N-(2-吡咯甲基)-1-苯基乙亞胺·二甲基鋁的合成、結(jié)構(gòu)及其催化丙交酯的活性
賈 斌 郝俊生 童紅波 魏學(xué)紅 周梅素 劉滇生*,
N-(2-吡咯甲基)-1-苯基乙亞胺·二甲基鋁的合成、結(jié)構(gòu)及其催化丙交酯的活性
賈 斌*,1,3郝俊生2童紅波3魏學(xué)紅2周梅素3劉滇生*,3
(1山西醫(yī)科大學(xué)基礎(chǔ)醫(yī)學(xué)院,太原 030001)
(2山西大學(xué)化學(xué)化工學(xué)院,太原 030006)
(3山西大學(xué)應(yīng)用化學(xué)研究所,太原 030006)
通過席夫堿配體N-(2-吡咯甲基)-1-苯基乙亞胺與三乙基鋁按物質(zhì)的量之比為1∶1在無氧無水的條件下反應(yīng),合成了席夫堿鋁的有機(jī)金屬化合物N-(2-吡咯甲基)-1-苯基乙亞胺·二甲基鋁。其結(jié)構(gòu)分別用核磁氫譜、碳譜,元素分析和X射線單晶衍射技術(shù)進(jìn)行了表征。鋁化合物在催化外消旋丙交酯開環(huán)聚合反應(yīng)中表現(xiàn)出了中等的活性并得到了以等規(guī)聚合為主的高聚物。
鋁;席夫堿;外消旋丙交酯;開環(huán)聚合
0 Introduction
Organoaluminum compounds have attracted increasing attention over the years for their use in the lactides polymerization[1-5],among which,organoaluminum compounds bearing salen[6-10],phenoxyimine[11-15],dialkylamino[16-24],β-diketiminate[25-32]and alkylimino[33]ligands with their derivatives are more prevalent in the literature. However, chiral organoaluminum compounds for the polymerization of rac-lactide have been rarely investigated.Recently,Du′s group used Chiral anilido-oxazolinate aluminum compounds for the ring-opening polymerization(ROP)of rac-lactide and showed hetero-and isotactic selectivities bymodulation of the substituent groups[34].Okuda and coworkers reported the chiral(OSSO)-type bis(phenolato)aluminum compounds which are active for the ROP of rac-lactide[35].Other chiral salen-based aluminum catalysts gave rise to isotactic(or block-isotactic)polylactide(PLA),but low to moderate catalytic activity[36-40].Recent research in our group has focused on chiralαphenylethylamine ligands because of electronics and steric bulk near the metal center is thought to be an important factor in promoting chain-end control of polymer tacticity with unique properties[41].Here we report the synthesis and structural features of aluminum alkylimino compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2.The catalytic activities of this compound toward ring-opening polymerization of rac-lactide have been investigated and it can be used as initiators to give the isotactic-rich polylactides.
1 Experimental
1.1 General comments
All manipulations were carried out under nitrogen atmosphere in the flamed Schlenk-type glassware on a dual manifold Schlenk line.Solvents purchased from commercial sources were distilled over standard drying agents under nitrogen from alkali metals directly and stored over 0.4 nm molecular sieves.All the chemicals used were of reagent grade,obtained from Aldrich.rac-Lactide was recrystallized with dry toluene and sublimed twice in vacuo at 50℃.Melting points were determined in sealed capillaries under argon on an electrothermal apparatus and uncorrected.1H and13C NMR spectra were recorded on a Bruker DRX 300(300.1 MHzfor1H,75.5 MHzfor13C)instrument and referenced internally to the residual solvent resonances (chemical shift data inδ).The homonuclear decoupled1H NMR spectra were recorded on a Bruker AV 400 spectrometer at 400 MHz.Elemental analyses were performed on a Vario EL-Ⅲinstrument.X-ray single crystal structures were determined on a Bruker Smart CCD APEX area detector.Polymerizations were carried out in Schlenk asks under nitrogen atmosphere strictly free of moisture and oxygen at room temperature or 70℃,as well as the polymerization factors of cLM/cLAand reaction time were tested.Gel permeation chromatography(GPC)analyses were carried out on a Waters 1515 Breeze Gel Permeation Chromatograph equipped with differential refractive index detectors.The GPC columns were eluted with tetrahydrofuran with 1 mL·min-1rate at 25℃and were calibrated with monodisperse polystyrene standards.
1.2 Preparation
1.2.1 Synthesis of(d,l)-N-(2-pyrrolylmethylene)-1-phenylethylamine
After the classical condensation reaction of aldehyde and amine,the Schiff base(d,l)-N-(2-pyrrolylmethylene)-1-phenylethylamine was isolated as yellow oily liquid.(18.22 g,Yield:95%).1H NMR(300 MHz,CDCl3):δ1.65,1.67(d,3 H,C H3),4.59,4.61(m,1 H,C H CH3),6.28,6.56,6.79(3 H,C H of pyrrolyl),7.29~7.45(m,5 H,Ar-H),8.26(s,1 H,N=C H),9.50(s,1 H,N H).13C NMR (75.5 MHz,CDCl3):δ27.1(C H3),71.6(C HCH3),112.3,117.4,124.8,129.6(pyrrolyl),128.4,131.2,132.9,147.7(Ar),153.4(C=N).Anal.Calcd.for C13H14N2(%):C,78.75;H,7.12;N,14.13.Found(%):C,78.84;H,7.06;N,14.10.
1.2.2 Synthesis of[C4H3N{CH=NCH(Me)C6H5}-2]
AlMe2
(d,l)-N-(2-pyrrolylmethylene)-1-phenylethylamine(0.61 g,3.0 mmol)was dissolved in hexane(25.0 mL),and the solution was cooled to 0℃.Trimethylaluminum(1.5 mL,3.0 mmol)was added to this solution slowly,and the mixture was stirred overnight at room temperature.The resulting precipitate was collected by filtration,washed with cold hexane(2×10 mL),and dried in vacuo(0.52 g, 2.0 mmol,68%).Crystals suitable for X-ray crystallographic analysis were grown from diethyl ether at ambient temperature.The synthetic procedures and transformation of compound are illustrated in Scheme 1.1H NMR (300 MHz,C6D6):δ-0.45,-0.29 (d,JHH=48.8 Hz,6 H;Al(CH3)2),1.36,1.38(d,JHH=7.0 Hz,3 H,CH3),4.15,4.17(sept,JHH=6.9 Hz,1 H,C H CH3),6.44,6.70,7.02 (m,3 H,C H of pyrrolyl),7.08~7.26 (m,5 H,Ar-H),8.0 (s,1 H,N=C H).13CNMR(75.5 MHz,C6D6):δ-9.25(Al(C H3)2),22.1(C H3),61.7(C HCH3),114.9,117.8,119.7,135.3(pyrrolyl),128.9,129.4,135.1,141.9(Ar),158.9(C=N).Anal.Calcd.for C15H19AlN2(%):C,70.84;H,7.53;N,11.02.Found(%):C,70.69;H,7.49;N,11.11.
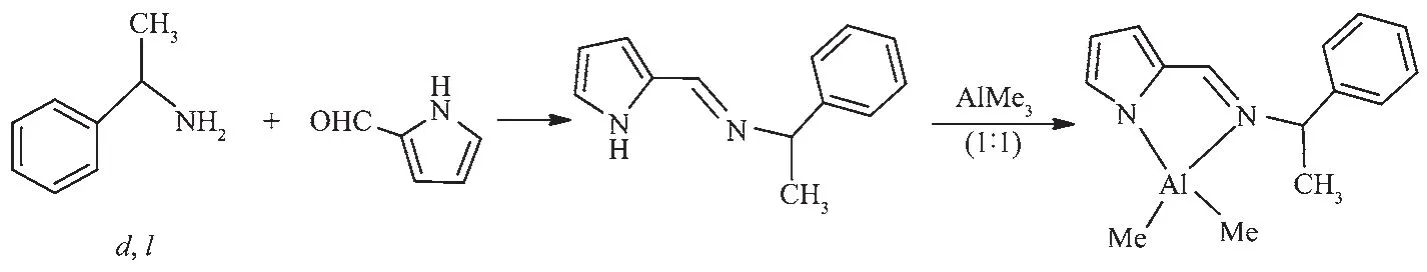
Scheme 1 General synthetic route for Aluminum compound
1.3 Typical polymerization procedure
A Schlenk tube previously dried at 150℃and cooled under nitrogen,and then the Schlenk flask was charged with a solution of the initiator in toluene(0.2 mL).To this solution was added rapidly a slurry of the monomer (rac-lactide)in the appropriate ratio in toluene(10.0 mL).The mixture was immediately stirred with a magnetic stir bar at room temperature or 70℃.After the desired time,the reaction was quenched by adding acidified methanol(ca.1.0 mL of 1.2 mol·L-1CH3COOH solution)and the polymer was precipitated with excess methanol(50.0 mL).The polymer was filtered and then dried in vacuo to constant weight.
1.4 X-ray crystallography
The single crystals of the Al compound suitable for X-ray diffraction studies were obtained.X-ray diffraction data were collected with graphite-monochromated Mo Kα radiation (λ=0.071 073 nm)on a Bruker Smart Apex CCD diffractometer at 213(2)K,equipped with an Oxford Cryosystems device.The collected frames were processed with the proprietary software SAINT and an absorption correction was applied(SADABS)to the collected reflections[42].The structure of this molecule was solved by direct methods and expanded by standard difference Fourier syntheses using the software SHELXTL[43-44].Structure refinements were made on F2using the full-matrix least-squares technique.All non-hydrogen atoms were refined with anisotropic displacement parameters.Hydrogen atoms were placed in their idealized positions and allowed to ride on the respective parent atoms.Pertinent crystallographic data and other experimental details are summarized in Table 1.
CCDC:854042.
2 Results and discussion
2.1 Molecular structure of[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2
X-ray quality crystals of compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2were grown from a concentrated hexane solution stored at-20℃.The molecular structure of compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2is shown in Fig.1 while relevant bond lengths and angles are shown in Table 2.The compound crystalizes in the monoclinic space group Pca21.The four-coordinated aluminum atom is wrapped by two N atoms of the one Schiff base ligand and two carbon atoms,which form a distorted tetrahedral.The bond distances for Al-N1(0.198 3(6)nm)and Al-N2(0.191 4(5)nm)are less to previously reported arylimine aluminum complexes[33].The Al(1)-C(16)/C(1)distances(0.196 0(7),0.193 8(8)nm)or the bond angle of N1-Al-N2(84.6(2)°)are more or less comparable to appropriate distances found for dimethylaluminumdistances previously reported[45].The aluminum atom is approximately coplanar with N1C9C10N2 by deviations of 0.001 7 nm.
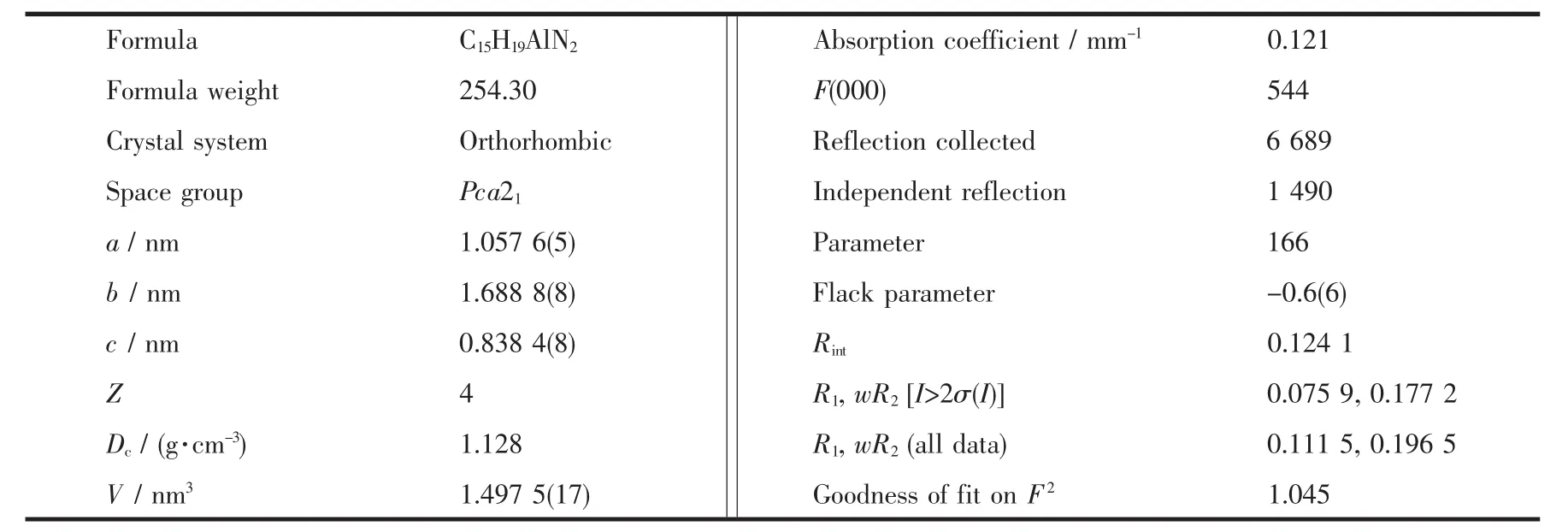
Table 1 Crystallographic data and structure refinement for Al compound

Table 2 Selected bond lengths(nm)and angles(°)for Al compound

Fig.1 Molecular structure of compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2 showing thermal ellipsoids at 50%probability level
2.2 Catalytic behavior of the catalysts
Compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2was used as initiators for catalytic ring-opening polymerization of rac-lactide.Polymerizations were carried out in Schlenk flask under nitrogen atmosphere strictly free of moisture and oxygen in toluene,as well as the polymerization factors of cLA∶cLM(100∶1 or 200∶1)and reaction temperature(room temperature or 70℃)were tested,which are summarized in Table 3.The polymerization data show that compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2can initiate the ring opening polymerization of rac-lactide under mild conditions.At room temperature,compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2is found to be low active at a cLA∶cLMof 100∶1 or 200∶1,but at 70 ℃,it shows high levels of activity.The molecular weight distributions(Mr/Mn,polydispersity index,PDI(1.63,1.64))are broad,indicating not well-controlled polymerization.

Table 3 Polymerization of rac-lactide catalyzed by Al compound
3 Conclusions
In conclusion,organoaluminum compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2has been readily prepared from the reactions of Schiff base ligands(d,l)-N-(2-pyrrolylmethylene)-1-phenylethylamine and trime-thylaluminum.It is an active initiator in the ring opening polymerization of rac-lactide under mild conditions,which gave PLAs with moderate molecular weight and broad molecular weight distributions(PDI=1.63,1.64).The resulting polylactides are isotacticrich determined by the ethane region of the homonuclear decoupled1H NMR experiments.
[1]Chen H L,Dutta S,Lin CC,et al.Organometallics,2012,31(5):2016-2025
[2]Schwarz A D,Chu Z,Mountford P.Organometallics,2010,29(5):1246-1260
[3]Liu Z,Gao W,Zhang J,et al.Organometallics,2010,29(22):5783-5790
[4]Chisholm M H,Lin C C,Gallucci J C,et al.Dalton Trans.,2003(3):406-412
[5]Kingsley NB,Doyon TJ,Shephard LE.J.Organomet.Chem.,2016,801(1):48-53
[6]Atwood D A,Jegier JA,Martin K J,et al.Organometallics,1995,14(4):1453-1460
[7]Atwood D A,Jegier J A,Rutherford D.Inorg.Chem.,1996,35(1):63-70
[8]Jakhar A,Sadhukhan A,Khan N H,et al.ChemCatChem,2014,6(9):2656-2661
[9]Duan R,Sun Z,Pang X,et al.Polymer,2015,77(23):122-128
[10]Gao B,Li D,Li Y,et al.New J.Chem.,2015,39(6):4670-4675
[11]Milione S,Grisi F,Centore R,et al.Eur.J.Inorg.Chem.,2008(35):5532-5539
[12]Iwasa N,Katao S,Liu J,et al.Organometallics,2009,28(7):2179-2187
[13]Flisak Z,Spaleniak GP,Bremmek M,et al.Organometallics,2013,32(14):3870-3876
[14]Han H L,Liu Y,Liu JY,et al.Dalton Trans.,2013,42(34):12346-12353
[15]Ternel J,Agbossou-Niedercorn F,Gauvin RM.Dalton Trans.,2014,43(11):4530-4536
[16]Contreras L,Cowley A H,Gabba?F P,et al.J.Organomet.Chem.,1995,489(1/2):C1-C3
[17]Cowley A H,Gabba?F P,Isom H S,et al.J.Organomet.Chem.,1995,500(1/2):81-88
[18]Isom H S,Cowley A H,Decken A,et al.Organometallics,1995,14(5):2400-2406
[19]B?ker C,Noltemeyer M,Gornitzka H,et al.Main Group Met.Chem.,1998,21(9):565-579
[20]Cui C,Roesky H W,Noltemeyer M,et al.Inorg.Chem.,2000,39(16):3678-3681
[21]Hair G S,Battle SL,Decken A,et al.Inorg.Chem.,2000,39(1):27-31
[22]Müller J,Schr?der R,Wang R M.Eur.J.Inorg.Chem.,2000(1):153-157
[23]Schumann H,Dechert S,Hummert M,et al.Z.Anorg.Allg.Chem.,2004,630(8/9):1196-1204
[24]Stender M,Segerer U,Sieler J,et al.Z.Anorg.Allg.Chem.,1998,624(1):85-90
[25]Radzewich C E,Guzei I A,Jordan R F.J.Am.Chem.Soc.,1999,121(37):8673-8674
[26]Singh S,Ahn H J,Stasch A,et al.Inorg.Chem.,2006,45(4):1853-1860
[27]Gong S,Ma H.Dalton Trans.,2008(25):3345-3357
[28]Yang Y,Schulz T,John M,et al.Inorg.Chem.,2008,47(7):2585-2592
[29]Li D,Peng Y,Geng C,et al.Dalton Trans.,2013,42(31):11295-11303
[30]Nako A E,Gates S J,White A J P,et al.Dalton Trans.,2013,42(42):15199-15206
[31]Liu Z,Lee JH Q,Ganguly R,et al.Chem.Eur.J.,2015,21(32):11344-11348
[32]HAO Peng-Fei(郝鵬飛),YANG Jun-Juan(楊俊娟),YANG Zhi(楊智),et al.Chinese J.Inorg.Chem.(無機(jī)化學(xué)學(xué)報(bào)),2014,30(12):2811-2817
[33]Beck J F,Schmidt J A R.Dalton Trans.,2012,41(3):860-870
[34]Shi B,A.Srinivas,Lu Z,et al.Organometallics,2014,33(10):2489-2495
[35]Lian B,Ma H,Spaniol T P,et al.Dalton Trans.,2009(41):9033-9042
[36]Nomura N,Ishii R,Akakura M,et al.J.Am.Chem.Soc.,2002,124(21):5938-5839
[37]Greco J F,McNevin M J,Hagadorn J R.Organometallics,2005,24(21):5167-5171
[38]Ahmed SA,Hill M S,Hitchcock PB,et al.Organometallics,2007,26(3):538-549
[39]Spassky N,Wisniewski M,Pluta C,et al.Macromol.Chem.Phys.,1996,197(9):2627-2637
[40]Radano C P,Baker G L,Smith M R.J.Am.Chem.Soc.,2000,122(7):1552-1553
[41]Jia B,Hao JS,Wei X H,et al.J.Organomet.Chem.,2017,831(1):11-17
[42]Bruker SMART Ver.5.0,SAINT Ver.6.02,Bruker AXS Inc.,Madison,WI,2000.
[43]Sheldrick G M.SADABS,SHELXS-97,SHELXL-97,University of G?ttingen,Germany,1997.
[44]Sheldrick G M.SHELXTL/PC,Ver.6.10,Bruker AXSInc.,Madison,WI,1999.
[45]Kingsley N B,Kirschbaum K,Mason M R.Organometallics,2010,29(22):5927-5935
Synthesis,Structure and Application in rac-Lactide Polymerization of Compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2
The crystalline compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2was synthesized by the reaction of the ligand(d,l)-N-(2-pyrrolylmethylene)-1-phenylethylamine and trimethylaluminum in high yield.And it was well characterized by1H NMR,13C NMR spectroscopy,elemental analyses and single crystal X-ray crystallography.Compound[C4H3N{CH=NCH(Me)C6H5}-2]AlMe2is an active initiator for the polymerization of rac-lactide leading to the isotactic-rich polylactides.CCDC:854042.
aluminum;Schiff base ligand;rac-lactide;ring-opening polymerization
O614.3+1;O631.5
A
1001-4861(2017)10-1876-05
10.11862/CJIC.2017.221
JIA Bin*,1,3HAO Jun-Sheng2TONGHong-Bo3WEI Xue-Hong2ZHOU Mei-Su3LIU Dian-Sheng*,3
(1School of Basic Medical Science,Shanxi Medical University,Taiyuan 030001,China)
(2School of Chemistry and Chemical Engineering,Shanxi University,Taiyuan 030006,China)
(3Institute of Applied Chemistry,Shanxi University,Taiyuan 030006,China)
2017-05-23。收修改稿日期:2017-07-19。
國家自然科學(xué)基金(No.21272142,21371111)和山西醫(yī)科大學(xué)博士基金(No.055208)資助項(xiàng)目。*
。 E-mail:jiabin@sxmu.edu.cn,dsliu@sxu.edu.cn

