Syntheses,Crystal Structures and Properties of Two d10Metal Complexes Constructed from 1,5-bis(2-ethyl-imidazolyl)pentane
CHEN Man-ShengHUANG Xiu-YuCHEN Xiao-LiLIU QinHE Xiang-LiangZENG Zhao-Jian
(1Key Laboratory of Functional Organometallic Materials of Hengyang Normal University,College of Hunan Province, College of Chemistry and Materials Science,Hengyang,Hunan 421008,China) (2School of Chemistry and Pharmaceutical Sciences,Guangxi Normal University,Guilin,Guangxi 541004,China)
Syntheses,Crystal Structures and Properties of Two d10Metal Complexes Constructed from 1,5-bis(2-ethyl-imidazolyl)pentane
CHEN Man-Sheng*,1,2HUANG Xiu-Yu1CHEN Xiao-Li1LIU Qin1HE Xiang-Liang1ZENG Zhao-Jian1
(1Key Laboratory of Functional Organometallic Materials of Hengyang Normal University,College of Hunan Province, College of Chemistry and Materials Science,Hengyang,Hunan 421008,China) (2School of Chemistry and Pharmaceutical Sciences,Guangxi Normal University,Guilin,Guangxi 541004,China)
Reaction of CdCl2or ZnCl2·4H2O,5-hydroxyisophthalic acid(5-OHH2IP)as well as 1,5-bis(2-ethylimidazolyl)pentane(BEIP)results in formation of a 1D[Cd(BEIP)(Cl)2]n(1)and 2D[Zn(BEIP)(5-OHIP)]n(2).X-ray diffraction crystal structure analysis shows that 1 crystallizes in orthorhombic system,space group Pca21,while 2 is of monoclinic,space group P21/n with β=100.542(4)°.In 1,the 1,5-bis(2-ethyl-imidazolyl)pentane links all the Cd atoms into a 1D chain.In 2,the carboxylate group with μ2-η1:η1coordination mode links metal atoms to give a 1D zigzag chain structure,which forming the 2D layer through Zn-N interactions by BEIP ligands.Finally the 2D layers are further assembled into 3D framework by the H-bond interaction.In addition,the properties of complexes 1 and 2 have been investigated,which exhibit good fluorescence in the solid state at room temperature.And complex 2 shows good photocatalytic activity for the degradation of methyl orange solution.CCDC:1523229,1;1523230,2.
1,5-bis(2-ethyl-imidazolyl)pentane;crystal structure;fluorescence
0 Introduction
Inrecent years,coordination polymers(CPs) based on multidentate N-heterocyclic ligands attracted anupsurgeofresearchinterestowingtotheir intriguingmoleculartopologiesandpotential applications in gas sorption and storage,catalysis, luminescence,and so on[1-8].On the other hand,the use of auxiliary ligands is also an effective method for the framework construction of coordination polymers attributing to the fact that they can satisfy and even adjust the coordination needs of the metal center and generate more meaningful architectures.Especially, bis(imidazole)with-CH2-spacers are good N-donor bridging ligands,and the flexible nature of-CH2-spacers allows the ligands to bend and rotate freely when coordinating to metal centers to conform to the coordination geometries of different metal ions[9-10]. Furthermore,systematic investigation of the role of auxiliary ligands in the formation of coordination frameworks is not well documented so far.From another point of view,CPs containing metal ions with a d10configuration,such as Zn(Ⅱ),Cd(Ⅱ)and Hg(Ⅱ),are potential materials for optical applications,such as fluorescenceprobesandnonlinearoptical materials[11-15].In order to further investigate the influence of organic ligands with different metal ions onthecoordinationarchitecturesandrelated properties,in this article,5-hydroxyisophthalic acid(5-OHH2IP)and 1,5-bis(2-ethyl-imidazolyl)pentane(BEIP) were employed to synthesize two new coordination polymers,namely[Cd(BEIP)(Cl)2]n(1)and 2D[Zn (BEIP)(5-OHIP)]n(2).Moreover,theluminescent property and photocatalytic activity for the degradation of methyl orange have also been investigated.
1 Experimental
1.1 Materials and instruments
The reagents were used as commercial sources without further purification.Elemental analyses were performedonaPerkin-Elmer240Celemental analyzer.The IR spectra were recorded on Bruker Vector22 FTIR spectrophotometer using KBr discs. Thermogravimetric analyses(TGA)were performed on a TGA V5.1A Dupont 2100 instrument heating from room temperature to 700℃under N2with a heating rate of 20℃·min-1.Fluorescence measurements were performedusinganF-7000Fluorescence Spectrophotometer at room temperature in the solid state.UV-Vis absorption spectra were measured with a Hitachi U-3010 UV-Vis spectrophotometer.
1.2 Syntheses of the complexes 1 and 2
A mixture of CdCl2(18.3 mg,0.1 mmol),5-OHIP (18.2 mg,1 mmol),BEIP(31.5 mg,0.1 mmol),NaOH (0.008 g,0.2 mmol),and deionized water(10 mL)was sealed in a 20 mL Teflon-lined stainless steel vessel and heated at 140℃for 96 h.After cooling to room temperature,colorless block crystals were obtained and washed with alcohol several times(Yield:40% based on Cd).Anal.Calcd.for C15H24CdN4Cl2(%):C 40.57;H 5.41;N 12.62;Found(%):C 40.52;H 5.49; N 12.57.The preparation of 2 is similar to that of 1 except that ZnCl2·4H2O(20.9 mg,0.1 mmol)was used instead of CdCl2.Colorless single crystals of 2 were collected by filtration and washed by water and ethanol for several times with a yield of 33%.Anal. Calcd.for C23H28ZnN4O5(%):C 54.56;H 5.54;N 11.07;Found(%):C 54.52;H 5.61;N 10.99.IR(KBr pellet,cm-1):1 672(m),1 563(s),1 429(m),1 397(m), 1 356(s),1 275(m),1 241(w),1 107(m),956(w),847 (w),781(m),733(m),664(w),592(w),433(m)for 1. 3 128(s),1 599(s),1 555(m),1 435(s),1 362(s),1 271 (w),1 157(m),1 113(m),997(m),929(m),829(w),736 (s),669(m),428(m)for 2.
1.3 X-ray crystallography
The data of two single crystals with dimensions of 0.30 mm×0.28 mm×0.24 mm for 1 and 0.20 mm× 0.15 mm×0.10 mm for 2,respectively,were carried out on a Bruker Smart ApexⅡCCD diffractometer equipped with a graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm).The data were integrated by using the SAINT program[16],which also did the intensitycorrectionsforLorentzandpolarization effect.An empirical absorption correction was applied using the SADABS program[17].The structures were solved by direct methods using the program SHELXS-97 and all the non-hydrogen atoms were refined anisotropically on F2by the full-matrix least-squares techniqueusingtheSHELXL-97crystallographic software package[18-19],all the hydrogen atoms were positioned geometrically and refined using a riding model.Atoms C12,C13,C14,and C12,C13,C14 in 1 were disordered with the site occupancy factors of 0.637(18)and 0.363(18),respectively.Crystal data and structure refinement parameters are listed in Table 1.The selected bond lengths are given in Table 2.
CCDC:1523229,1;1523230,2.

Table 1Crystal data and structure parameters for the title complexes

Table 2Selected bond lengths(nm)for complexes 1 and 2
1.4 Photocatalytic activity
In the process of photocatalysis,40 mg complex 2 was suspended in 1.5×10-5mol·L-1MO aqueous solution(100 mL),then magnetically stirred in the dark for about 30 min to ensure the establishment of an adsorption-desorptionequilibrium.Afterthat,the mixture was stirred and continuously exposed to UV irradiation from a 300 W high pressure mercury vapor lamp equipped with cool water circulating.The MO concentrationwasdeterminedbymeasuringthe maximum absorbance at 465 nm,and all the tests were carried out three times to ensure the reproducibility.
2 Results and discussion
2.1 Structure description
The results of structural analysis revealed that complex 1 contains one Cd(Ⅱ)cation,one 1,5-bis(2-ethyl-imidazolyl)pentane ligand and two coordinated Cl-.As shown in Fig.1a,the metal Cd2+ion center is located in a slightly distorted tetrahedral coordination geometry environment with two Cl atoms and two N atom from two different BEIP ligands.Then,aninfinite chain built up by Cd(Ⅱ)ions and BEIP through the μ1-η1:η1coordination modes of 2-ethylimidazolyl groups(Fig.1b).Finally,the 1D chains of 1 connect toether through the C-H…Cl non-classic hydrogenbondinginteractionstogivethe2D supramolecular structure(Fig.2).

Fig.1Coordination environment of the Cd(Ⅱ)ion in 1 showing 30%probability displacement ellipsoids.(b)1D chain structure of 1

Fig.22D layer structure linked by C-H…Cl H-bonding
Complex2wassynthesizedinasimilar procedure as complex 1,but here ZnCl2·4H2O was used instead of CdCl2.The crystallographic data showed that complex 2 crystallizes in monoclinic with P21/n space group.There are one Zn(Ⅱ),one 5-OHIP2-ligand,one BEIP ligand in the asymmetric unit of 1. Each Zn2+ion is four-coordinated in a distorted tetrahedral geometry by two nitrogen atoms from two BEIP ligands and two oxygen atoms from two 5-OHIP2-ligands.The bond angles around the Zn2+ion are in the range of 100.00(9)°~122.19(9)°.The bond lengths of Zn-O are 0.194 1(2)~0.198 92(19)nm and Zn-N are 0.200 3(2),0.201 3(2)nm,respectively.In 2,each 5-OHIP2-anion serves as a μ2-bridge linking two adjacent Zn2+ions in the bismonodentate mode to give rise to a 1D zigzag chain,while such 1D chains are further double-bridged by BEIP ligands into a 2D layer(Fig.3b).It is note that each flexible BEIP is ligated to two Zn2+ions with two terminal imidazole groups assuming the anti-gauche conformation and a pair of oppositely arranged μ2-BEIP ligands bind two Zn2+ions from adjacent chains to form a[Zn2(BEIP)2] 24-memberedmetallomacrocyclering.Fromthe viewpoint of network topology[20],if the Zn2+ions are considered as 3-connected nodes,5-OHIP2-and BEIP ligands as linear connectors,the whole structure canbe simplified to a 3-connected 63topological net(Fig. 3c).The2Dlayersarefurtherassembledby intermolecular hydrogen bonds with a H(5)…O(2) distance of 0.190 nm and the angle of 160°(O(5)-H(5)…O(2)),leading to formation of a 3D framework (Fig.3d).
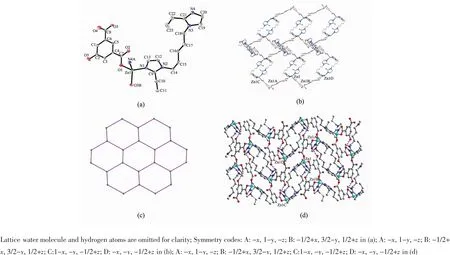
Fig.3(a)ORTEP drawing of 2 with 30%thermal ellipsoids;(b)2D layer constructed by Zn(Ⅱ)and 5-OHIP2-and BEIP ligands; (c)Topological representation of the 2D structure of 2;(d)View of the 3D framework of 2 with the H-bond interaction
2.2 Thermal gravimetric analyses andphotoluminescence property
Thermalgravimetricanalyses(TGA)were performedtoverifythethermalstabilityofthe coordination polymers.Since there are not any solvent molecules in the two complexes,the results indicated that they exhibit excellent thermal stability as no weight loss step occurs below 310 and 380℃for 1 and 2,respectively.When the temperature rises up, the whole framework begins to collapse(Fig.4).
On the other hand,CPs with d10metal centers have been investigated for their photoluminescent propertiesandpotentialapplicationsinchemical sensorsandphotochemistry[21-22].Thesolid-state luminescent properties of 1,2 and ligand BEIP were investigated at room temperature and the solid-state emission spectra are shown in Fig.5.It shows that there are emission bands at 463 nm(λex=370 nm)for 1,468 nm(λex=370 nm)for 2.Such fluorescent emissions may be assigned to intra-ligand π-π* transitions,since the free BEIP ligand exhibited a similar broad emission at 448 nm upon excitation at 360 nm.It is noteworthy that the emission bands of both complexes is 15 nm and 20 nm red-shifted compared with the free BEIP ligand.The results suggestthatcomplexes1and2maybegood candidate of potential fluorescent materials.
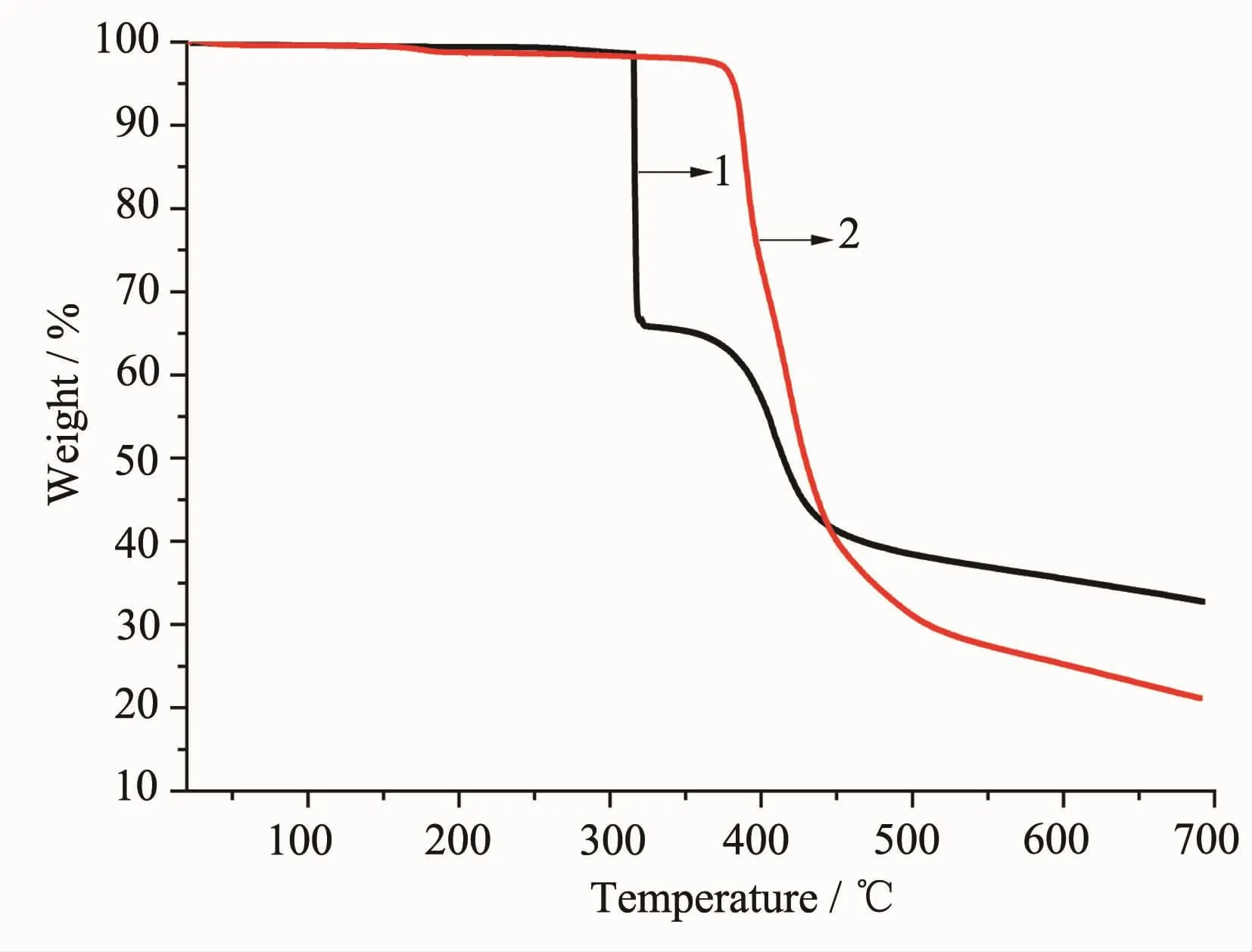
Fig.4TG curves of complexes 1 and 2
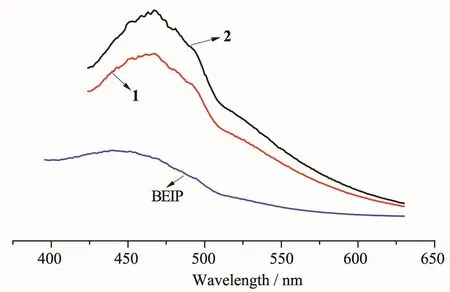
Fig.5Emission spectra of 1~2 and ligand(BEIP)in the solid state at room temperature
2.3 Photocatalytic activity
Methyl orange(MO)is a model of dye pollutant, which can be used to evaluate the effectiveness of photocatalysts in the wastewater[23-26].And the diffuse reflection spectra of complex 2 was explored.In Fig. 6a,the newly appeared absorption at 352 nm should be assigned to LMCT and the value of the band gap was calculated as 2.95 eV,which indicated complex 2 might be a photocatalyst for MO.
Thus,the photocatalytic activity of as-prepared 2 was tested by the degradation of MO solution under 365nmultraviolet(UV)lightirradiation.As illustrated in Fig.6b and 6c,the change in the concentration of MO solution is obvious with the use of complex 2 as photocatalyst.It can be seen that approximately 79.8%of MO has been decomposed after 3.0 h of irradiation rather than the blank experiment.The result indicates that complex 2 is active for the decomposition of MO under UV light irradiation.

Fig.6UV-Vis spectra of complex 2 and ligand 5-OHIP(a),absorption spectra of the MO solution during the decomposition reaction with the use of complex 2(b)and degradation of MO with complex 2(c)
3 Conclusions
Insummary,twonewCPs withdifferent frameworkstructureshavebeensuccessfully constructedbasedontheconnectivityco-effect between the flexible BEIP ligand and carboxylates togetherwithmetalsaltsunderhydrothermal conditions.The results suggest that the structural diversification of CPs may result from the different metal ions.Moreover,complexes 1 and 2 both exhibit photoluminescenceproperty,whichappeartobe potentialhybridinorganic-organicphotoactive materials.Andcomplex2exhibitsphotocatalytic activity for the degradation of MO solution under ultraviolet light irradiation.
[1]Cotton F A,Lin C,Murillo C.Acc.Chem.Res.,2001,34: 759-771
[2]Stock N,Biswas S.Chem.Rev.,2012,112:933-969
[3]Rowsell J L C,Yaghi O M.J.Am.Chem.Soc.,2006,128: 1304-1315
[4]Ockwig N W,Delgado-Fridrichs O,OKeeffe M,et al.Acc. Chem.Res.,2005,38:176-182
[5]Deng M L,Yang P,Liu X F,et al.Cryst.Growth Des.,2015, 15:1526-1534
[6]Banerjee R,Phan A,Wang B,et al.Science,2008,319:939-943
[7]Zhang W,Xiong R G,Huang S D.J.Am.Chem.Soc.,2008, 130:10468-10469
[8]Wriedt M,Yakovenko A A,Halder G J,et al.J.Am.Chem.Soc.,2013,135:4040-4050
[9]Liu J Q,Huang Y S,Zhao Y Y,et al.Cryst.Growth Des., 2011,11:569-572
[10]Liu T F,Wu W F,Wan C Q,et al.J.Coord.Chem.,2011, 64:975-981
[11]Zhang J,Chen S M,Wu T,et al.J.Am.Chem.Soc.,2008, 130:12882-12883
[12]Yang W,Schmider H,Wu Q,et al.Inorg.Chem.,2000,39: 2397-2402
[13]Gong Y Q,Wang R H,Zhou Y F,et al.J.Mol.Struct., 2004,705:29-36
[14]Lin W,Evans O R,Xiong R G,et al.J.Am.Chem.Soc., 1998,120:13272-13273
[15]Lin W,Wang Z,Ma L.J.Am.Chem.Soc.,1999,121:11249-11250
[16]SAINT Version 6.02a,Bruker AXS Inc.,Madison,W1,2002.
[17]Sheldrick G M.SADABS,Program for Bruker Area Detector Absorption Correction,University of G?ttingen,G?ttingen, Germany,1997.
[18]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,UniversityofG?ttingen,G?ttingen,Germany, 1997.
[19]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,UniversityofG?ttingen,G?ttingen,Germany,1997.
[20]Blatov V A.IUCr CompComm Newsl.,2006,7:4-10
[21]Dong Y B,Wang P,Huang R Q,et al.Inorg.Chem.,2004, 43:4727-4739
[22]Chu Q,Liu G X,Huang Y Q,et al.Dalton Trans.,2007, 4302-4311
[23]Gong Y,Wu T,Lin J H.CrystEngComm,2012,14:3727-3736
[24]Hu Y,Luo F,Dong F F.Chem.Commun.,2011,47:761-763
[25]FEI Bao-Li(費(fèi)寶麗),WANG Ping-Ping(王平平),WANG Hao-Rong(王浩榮),et al.Chinese J.Inorg.Chem.(無機(jī)化學(xué)學(xué)報(bào)),2015,31(2):399-404
[26]LIU Xiang(劉祥),HAN Jing(韓晶),YU Zhong(余中),et al. Chinese J.Inorg.Chem.(無機(jī)化學(xué)學(xué)報(bào)),2016,32(11): 1931-1941
柔性1,5-二(2-乙基咪唑)戊烷配體構(gòu)筑的兩個(gè)d10金屬配合物的合成、結(jié)構(gòu)及性質(zhì)
陳滿生*,1,2黃秀玉1陳小利1劉琴1何湘良1曾昭建1
(1功能金屬有機(jī)材料湖南省普通高等學(xué)校重點(diǎn)實(shí)驗(yàn)室,衡陽師范學(xué)院化學(xué)與材料科學(xué)學(xué)院,衡陽421008) (2廣西師范大學(xué)化學(xué)與藥學(xué)學(xué)院,桂林541004)
利用過渡金屬鎘(鋅)鹽與1,5-二(2-乙基咪唑)戊烷(BEIP)、5-羥基間苯二甲酸(5-OHH2IP)在水熱條件下合成了配合物[Cd (BEIP)(Cl)2]n(1)和[Zn(BEIP)(5-OHIP)]n(2),并對(duì)其進(jìn)行了元素分析、IR及X射線衍射法表征。晶體結(jié)構(gòu)研究表明:配合物1屬于正交晶系,Pca21空間群。配合物2屬于單斜晶系,P21/n空間群,β=100.542(4)°。配合物1是由配體1,5-二(2-乙基咪唑)戊烷連接鎘離子形成一維鏈狀結(jié)構(gòu)。而配合物2是由配體間苯二甲酸連接鋅離子形成一維鏈狀結(jié)構(gòu),該一維鏈通過1,5-二(2-乙基咪唑)戊烷連接成二維網(wǎng)絡(luò)結(jié)構(gòu),進(jìn)而通過氫鍵連接成三維超分子結(jié)構(gòu)。此外,配合物1和2具有較高的穩(wěn)定性和較好的熒光性能,配合物2對(duì)甲基橙染料有一定的降解作用。
1,5-二(2-乙基咪唑)戊烷;晶體結(jié)構(gòu);熒光
O614.24+2;O614.24+1
A
1001-4861(2017)06-1090-07
2016-03-19。收修改稿日期:2017-04-28。
10.11862/CJIC.2017.128
國家自然科學(xué)基金項(xiàng)目(No.21461004)、中國博士后科學(xué)基金(No.2014M552534XB)、衡陽師范學(xué)院省級(jí)平臺(tái)開放基金(No.GN16K02)和湖南省大學(xué)生研究性學(xué)習(xí)與創(chuàng)新性實(shí)驗(yàn)項(xiàng)目(No.CX1615)資助。
*通信聯(lián)系人。E-mail:cmsniu@163.com;會(huì)員登記號(hào):S06N7223M1009。
 無機(jī)化學(xué)學(xué)報(bào)2017年6期
無機(jī)化學(xué)學(xué)報(bào)2017年6期
- 無機(jī)化學(xué)學(xué)報(bào)的其它文章
- 過渡元素?fù)诫sFe3O4(001)表面磁電性能的理論研究
- 熔鹽法合成電化學(xué)性能優(yōu)異的富鋰層狀正極材料Li1.5Ni0.25Mn0.75O2.5
- Efficient Synthesis and Application in Heck Reaction of Pd/Fe3O4Magnetic Nanoparticles
- Syntheses,Crystal Structures and Catalytic Activity of Rhenium Carbonyl Complexes Containing Aryl-Substituted Tetramethylcyclopentadienyl Ligands
- Characterization,Photocatalytic Property and Kinetics of ZnO Nanoparticles Synthesized by One Step Solid State Reaction
- Syntheses,Characterization and Radical Scavenging Activity of Two Copper(Ⅱ)Complexes Containing Pyrazoles
