Polymorphism of D-mannitol:Crystal structure and the crystal growth mechanism☆
Weiyi Su ,Na Jia ,Hongshi Li,Hongxun Hao ,Chunli Li,*
1 School of Chemical Engineering and Technology,Hebei University of Technology,Tianjin 300130,China
2 School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
1.Introduction
Polymorphism,defined as the ability of a material to crystallize in different crystal structures,has gained more and more attention especially in the field of pharmaceuticals[1].It has been reported that 80%of the marketed pharmaceuticals exhibit polymorphs under experimentally accessible conditions[2],and some of them even showed different functionalities and bioavailabilities from the previously launched products.D-mannitol(mannitol)is a natural hexahydric alditol which has been widely used in pharmaceutical industry as a nephropathy treatment medicine and also an excipient in the formulation of various tablets and granulated powders[3].In addition to those,mannitol is a commonly used sugar replacer in food industry.The molecular structure of D-mannitol is shown in Fig.1.
It has been reported that mannitol has three anhydrous polymorphs[4–6]and a hemihydrate[7].However the nomenclature of mannitol polymorphs is often diverse in different literatures[8,9]in despite of some similar unit cell parameters based on X-ray powder diffraction(XRPD).Therefore a review of mannitol polymorphism is initially presented along with the preparation procedures of different forms.
Crystallization kinetics is more complicated when polymorphism is involved since on one hand,all polymorphs compete to nucleate at certain supersaturation[10,11],and on the other hand,a metastable form has thermodynamic tendency to transform to the stable one[12],which could somehow affect the crystal growth process[13–15].Therefore it is difficult to investigate the crystal growth kinetics especially for a metastable polymorph[16–18].For example,in the research of Schollet al.[19]a traditional desupersaturation was used to determine the growth kinetics of metastable α-glutamic acid,but it only worked at low temperature when the solvent-mediated polymorphic transformation(SMPT)could be ignored.Thus the research to the crystal growth of mannitol polymorphs was reviewed in this work for a clear perspective.Furthermore a method reported by Kuldipkumar[20]was extended to show the growth mechanism of metastable δ mannitol based on our previously collected data.
2.Polymorphism of D-mannitol
It has been con firmed that there are only three pure anhydrous polymorphs of mannitol even though numerous names have been given[6,8].Hereby,for clarity and to avoid any confusion,the unite cell parameters of these mannitol polymorphs are summarized in Table 1.It is clear in Table 1 that the first six β references have a similar structure,which is named β in our work.While focusing on the polymorphs from the 7th to 9th in Table 1,the parameters were nearly the same disregarding what they were called in the original literature.So this form is referred to as α mannitol here.Finally,the last three items are significantly distinct from the others,and they are referred to as the δ form of mannitol in this paper.
The polymorphism of D-mannitol was firstly reported in Groth's work[27]in 1910.Then as X-ray technology started to be introduced into the analysis of crystal structures,Marwick[21]examined the X-ray pattern of the stable β form in 1931 as shown in Table 1.The results were consistent with those in a subsequent paper[22]in 1952.Then in 1968,the single crystal data of the β form crystallized from an aqueous ethanol solution were con firmed by X-ray diffraction(XRD)with CuKαradiation by Bermanet al.[23]Following,a new XRD instrument(with MoKαradiation)was used by Kaminsky[24]to determine and refine the same polymorph in 1997,and the similar crystal lattice parameters and an improvedR-value were obtained.Thus based on the consistent crystal unit cell parameters and mostly used names in the literatures shown in Table 1,it is reasonable to name these structures β form.
The α form of mannitol was also mentioned by Groth[27]in 1910,but the unit cell parameters were first given by Walter-Levy[4]in 1968 as shown in Table 1.In the same year,a slow evaporation of mannitol and boric acid solution in methanol was applied to get the α form(namedKin the original literature)by Kimet al.[25]In addition to the similar unit cell parameters in Table 1,a thermoanalytical study performed by Pitkanenet al.[28]also con firmed that the α form in Walter-Levy's work is the same with theKform in Kim's work.Moreover,the unit cell data are also found consistent with those collected by Fronczeket al.[5]in 2003.It is worth noting that Reyet al.[22]obtained a mannitol polymorph named α in 1952,while Bermanet al.[23]prepared one named α′in 1968.The two substances have similar structure according to Bermanet al.[23],but they should not be the α form of mannitol defined in our work since Grindleyet al.[29]have testified by C-MNR that those two forms have different structures with the classical α form in Walter-Levy's work.Grindleyet al.also mentioned that those two forms might be the δ form produced by Walter-Levy,but we think it is not appropriate due to the different crystal symmetry between these two(monoclinic)and the δ form(orthorhombic).Therefore it is reasonable to believe that these two forms might be mixtures.Based on Table 1,it is clear that the β and α forms of mannitol belong to the same crystal system(orthorhombic)and the same P212121space group with only a little difference on the length of the unit cell edges.Thus the two forms should have the same molecular conformation but different orientations of the hydrogen in the hydroxyl groups and hydrogen bonds[28].
The δ form of mannitol is difficult to isolate compared to the other two.It was firstly obtained by Walter-Levy[4]by gradual evaporation of a mannitol aqueous solution in a watch glass in 1968,where the calculated crystal lattice parameters from the XRPD data showed that it belongs to P21space group as illustrated in Table 1.However this procedure was found difficult to follow as other researchers[30]could only obtain a mixture of the δ and α forms instead of pure δ mannitol with the same method.Pitkanenet al.[28]then prepared δ mannitol by slow cooling a pure melt in 1993,still different polymorphs or mixtures were obtained while the cooling rate fluctuated.Then a freeze-dryer was finally applied to get the δ form with high reproducibility[26,31].After that,a handy anti-solvent crystallization[6]was reported to produce the δ form(named form III in the original literature),during which the solid had to befiltered and dried immediately in order to prevent any polymorphic transformation.Recently,Sullivanet al.[32]successfully produced δ mannitol by cooling a saturated solution rapidly in dilute aqueous ethanol to below 0°C.In all the work mentioned above,the unit cell parameters or XRPD patterns of δ form are consistent to those shown in Table 1.It is also clear in Table 1 that the δ form displayed significant difference in the hydrogen bond situation from the previously known α and β forms.In 2003,the XRD patterns of the three forms were again determined by Fronczeket al.[5]at 100 K.The results indicate that the parameters in Table 1 are accurate for each of the mannitol polymorph.
Even though different nomenclatures have been used in various literatures for mannitol polymorphs or their mixtures,such asCandDin Giron's work[33]or γ in Rye's work[22](all should be mixtures),the summarization based on the crystal structural parameters in this paper should provide a clear perspective for the polymorphism of mannitol.
3.Crystal Growth Kinetics of Mannitol Polymorphs
The crystal growth process is difficult to follow for polymorphic material due to the potential transformation tendency as mentioned before.As in mannitol polymorphs,limited researches have been reported on the growth kinetics in different crystallization processes.
Nakagawaet al.[34]studied the crystal behavior of mannitol polymorphs in a freezing solution,and found that the amount of each polymorph was correlated to the ice crystal nucleation temperature and the cooling rate.To elucidate the results,they did a qualitative estimation to the crystal growth at the liquid–solid interface.Even though it was reasonable to believe that the cooling rate could influence both the polymorphic transition and crystal growth and then cause the amount difference of various polymorphs along the freezing-dried cake,it didn't provide detailed growth kinetics to each polymorphs.In another freeze drying process,Liaoet al.[35]found that annealing could facilitate both mannitol nucleation and crystal growth even when a crystallization inhibitor,an active pharmaceutical ingredient,was present.Similarly,Dixonet al.[36]investigated the influence of protein on the polymorphism of mannitol during lyophilization,and the results illustrated that more δ and less β mannitol were produced when the protein concentration increased from 1 to 5 mg·ml?1.But as the protein concentration was above 10 mg·ml?1,the nucleation and growth of all mannitol forms were inhibited.

Fig.1.Molecular structure of D-mannitol.

Table 1The review of unit cell parameters and nomenclature of D-mannitol anhydrous polymorphs in literatures
In addition to freezing experiments,the crystallization mechanism of mannitol in a microdroplet evaporation was discussed by Poornacharyet al.[37],where the growth rate along the needle axis was firstly identified as(1.5 ± 0.2)μm·s?1and(0.3 ± 0.1)μm·s?1for the δ and β forms,respectively.Typically the faster growth rate was considered the reason to cause the appearance of the δ mannitol in the solution–substrate contact line where the local supersaturation was usually high due to the Marangni-driven convection.Moreover,while focusing on the nano-cystalline cluster of mannitol,Hammondet al.[38]found that the conformational variability was higher for the stable β form in the early stages of crystal growth compared to the metastable δ form.
In bulk aqueous solution,the solvent-mediated polymorphic transformation is quite obvious for mannitol metastable forms[39,40]especially when the temperature is not low,which makes it even more difficult to clarify the growth mechanism.Crispet al.[41]reported that the growth of mannitol polymorphs could be affected by the preferred orientation effects,thus the amount of δ form increased with the content of the antisolvent(e.g.acetone)during crystallization.In another study,Cornelet al.[8]used the growth rates of these mannitol polymorphs for a transformation model,but the parameters were only selected qualitatively to satisfy the nucleation experiments without any precise calculation.Additionally,O′Sullivanet al.[40]detected the growth of the metastable δ form clearly by focused beam reflectance measurement(FBRM)in a polymorphic transformation process,but unfortunately the kinetics were not con firmed.According to the literatures,it is obvious that the growth kinetics of mannitol,especially of the metastable forms,haven't been investigated clearly even though bulk crystallization is quite common in industry to produce mannitol polymorphs.Thus in order to enlarge the acquaintance to the growth of δ mannitol,the induction time measured in our previous work[42]was reanalyzed here to provide the growth mechanism following a reported method by Kuldiplumaret al.[20].
4.Crystal Growth Mechanism of δ Mannitol by Induction Time Measurement
4.1.Theory
Generally the induction time can be considered as being made up of several parts,such as[43]the times for the system to achieve a quasi-steady state distribution of molecular clusters,for the formation of a stable nucleus,and for the nucleus to grow to a detectable size.Thus it is related to the rates of both nucleation and growth of crystals:

whereJis the rate of nucleation,Vis the volume of the system,α is the volume fraction of the new formed phase,Gis the rate of crystal growth,anis a factor related to the crystal shape withn=mν+1(mindicates the dimensionality of growth while ν equals 0.5 or 1 depending on the crystal growth is controlled by volume diffusion or surface reaction,respectively).Generally the first term is usually negligible compared to the second especially when the supersaturation is not too low as in our case,which leaves Eq.(1)to the following:

In this scenario,the steady state nucleation rate is usually described as below:

wheresis the supersaturation,KJis the nucleation rate constant,andBis a constant composed of the shaper factors(fs,the surface shape factor,andfv,the volume shape factor),molecular volume(v),and the interfacial free energy(γ).

whereKGis the growth rate constant andf(s)is a function of supersaturation depending on specific growth mechanism.Combining Eqs.(2),(3)and(4),it can be obtained:
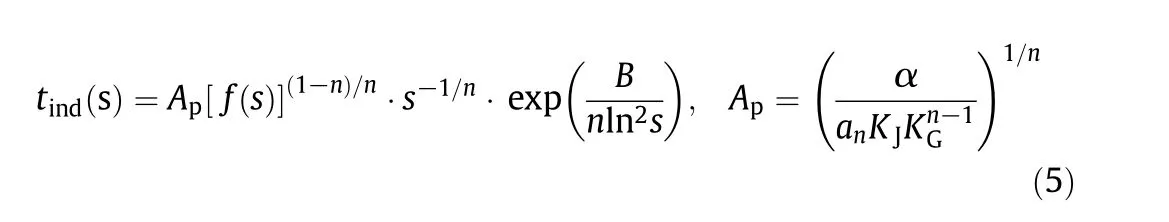
It is clear that the induction time(tind)and supersaturation(s)can be related to Eq.(5)once the crystal growth mechanism is fixed.Generally there are four kinds of growth mechanismsf(s)in the literature[20]as listed in Table 2.
Since the δ form of mannitol is thin rod like[39],the dimensionality of growth is set tom=1.In the normal growth mechanism,the spiral growth mechanism,and the 2D nucleation-mediated mechanism,the growth rate is technically determined by the surface reaction in a growth unit,thus ν=1 and the factornis correspondingly 2 as displayed in Table 2.While the growth is controlled by the transport of growth units through the solution to the crystal surface as in the volume diffusion-controlled mechanism,one can accordingly get that ν=0.5 andnis 1.5 as shown in Table 2.
By introducing differentf(s)in Table 2 to Eq.(5),it is possible to relate the supersaturation and the induction time under different mechanisms[44].To easily do this,another functionF(s)[20]was derived by rearranging Eq.(5)for normal,spiral,and volume diffusion-controlled growth:

Thus the plot ofF(s)versus 1/(lns)can be fitted by a parabolic curve.Depending on the goodness of the fit to Eqs.(6)or(7),it should be identified the growth mechanism as one of the four above once the correlation index(R2)is in the tolerant range.The expressions ofF(s)for different operating mechanisms are also shown in Table 2.
4.2.Results and discussion
The determination of induction time for metastable polymorph is difficult due to the transition tendency,that is why thein situRaman spectroscopy and FBRM were combined to distinguish the polymorph and measure the induction time simultaneously in our previous work[42].Here in this paper,the induction time and supersaturation data were reanalyzed in order to fulfill different growth mechanisms.Specifically the calculatedF(s)are plotted against1/(ln2s)for the normal,spiral,and volume diffusion-controlled growth mechanism as shown in Fig.2,whileF(s)against1/(lns)is plotted in Fig.3 following the 2D nucleation-mediated growth mechanism.

Table 2Functions and parameters for different crystal growth mechanisms

Fig.2.Plots of F(s)versus 1/ln2s for δ form of mannitol under(a)the normal growth mechanism,(b)the spiral growth mechanism,(c)the volume diffusion controlled growth mechanism.

Fig.3.Plot of F(s)versus 1/ln s for δ form of mannitol under the 2D nucleation-mediated growth mechanism.
It is clear that the three linear fittings in Fig.2 are quite different.The correlation coefficient is as poor as 0.9058 in Fig.2(b),which means that the growth of the δ form of mannitol is barely following the spiral growth mechanism.The data points in Fig.2(a)are fitted to the normal growth mechanism while those in Fig.2(c)are fitted to the volume diffusion controlled growth mechanism.It can be seen that the correlation coefficients of these two fittings are better than that in the spiral growth mechanism.But the largestR2of 0.9912 appears when the data are fitted to the 2D nucleation-mediated mechanism as shown in Fig.3.Therefore it is reasonable to believe that the growth mechanism of the metastable δ form of mannitol should be 2D nucleation-mediated in a cooling crystallization.Since δ mannitol only nucleates at high initial concentration in aqueous solution as mentioned in our previous paper[42],it seems that once this metastable form nucleates from the solution,the growth is mediated by the formation and spread of numerous 2D nuclei.
The method used in this work for crystal growth mechanism investigation has only been used in material without polymorphism phenomenon in the literature[20,44].However the high correlation index indicates that it should be suitable for polymorphic materials.To testify this,more detailed and microscopic work will be done in the future.
6.2.2.3 冷藏冷凍商品貯存倉庫、陳列柜和熱熟食展示柜都有功能正常的溫度顯示器,并且溫度滿足產品要求,定時做好冷藏冷凍庫(柜)和熱展示柜的溫度監控記錄。熱展示柜的溫度在60℃以上,冷藏溫度應為0℃~8℃;冷凍溫度應為-20℃~-1℃, 宜低于-12℃。
5.Conclusions
The nomenclature of D-mannitol polymorphs is summarized in this paper due to the confusion of polymorph identification.It was con firmed that there are three pure anhydrous forms of mannitol based on the unit cell parameters even though some of them have been given various names in different literatures.The three pure mannitol polymorphs are named β,α,and δ in this work based on the review to many publications.Moreover the literatures on crystal growth of mannitol polymorphs are reviewed,and it was found the growth can kinetically be affected by many factors.After that,the induction time previously determined by us is applied to investigate the crystal growth mechanism of the δ mannitol polymorph of mannitol in a bulk crystallization.And it was found that the growth of the metastable δ form should be 2D nucleation-mediated.
Nomenclature
athe molecular area,m2
ana factor related to the crystal shape in the expression of induction time
Ba constant related to the nucleation rate
Dxcrystal density,kg·m?3
fsthe surface shape factor
fvthe volume shape factor
Grate of crystal growth,m·s?1
Jrate of nucleation,m?3·s?1
KGgrowth rate constant,m·s?1
KJnucleation rate constant,m?3·s?1
mthe dimensionality of growth
sSupersaturation(s=c/c*)
tindinduction time,s
Vsystem volume,m3
vmolecular volume,m3
α volume fraction of the new formed phase
β2Da numerical 2D shape factor
γ interfacial free energy,J·m?2
κ specific edge free energy of the nuclei,J·m?2
[1]J.Bernstein,Polymorphism in Molecular Crystals,Oxford University Press,USA,2002.
[2]S.Datta,D.J.W.Grant,Crystal structures of drugs:Advances in determination,prediction and engineering,Nat.Rev.Drug Discov.3(1)(2004)42–57.
[3]B.O'Sullivan,The Application of In situ Analysis to Crystallization Process Development.Ph.D.Thesis University College Dublin,Ireland,2005.
[4]L.Walter-Levy,The crystalline varieties of D-mannitol,C.R.Acad.Sc.Paris,Ser.C.267(1968)1779.
[5]F.R.Fronczek,H.N.Kamel,M.Slattery,Three polymorphs(alpha,beta and delta)of D-mannitol at 100 K,Acta Crystallogr.Sect.C:Cryst.Struct.Commun.59(10)(2003)o567–o570.
[6]A.Burger,J.O.Henck,S.Hetz,J.M.Rollinger,A.A.Weissnicht,H.Stottner,Energy temperature diagram and compression behavior of the polymorphs of D-mannitol,J.Pharm.Sci.89(4)(2000)457–468.
[7]C.Nunes,R.Suryanarayanan,C.E.Botez,P.W.Stephens,Characterization and crystal structure of D-mannitol hemihydrate,J.Pharm.Sci.93(11)(2004)2800–2809.
[8]J.Cornel,P.Kidambi,M.Mazzotti,Precipitation and transformation of the three polymorphs of D-mannitol,Ind.Eng.Chem.Res.49(12)(2010)5854–5862.
[9]W.Su,C.Li,H.Hao,J.Whelan,M.Barrett,B.Glennon,Monitoring the liquid phase concentration by Raman spectroscopy in a polymorphic system,J.Raman Spectrosc.46(11)(2015)1150–1156.
[10]I.S.Lee,A.Y.Lee,A.S.Myerson,Concomitant polymorphism in con fined environment,Pharm.Res.25(4)(2008)960–968.
[11]M.Svard,F.L.Nordstrom,T.Jasnobulka,A.C.Rasmuson,Thermodynamics and nucleation kinetics of m-Aminobenzoic acid polymorphs,Cryst.Growth Des.10(2010)195–204.
[12]W.Ostwald,Uber die vemeintliche Isomerie des roten und gelben quecksilberoxyds und die ober flachen-spannung fester korper,Z.Phys.Chem.34(1900)495–512.
[13]M.Kitamura,Crystallization behavior and transformation kinetics of L-histidine polymorphs,J.Chem.Eng.Jpn26(3)(1993)303–307.
[14]T.Ono,H.J.M.Kramer,J.H.terHorst,P.J.Jansens,Process modeling of the polymorphic transformation of L-glutamic acid,Cryst.Growth Des.4(6)(2004)1161–1167.
[15]M.W.Hermanto,N.C.Kee,R.B.H.Tan,M.S.Chiu,R.D.Braatz,Robust Bayesian estimation of kinetics for the polymorphic transformation of L-glutamic acid crystals,AIChE J54(12)(2008)3248–3259.
[16]M.Kitamura,T.Ishizu,Growth kinetics and morphological change of polymorphs of L-glutamic acid,J.Cryst.Growth209(1)(2000)138–145.
[17]N.C.S.Kee,P.D.Arendt,L.May Goh,R.B.H.Tan,R.D.Braatz,Nucleation and growth kinetics estimation for l-phenylalanine hydrate and anhydrate crystallization,CrystEngComm13(4)(2011)1197–1209.
[18]L.Carpentier,K.Filali Rharrassi,P.Derollez,Y.Guinet,Crystallization and polymorphism of l-arabitol,Thermochim.Acta556(0)(2013)63–67.
[19]J.Scholl,C.Lindenberg,L.Vicum,J.Brozio,M.Mazzotti,Precipitation of alpha L-glutamic acid determination of growth kinetics,Faraday Discuss.136(2007)247–264.
[20]A.Kuldipkumar,G.S.Kwon,G.G.Z.Zhang,Determining the growth mechanism of tolazamide by induction time measurement,Cryst.Growth Des.7(2)(2007)234–242.
[21]T.C.Marwick,An X-ray study of mannitol,dulcitol,and mannose,Proc.R.Soc.London,Ser.A131(818)(1931)621–633.
[22]A.Rye,H.Sorum,Crystalline modifications of D-mannitol,Acta Chem.Scand.6(1952)1128–1129.
[23]H.M.Berman,G.A.Jeffrey,R.D.Rosenstein,The crystal structures of the alpha and beta forms of D-mannitol,Acta Crystallogr.Sect.B:Struct.Sci.B24(1968)442–449.
[24]W.Kaminsky,Crystal optics of D-mannitol,C6H14O6crystal growth,structure,basic physical properties,birefingence,optical activity,Faraday effect,electro-optic effects and model calculations,Z.Kristallogr.212(1997)283–296.
[25]H.S.Kim,G.A.Jeffrey,R.D.Rosenstein,The crystal structure of the K form of D-mannitol,Acta Crystallogr.Sect.B:Struct.Sci.B24(1968)1449–1455.
[26]C.E.Botez,P.W.Stephens,C.Nunes,R.Suryanarayanan,Crystal structure of anhydrous delta D-mannitol,Powder Diffract.18(3)(2003)214–218.
[27]P.Groth,Chemical Crystallography,Part Three:Aliphatic and Aromatic Hydrocarbon Compounds,Verlag von Wilhelm Engelmann,Leipzig,1910.
[28]I.Pitkanen,P.Perkkalainen,H.Rautiainen,Thermoanalytical studies on phases of D-mannitol,Thermochim.Acta214(1)(1993)157–162.
[29]T.B.Grindley,M.S.McKinnon,R.E.Wasylishen,Towards understanding13C-NMR chemical shifts of carbohydrates in the solid state.The spectra of D-mannitol polymorphs and of DL-mannitol,Carbohydr.Res.197(1990)41–52.
[30]B.Debord,C.Lefebvre,A.M.Guyot-Hermann,J.Hubert,R.Bouché,J.Guyot,Study of different crystalline forms of mannitol:Comparative behaviour under compression,Drug Dev.Ind.Pharm.13(9–11)(1987)1533–1546.
[31]A.I.Kim,M.J.Akers,S.L.Nail,The physical state of mannitol after freeze-drying:Effects of mannitol concentration,freezing rate,and a noncrystallizing cosolute,J.Pharm.Sci.87(8)(1998)931–935.
[32]B.O'Sullivan,P.Barrett,G.Hsiao,A.Carr,B.Glennon,In situ monitoring of polymorphic transitions,Org.Process.Res.Dev.7(2003)977–982.
[33]D.Giron,Thermal-analysis and calorimetric methods in the characterization of polymorphs and solvates,Thermochim.Acta248(1995)1–59.
[34]K.Nakagawa,W.Murakami,J.Andrieu,S.Vessot,Freezing step controls the mannitol phase composition heterogeneity,Chem.Eng.Res.Des.87(2009)1017–1027.
[35]X.Liao,R.Krishnamurthy,R.Suryanarayanan,Influence of the active pharmaceutical ingredient concentration on the physical state of mannitol-implications in freezedrying,Pharm.Res.22(11)(2005)1978–1985.
[36]D.Dixon,S.Tchessalov,A.Barry,N.Warne,The impact of protein concentration on mannitol and sodium chloride crystallinity and polymorphism upon lyophilization,J.Pharm.Sci.98(9)(2009)3419–3429.
[37]S.K.Poornachary,J.V.Parambil,P.S.Chow,R.B.H.Tan,J.Y.Y.Heng,Nucleation of elusive crystal polymorphs at the solution–substrate contact line,Cryst.Growth Des.13(3)(2013)1180–1186.
[38]R.B.Hammond,K.Pencheva,K.J.Roberts,Structural variability within,and polymorphic stability of,nano-crystalline molecular clusters of L-glutamic acid and D-mannitol,modelled with respect to their size,shape and ‘crystallisability’,CrystEngComm14(3)(2012)1069–1082.
[39]W.Y.Su,H.X.Hao,M.Barrett,B.Glennon,The impact of operating parameters on the polymorphic transformation of D-mannitol characterized in situ with Raman spectroscopy,FBRM,and PVM,Org.Process.Res.Dev.14(6)(2010)1432–1437.
[40]B.O'Sullivan,B.Glennon,Application ofin situ FBRMand ATR-FTIR to the monitoring of the polymorphic transformation of D-mannitol,Org.Process.Res.Dev.9(6)(2005)884–889.
[41]J.L.Crisp,S.E.Dann,C.G.Blatchford,Antisolvent crystallization of pharmaceutical excipients from aqueous solutions and the use of preferred orientation in phase identification by powder X-ray diffraction,Eur.J.Pharm.Sci.42(5)(2011)568–577.
[42]W.Su,H.Hao,B.Glennon,M.Barrett,Spontaneous polymorphic nucleation of D-mannitol in aqueous solution monitored with Raman spectroscopy and FBRM,Cryst.Growth Des.13(12)(2013)5179–5187.
[43]J.W.Mullin,Crystallization,4th Ed,London,2001.
[44]M.Zhi,Y.Wang,J.Wang,Determining the primary nucleation and growth mechanism of cloxacillin sodium in methanol–butyl acetate system,J.Cryst.Growth314(1)(2011)213–219.
 Chinese Journal of Chemical Engineering2017年3期
Chinese Journal of Chemical Engineering2017年3期
- Chinese Journal of Chemical Engineering的其它文章
- A novel green inhibitor for C-steel corrosion in 2.0 mol·L?1 hydrochloric acid solution
- An ecofriendly approach for corrosion control of 6061 Al-15%(v)SiC(P)composite and its base alloy
- Bulk and bubble-scale experimental studies of influence of nanoparticles on foam stability
- Simulation and optimization of an industrial gas condensate stabilization unit to modify LPGand NGL production with minimizing CO2 emission to the environment
- Efficient decolorization of dye-containing wastewater using mycelial pellets formed of marine-derived Aspergillus niger☆
- Characterization of pyrolytic lignins with different activities obtained from bio-oil☆
