基于全氟酞菁銅和碗烯雙分子體系在銀和石墨表面自組裝行為的低溫掃描隧道顯微鏡研究
郭瑞 張嘉霖, 趙宋燾 余小江 鐘舒 孫碩 李震宇 陳偉,,5,6,*
(1新加坡國立大學化學系,新加坡117543;2新加坡國立大學物理系,新加坡117542;3中國科學技術大學,合肥微尺度物質科學國家實驗室,中國科學院量子信息與量子科技前沿卓越創新中心,合肥23026;4新加坡國立大學,新加坡同步加速器光源中心,新加坡117603;5新加坡國立大學,先進二維材料石墨烯研究中心,新加坡117546;6新加坡國立大學蘇州研究院,江蘇蘇州215123)
基于全氟酞菁銅和碗烯雙分子體系在銀和石墨表面自組裝行為的低溫掃描隧道顯微鏡研究
郭瑞1張嘉霖1,2趙宋燾3余小江4鐘舒1孫碩2李震宇3陳偉1,2,5,6,*
(1新加坡國立大學化學系,新加坡117543;2新加坡國立大學物理系,新加坡117542;3中國科學技術大學,合肥微尺度物質科學國家實驗室,中國科學院量子信息與量子科技前沿卓越創新中心,合肥23026;4新加坡國立大學,新加坡同步加速器光源中心,新加坡117603;5新加坡國立大學,先進二維材料石墨烯研究中心,新加坡117546;6新加坡國立大學蘇州研究院,江蘇蘇州215123)
由于其獨特的分子構型和電子結構,碗烯被認為是組成有機分子電子器件的一種重要的結構單元。在不同金屬表面上單一組分的碗烯或其衍生物進行自組裝的行為,及其所形成自組裝薄膜的電子結構已經被廣泛研究。這里我們利用低溫掃描隧道顯微鏡(LT-STM),對全氟酞菁銅和碗烯兩種組分在高定向熱解石墨和銀(111)兩種不同襯底上的自組裝結構進行了報道。在石墨襯底上,全氟酞菁銅和碗烯分子間形成的氫鍵成為雙分子網絡結構能夠形成的關鍵;同時,由于這種分子間氫鍵的存在,碗烯分子大多采取“開口朝下”的空間構型,以保證分子間氫鍵最大限度的形成。但在銀襯底上觀察到的碗烯分子則隨機采取“開口向上”或“開口向下”兩種構型,并沒有一種優勢構型的存在。我們認為此時銀(111)襯底和有機分子間強烈的相互作用限制了碗烯兩種構型之間的翻轉,使得碗烯分子一旦被吸附就只能保持其原本的構型,從而導致了在結果上兩種構型的隨機分布。
分子自組裝;雙分子網絡結構;碗烯;低溫掃描隧道顯微鏡;分子間氫鍵
1 Introduc tion
Corannulene(COR)is a bow l-shaped molecule and can be regarded asa fragmentof fullerene,asshown in Fig.1a.Since its first successful synthesis in 19661,COR has attracted intensive attention due to several intriguing properties.W ith fivefold symmetry,COR provides a unique opportunity to study the symmetrymismatching betweenadsorbateand substrate,given the incom patibility betw een the fivefold rotational symmetry of moleculeand translationalorder of theunderneath crystal lattice2. The combination of non-planar shapeand aromaticitymakes COR an interesting system w ith unique geometry and electronic properties2c.Specificπ-πinteractionsbetween curved and planar structures give rise tofascinating photoelectric properties3. Buckybow ls also serve as ideal hosts toform the host-guest complexes in supramolecular chem istry4.COR has alw ays been regarded as a fragment of C60molecule for its symmetry and conformation.Butconsidering itshigh solubility inmost common solvents2c,COR can be a better choice than fullerenes as a prom ising candidate for acceptormaterials in organic optoelectronic devices2c.Itshould bementioned thatKuvychko etal.5have recently reported a COR derivative(w ith electron w ithdraw ing groups)that has a higher electron affinity and thus can be a strongerelectron acceptor than thewell-studied C60.
Two-dimensional(2D)self-assembly of functional organic molecules into ordered arrays represents one of themost promising strategies tofabricate functionalmolecular nanostructures overmacroscopicareas6.Modification ofmetalsurfaceswith COR and its derivativeshasbeen studied for symmetrym ismatch between substrate and adsorbate2a,7,multi-component packing4,8, templated assembly8a,9,interface dipole formation10,aswell as2D phase transitions6c,11.The assembly behaviors of the single-componentmoleculeswith fivefold symmetry on surfaceareof fundamental interest12.The structureof self-assembled CORmonolayer on Cu(111)and Cu(110)has been reported.On Cu(111), each CORmolecule adsorbson either fcc orhcp threefold hollow sitewith itsbow lopening pointing up11b.Oneof the fivehexagonal ringsorients parallel to the surface planeand thereforea tiltbetween molecular bow l w ith respect to the surface exists.A temperature-controlled reversible phase transitionwasalso observed in this system.It isexplained that low tem perature constrains the vibration of COR molecules,thus leads to amore effective intermolecular attraction,and finally results in the transition to the phasewith higher packing density11b.On Cu(110),asimilarquasihexagonal lattice with slightly tilted COR moleculeswas observed2a.In addition tomonolayer,a bilayer bow l-in-bow l stacking structure of COR wasalso reported on Cu(111)at low temperature13.Each second-layermolecule locatesdirectly aboveone firstlayermolecule,leading to the formation ofabow l-in-bow ldimer.
In contrast to the intensive studies on single component selfassembly of COR and its derivatives,investigation on multicomponentmolecular assembled system consisting of COR is rarely reported.Multicomponent 2D assemblies providemore functionality and tunability for themolecular nanostructures14. Calmettes etal.8areported binarymolecular networks comprising 2,3,9,10,16,17,23,24-octachlorozinc phthalocyanine(ZnPcCl8)and the COR derivative of 1,3,5,7,9-penta-tertbutylcorannulene (PTBC).In this case,themetastable phaseof ZnPcCl8can beused asa flexible template to realize the controllable insertion of PTBC molecule.By selecting different phases formed by ZnPcCl8,the final bimolecular 2D structure,w hich resembles the original packing of template,canbe regulated.Xiao etal.4reported aCORC60buckybow l-buckyballhost-guestcomplexesby depositing C60onto the ordered monolayer of COR on Cu(110).The concave structure of COR is optimal to realize a“face-to-face”contact w ith the convex surface of C60and their com plementary electron environmentsare favorable for binding.Via thermalactivation,a strongly bound COR-C60host-guest system is formed.Delicate balance betw een various intermolecular and interfacial interactions plays essential role in tailoring these supramolecular structures6b,8a,14a,15.
Herein we report the formation of self-assembled binarymolecular networks of COR and copper hexadecafluorophthalocyanine(F16CuPc)on the highly oriented pyrolytic graphite(HOPG) and Ag(111).Thegeometrical arrangementsof the binary system on differentsubstrateswere systematically investigated by lowtemperature scanning tunneling m icroscopy/spectroscopy(LT-STM/STS).
2 Experim en talm ethods
TheAg(111)and HOPG single crystalsubstratesare purchased from MaTeck Material-Technologie&Kristalle GmbH.The F16CuPc molecules are tw ice sublimed and purchased from CREAPHYS.Both sample preparation and investigation were performed in an ultrahigh vacuum system at a base pressure around 10-10mbar(1mbar=101Pa).TheAg(111)surfacewas prepared via repeated cycles of sputtering by Ar+and then annealing to 750K.Freshly cleaved HOPGwas thoroughly degassed in UHV at800 K overnight.COR and F16CuPc were thermally evaporated from separate Knudsen cells at 380 and 670 K,respectively,onto the substrate(keptatroom temperature).
In-situ STM investigation was carried out in a custom-designed Om icron LT-STM w ith an electrochem ically etched tungsten tip scanning at77K.All STM imageswere obtained under constant currentmodewith biasvoltagesapplied to the tip.To collect the differential conductance d I/d V(local density of states),a lock-in techniquewasadopted togetherwith amodulation voltageof 50 m V and a frequency of 625 Hz.When ram ping the voltage,the feedback loopwasopened16.
3 Resu lts and discussion
F16CuPc,asshown in Fig.1bwas firstdeposited onto HOPG toform a self-assembledmonolayer.STM image(Fig.1c)clearly revealsa typical close packing structurewhere F16CuPcmolecules lie flat on substrate with theirmolecular planes parallel to the substrate,arising from the interfacialπ-πinteraction17.A unitcell w ith a=1.66 nm,b=3.5 nm,θ1=108°isoutlined in Fig.1c and schematic packingmodelof oneunitcell is shown in Fig.1d.Two differentmolecular orientations exist in F16CuPcmonolayer on HOPG,which hasbeen concretely analyzed in previous report17. In oneunit cell,theorientation of four F16CuPcmoleculeson the corner is deviated from thatof two F16CuPcmolecules centered at the b edge.Then COR molecules were evaporated onto the F16CuPc covered HOPG.Co-assembly of F16CuPc and COR,as shown in Fig.1eand 1f,formsa long range-ordered structurewith an intermixing ratio of 1:2.A unitcellwith c=2.87 nm,d=2.17 nm,θ2=114°is highlighted in Fig.1f and the schematic packing modelof one unit cell is shown in Fig.1g.It isnoteworthy that in the supramolecular structure,only one orientation of F16CuPc molecule isobserved and each F16CuPcmolecule issurrounded by 6CORmolecules.
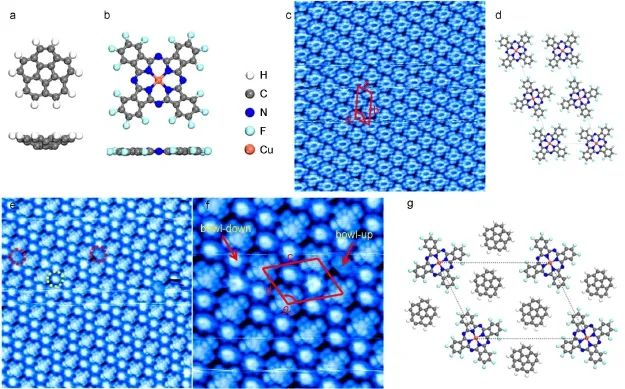
Fig.1 M olecu lar structures for(a)COR and(b)F16CuPc;(c)STM imageof F16CuPcmonolayer deposited on HOPG;(d)schematic packing structure for F16CuPcm oleculeon HOPG;(e,f)STM images of long range-ordered binarym olecular networks form ed by F16CuPc and CORw ith amolecular ratio of 1:2 on HOPG;(g)schematic packingmodel for the F16CuPc-COR binary structureon HOPGIn figure c:Theunitcell ishighlightedwith a=1.66 nm,b=3.5 nm,θ1=108°.Vtip=1.654V,20 nm×20 nm.In figurese,f:TheCORmoleculesadopting thebow lup and bow ldown configurationsare indicated by thearrows.Theunitcellishighlighted in the imagewith c=2.87 nm,d=2.17 nm,θ2=114°.Vtip=1.496V,20nm×20 nm;Vtip=1.496V,10 nm×10 nm
Herewe observe two kinds of dotsaround F16CuPc,including the dots thatarebrighterand the dots thatare slightly darker.We propose both kinds of dots are CORmoleculesbutw ith different configurations:bow lopening pointing up and pointing down.STS measurements(Fig.2b)confirm this assumption and reveals the highest occupied molecular orbital(HOMO)-the lowest unoccupiedmolecular orbital(LUMO)gap of around 3.10 eV,which agreesw ith the theoretically calculated HOMO-LUMO gap of CORmolecule18.The simulated topographic STM imagesof COR,based on semiempiricalextended H?ckel calculation,have been used to determine the configuration of adsorbed COR by Parschau etal.2a.For bow lup configuration,both the HOMO and LUMO topographic simulated images show a density m inimum at the centerof themolecule togetherwith a distinct fivefold doughnut shape.On the contrary,for bow l down configuration,both the HOMO and LUMO topographic simulated imagesshow a density maximum at the centerof themolecule and theoutline of COR molecule is rather vague2a.Hence by com paring the simulated STM imagewith our high resolution STM results in Fig.2a,we assign these brighter dots to COR with bow lopening down and darkerdots to CORwith bow lopening up.

Fig.2(a)High resolution STM image of the F16CuPc-COR binarymolecu lar networkson HOPG; (b)d I/d V spectra recorded on the bow l-up and bow l-down COR molecules
It is noticed that in the F16CuPc-COR binary molecular networks on HOPG,COR molecules that adopt bow l-down configuration hold majority.We propose that this configuration preferencemay arise from the formation ofmultiple intermolecular hydrogen bonding.As F16CuPcmolecules lie flat on the plane, peripheral hydrogen atoms of CORmolecule w ith bow l-down configuration can stand closer to the neighboring F16CuPc,which facilitates the formation of multiple intermolecular hydrogen bonding between neighboring F16CuPc and COR.In thisway,the binary supramolecular structure iseffectively stabilized and bow ldown configuration of COR thus isenergetically favorable.
Wealsogrew the same F16CuPc-COR binary system on Ag(111) to compare the co-assembly structureson differentsubstrates.Ag (111)has shownmuch strongermolecule-substrate interactions for variousorganic adsorbates19,compared w ith HOPG.Hencewe were able to grow amonolayer of COR onto Ag(111).A large scaleand thecorresponding closeup STM imagesof COR on Ag (111)are shown in Fig.3(a,b)with aunitcelloutlined(e=1.02 nm,f=1.17 nm,θ3=73°).Each CORmolecule isshared by four unit cells(Fig.3c).Likew ise,w e observe brighter and slightly darker dots in the STM image of COR monolayer.Careful inspection of high-resolution STM(Fig.3d)confirms the co-existenceof CORmoleculeswith differentconfigurations.Herein the brighter dot obviously has an intensity minimum in the center. Hence by using the aforementioned comparison of high-resolution STM imageswith simulated results,these brighter dots should be assigned to CORmoleculeswith bow ls opening up and the darker and vague dots should be COR moleculesw ith bow ls opening down.

Fig.3(a)Large scale STM imageofCORmonolayer deposited on Ag(111);(b)closeup STM image of COR on Ag(111); (c)proposed schem atic packingm odel for COR on Ag(111);(d)high resolution STM im ageof COR on Ag(111)w ith the bow l-up and bow l-down configurations indicated by red arrowsIn figurea:Vtip=1.0V;60 nm×60 nm.In figureb:Theunitcellishighlighted by the red rhombusw ith e=1.02 nm,f=1.17 nm,θ3=73°; Vtip=1.0V,10 nm×10 nm.In figured:Vtip=-1.5V;6nm×6 nm
Tofurther confirm our assignment,a comparison of the brighter dotson HOPG and Ag(111)underhigh-resolution STM is shown in Fig.4.It isobvious thatin Fig.4a,the CORmoleculewithbow lup configuration possesses a hollow center w ith a rough pentagonal doughnutshape,which is consistentwith features of thesimulated bow l-up COR.While in Fig.4b,the COR molecule accounted asbow l-dow n configuration ismore protruding in the center and themolecule shape is obscure,which also resembles simulated bow l-down topography.We noted that on Ag(111) substrate,the configuration preference of COR disappears:both bow l-up and bow l-down COR exist in almostequalamount.In otherwords,the adoption of bow l-up or bow l-down configuration is random.We suggest that the strong COR-Ag(111)interfacial interaction constrains themovementand bow l inversion of COR molecules.Onceadsorbed on Ag(111),CORmolecule could only retain its initial configuration and therefore both configurations have equal chance to appear.

Fig.4 Com parison of the brighter dotsunder high-resolution STM(a)CORmoleculew ith bow l-up configurationon Ag(111); (b)CORmoleculewith bow l-down configuration on HOPG
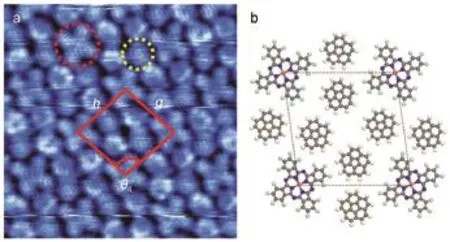
Fig.5(a)High resolution STM imageof F16CuPc-COR binary m olecular networks on Ag(111)w ith am olecular ratio of 1:4; (b)schem atic pack ingm odel for the F16CuPc-COR b inary structureon Ag(111)In figurea:Theunitcellishighlighted by the red rhombusw ith g=h=2.73 nm,θ4=100°.Vtip=1.26V,10 nm×10 nm
Co-assemblymonolayerof F16CuPc and COR on Ag(111)was also prepared by furtherevaporating F16CuPcmolecules onto the COR covered substrate.STM image reveals the long range-ordered binarymolecularnetworksw ith amolecular ratio of 1:4. The unit cell isoutlined in Fig.5aw ith features including g=h= 2.73 nm andθ4=100°.Corresponding schematic packingmodel for the binary structure is shown in Fig.5b.All the F16CuPc molecules lie in the same orientation and each F16CuPcmolecule issurrounded by 8CORmolecules.
4 Conc lusions
In summary,we have investigated the binary supramolecular structureof F16CuPc-CORmonolayerassembled on HOPGand Ag (111)substrates.The formation of multiple intermolecular hydrogen bonding between F16CuPc and COR could result in a preferred bow l-dow n configuration for COR molecules on the weakly interacting HOPG.In contrast,this configuration preference disappears on Ag(111)substrate where the adoption of bow l-up orbow l-down configuration is random,resulting from the strongmolecule-substrate interactions.Ourwork would further reinforce themodification of surfacew ith binarymolecular networks consisting ofCOR and itsderivatives.
(1)Barth,W.E.;Law ton,R.G.J.Am.Chem.Soc.1966,88(2), 380.doi:10.1021/ja00954a049
(2)(a)Parschau,M.;Fasel,R.;Ernst,K.H.;Gr?ning,O.; B randenberger,L.;Schillinger,R.;Greber,T.;Seitsonen,A.P.; Wu,Y.T.;Siegel,J.S.Angew.Chem.-Int.Edit.2007,46(43), 8258.doi:10.1002/anie.200700610
(b)Shechtman,D.;Blech,I.;Gratias,D.;Cahn,J.W.Phys.Rev. Lett.1984,53(20),1951.doi:10.1103/PhysRevLett.53.1951
(c)Bauert,E.FundamentalAspectsof the Self-assembly Behaviorand Electronic Propertiesof Corannulenes.Ph.D. Dissertation,University of Zurich,Zurich,2011.
(3)Li,J.;Liu,Y.;Qian,Y.;Li,L.;Xie,L.;Shang,J.;Yu,T.;Yi,M.; Huang,W.Phys.Chem.Chem.Phys.2013,15(30),12694. doi:10.1039/C3CP51095F
(4)Xiao,W.;Passerone,D.;Ruffieux,P.;A?t-M ansour,K.; G r?ning,O.;Tosatti,E.;Siegel,J.S.;Fasel,R.J.Am.Chem. Soc.2008,130(14),4767.doi:10.1021/ja077816l
(5)Kuvychko,I.V.;Dubceac,C.;Deng,S.H.;Wang,X.B.; G ranovsky,A.A.;Popov,A.A.;Petrukhina,M.A.;Strauss,S. H.;Boltalina,O.V.Angew.Chem.-Int.Edit.2013,52(29), 7505.doi:10.1002/anie.201300796
(6)(a)Baris,B.;Jeannoutot,J.;Luzet,V.;Palmino,F.;Rochefort, A.;Cherioux,F.ACSNano 2012,6(8),6905.doi:10.1021/ nn301827e
(b)Mali,K.S.;De Feyter,S.Phil.Trans.R.Soc.A 2013,371 (2000),20120304.doi:10.1098/rsta.2012.0304
(c)Zoppi,L.;Bauert,T.;Siegel,J.S.;Baldridge,K.K.;Ernst, K.H.Phys.Chem.Chem.Phys.2012,14(38),13365. doi:10.1039/C2CP41732D
(7)Guillermet,O.;Niem i,E.;Nagarajan,S.;Bouju,X.;Martrou, D.;Gourdon,A.;Gauthier,S.Angew.Chem.-Int.Edit.2009,48 (11),1970.doi:10.1002/anie.200805689
(8)(a)Calmettes,B.;Nagarajan,S.;Gourdon,A.;Abel,M.;Porte, L.;Coratger,R.Angew.Chem.-Int.Edit.2008,47(37),6994. doi:10.1002/anie.200802628
(b)Yokoi,H.;Hiraoka,Y.;Hiroto,S.;Sakamaki,D.;Seki,S.; Shinokubo,H.Nat.Commun.2015,6.doi:10.1038/
ncomms9215
(9)Balandina,T.;Tahara,K.;Sandig,N.;Blunt,M.O.;Adisoejoso, J.;Lei,S.;Zerbetto,F.;Tobe,Y.;De Feyter,S.ACSNano 2012, 6(9),8381.doi:10.1021/nn303144r
(10)Bauert,T.;Zoppi,L.;Koller,G.;Garcia,A.;Baldridge,K.K.; Ernst,K.H.J.Phys.Chem.Lett.2011,2(21),2805. doi:10.1021/jz2012484
(11)(a)M erz,L.;Bauert,T.;Parschau,M.;Koller,G.;Siegel,J.S.; Ernst,K.H.Chem.Commun.2009,(39),5871.doi:10.1039/ B911056A (b)M erz,L.;Parschau,M.;Zoppi,L.;Baldridge,K.K.;Siegel, J.S.;Ernst,K.H.Angew.Chem.-Int.Edit.2009,48(11),1966. doi:10.1002/anie.200804563
(12)(a)Bauert,T.;Merz,L.;Bandera,D.;Parschau,M.;Siegel,J.S.; Ernst,K.H.J.Am.Chem.Soc.2009,131(10),3460. doi:10.1021/ja8101083. (b)Merz,L.;Parschau,M.;Siegel,J.S.;Ernst,K.H.Chimia 2009,63(4),214.doi:10.2533/chim ia.2009.214
(13)Bauert,T.;Baldridge,K.K.;Siegel,J.S.;Ernst,K.H.Chem. Commun.2011,47(28),7995.doi:10.1039/C1CC12540K.
(14)(a)De Oteyza,D.G.MulticomponentAssembly Strategies for Supramolecular Systems.In SupramolecularMaterialsforOpto-Electronics;Nobert Korch;Royal Society of Chem istry: Cambridge,2014;pp 53-97.doi:10.1039/9781782626947-00053 (b)Huang,Y.L.;Chen,W.;Li,H.;Ma,J.;Pflaum,J.;Wee,A.T. S.Small2010,6(1),70.doi:10.1002/sm ll.200901291
(15)Zhong,J.Q.;Qin,X.;Zhang,J.L.;Kera,S.;Ueno,N.;Wee,A. T.S.;Yang,J.;Chen,W.ACSNano 2014,8(2),1699. doi:10.1021/nn406050e
(16)Zhang,J.;Wang,Z.;Niu,T.;Li,Z.;Chen,W.Appl.Phys.Lett. 2014,104(11),113506.doi:10.1063/1.4869115
(17)Huang,Y.L.;Chen,W.;Chen,S.;Wee,A.T.S.Appl.Phys.A 2009,95(1),107.doi:10.1007/s00339-008-5000-6
(18)dos Santos,R.B.;Rivelino,R.;de M ota,F.B.;Gueorguiev,G. K.J.Phys.Chem.A 2012,116(36),9080.doi:10.1021/ jp3049636
(19)(a)Lackinger,M.;Griessl,S.;Heckl,W.M.;Hietschold,M. J.Phys.Chem.B 2002,106(17),4482.doi:10.1021/jp014275s (b)Lackinger,M.;Hietschold,M.Surf.Sci.2002,520(1), L619.doi:10.1016/S0039-6028(02)02269-0
LT-STM Investigation of the Self-Assem bled F16CuPc-Co rannu lene Binary System on Ag(111)and Graphite Surfaces
GUO Rui1ZHANG Jialin1,2ZHAO Songtao3YU Xiaojiang4ZHONG Shu1SUN Shuo2LIZhenyu3CHENWei1,2,5,6,*
(1DepartmentofChemistry,NationalUniversity ofSingapore,3Science Drive 3,117543,Singapore;2DepartmentofPhysics,NationalUniversity ofSingapore,2Science Drive 3,117542,Singapore;3HefeiNational Laboratory for PhysicalSciencesat the Microscale,CASCentre for Excellence and Synergetic Innovation Center of Quantum Information and Quantum Physics,University ofScience and Technology ofChina,Hefei230026,P.R.China;
4Singapore Synchrotron LightSource,National University ofSingapore,5 Research Link,117603,Singapore;
5Centerfor Advanced 2DMaterialsand Graphene Research Center,NationalUniversity ofSingapore,3 Science Drive 3,117546,
Singapore;6NationalUniversity ofSingapore(Suzhou)Research Institute,Suzhou 215123,Jiangsu Province,P.R.China)
Mo lecularassembly;Binarymolecularnetworks;Corannulene;Low-temperature scanning tunne lingm icroscopy;Intermo lecularhyd rogen bonding
O647
10.3866/PKU.WHXB201612051
www.whxb.pku.edu.cn
Received:September 29,2016;Revised:December 2,2016;Published online:December 5,2016.
*Corresponding author.Email:phycw@nus.edu.sg;Tel:+65-65161879.
Theprojectwas supported by theNational Key Basic Research Program of China(973)(2015CB856505),SingaporeMOE(R143-000-652-112),
Singapore NRF-CRPgrantof“Doped Contacts and Heterostructures for Solution-Processable Plastic Electronics”(R143-001-608-281),Jiangsu
Province GovernmentResearch Platform Grant,China,and NUSRISeed Fund.
國家重點基礎研究發展規劃項目(973)(2015CB856505),新加坡教育部(MOE,Tier II,R143-000-652-112),新加坡國家研發基金會(NRF,R143-001-608-281),江蘇省平臺建設項目和新加坡國立大學蘇州研究院資助?Editorialoffice of Acta Physico-Chim ica Sinica
Abstract:Corannulene(COR)is considered a prom isingmolecularbuilding block fororganic electronics owing to its intriguing geome trical and e lec tronic p roperties.Intensive research e fforts have been devoted to understanding the assemb ly behavior and e lectronic structure of COR and its derivatives on variousmeta l surfaces via low-temperature scanning tunne lingm icroscopy(LT-STM).Here we report the formation ofbinary mo lecular networks of copperhexadeca fluorophtha locyanine(F16CuPc)-COR self-assembled on the highly oriented pyrolytic graphite(HOPG)and Ag(111)substrates.Intermo lecularhydrogen bonding between F16CuPc and COR facilitates the formation ofbina rymolecular networks on HOPG and further induces a pre ference for bow l-down configured CORmolecules.This observed configuration preference disappears on Ag(111)substrate, where CORmolecules lie on the substrate with theirbow lopenings pointing up and down random ly.We propose tha tstrong interfacia l interactions betw een them olecule and Ag(111)su rface constrain the bow l inve rsion of the CORmo lecule,which thus retains its initialconfiguration upon adsorption.

