-1).The interaction enthalpies of compounds 1 and 2 were higher than those of their precursors,while the interaction enthalpy of compound 3 was lower than that of its precursor.
Acyl hydrazone;Crystal structure;Thermogravimetry;Microcalorimetry;DNA binding
O641
10.3866/PKU.WHXB201610191
Received:September 5,2016;Revised:October 19,2016;Published online:October 19,2016.
*Corresponding author.Email:xkchemistry@163.com;Tel:+86-29-85583183.
The project was supported by the National Natural Science Foundation of China(21103135,21073139,21301139),Natural Science Basic Research Plan in Shaanxi Province,China(2016JM2011),and Scientific Research Program Funded by Shaanxi Provincial Education Commission,China (15JK1492).
國家自然科學基金(21103135,21073139,21301139),陜西省自然科學基礎研究計劃(2016JM2011)及陜西省教育廳自然科學專項(15JK1492)資助項目? Editorial office of Acta Physico-Chimica Sinica

