加堿對(duì)豬場(chǎng)廢水厭氧消化液好氧處理過程酸化改進(jìn)作用及其對(duì)菌群結(jié)構(gòu)的影響
王 伸, 鄧良偉, 姜奕圻, 王 霜, 徐 則 , 鄭 丹
( 1.農(nóng)業(yè)部沼氣科學(xué)研究所, 成都 610041; 2.農(nóng)業(yè)部農(nóng)村可再生能源開發(fā)利用重點(diǎn)實(shí)驗(yàn)室, 成都 610041)
加堿對(duì)豬場(chǎng)廢水厭氧消化液好氧處理過程酸化改進(jìn)作用及其對(duì)菌群結(jié)構(gòu)的影響
王 伸1,2, 鄧良偉1,2, 姜奕圻1,2, 王 霜1,2, 徐 則1,2, 鄭 丹1,2
( 1.農(nóng)業(yè)部沼氣科學(xué)研究所, 成都 610041; 2.農(nóng)業(yè)部農(nóng)村可再生能源開發(fā)利用重點(diǎn)實(shí)驗(yàn)室, 成都 610041)

豬場(chǎng)廢水厭氧消化液; SBR; 短程硝化; pH值; 加堿
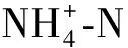
筆者對(duì)加堿改進(jìn)豬場(chǎng)廢水厭氧消化好氧處理效果進(jìn)行了長(zhǎng)期、比較系統(tǒng)的研究,并對(duì)加堿處理后的微生物群落進(jìn)行分析。希望能為厭氧消化液好氧后處理工程應(yīng)用提供參考。
1 試驗(yàn)材料和方法
1.1 污泥和污水
試驗(yàn)所用接種污泥來源于實(shí)驗(yàn)室培養(yǎng)的好氧污泥(具有硝化、反硝化活性)。試驗(yàn)進(jìn)水為四川邛崍某豬場(chǎng)廢水處理沼氣工程厭氧反應(yīng)器出水(厭氧消化液)。
1.2 試驗(yàn)裝置
試驗(yàn)采用SBR工藝,實(shí)驗(yàn)裝置為內(nèi)徑為12 cm,外徑為18 cm,高度100 cm的有機(jī)玻璃圓柱體,總?cè)莘e11.3 L,反應(yīng)容積為9.5 L。反應(yīng)器外設(shè)夾套以及加熱循環(huán)水進(jìn)出口各一個(gè),通過循環(huán)電子恒溫水浴鍋(型號(hào)HH-W21,北京中興偉業(yè)儀器有限公司)內(nèi)熱水以實(shí)現(xiàn)對(duì)溫度的控制。在反應(yīng)器底部布置定制的直徑為9cm的曝氣盤,曝氣盤連接空氣壓縮機(jī)(型號(hào)ACO-002,天津森森集團(tuán)股份有限公司)進(jìn)行曝氣。底部曝氣盤上設(shè)微型潛水泵(型號(hào)At-104,香港創(chuàng)星制品有限公司)作為攪拌裝置。采用蠕動(dòng)泵(型號(hào)WT600-2J,保定蘭格集團(tuán))進(jìn)行進(jìn)出料。
1.3 試驗(yàn)方法
處理系統(tǒng)酸化預(yù)實(shí)驗(yàn):在1個(gè)50 L桶里面加入生活污水處理廠曝氣池污泥,進(jìn)水為豬場(chǎng)廢水厭氧消化液,只進(jìn)水不出水,充分曝氣培養(yǎng),5 d后桶里面混合pH值降至6.0以下,視為酸化。
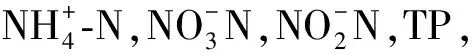
1.4 檢測(cè)項(xiàng)目及分析方法
1.5 微生物高通量測(cè)序分析
從反應(yīng)開始時(shí)的接種污泥、穩(wěn)定運(yùn)行203 d時(shí)反應(yīng)器混合液取樣。對(duì)所取樣品采用CTAB方法進(jìn)行基因組DNA提取,隨后利用瓊脂糖凝膠電泳檢測(cè)其DNA的濃度和純度。使用聚合酶鏈?zhǔn)椒磻?yīng)(PCR)技術(shù)擴(kuò)增細(xì)菌的16SrRNA基因。以16S rRNA V3~V4 區(qū)內(nèi)338F (5′-ACTCCT ACGGGAGGCAGCA-3′)和806R (5′-GGACTAC HVGGGTWTCTAA T-3′)為特征引物。PCR 反應(yīng)體系和程序(30μL)為: Phusion Master Mix(2×) 15 μL ;Primer(2μM) 3 μL(6μM) gDNA(1 ng·μL-1) 10 μL(5~10 ng) H2O 2 μL ;98℃預(yù)變性1 min;30 個(gè)循環(huán)包括(98 ℃,10 sec;50 ℃,30 sec;72 ℃,30 sec);72℃,超純水滅菌5 min;用2%濃度的瓊脂糖凝膠進(jìn)行電泳檢測(cè)PCR的產(chǎn)物;使用Thermo Scientific公司的GeneJET膠作為回收試劑盒對(duì)PCR擴(kuò)增產(chǎn)物進(jìn)行回收。委托北京諾禾致源生物信息科技有限公司基于Illumina Hiseq 2500平臺(tái)對(duì)PCR 擴(kuò)增產(chǎn)物的高通量測(cè)序,在多樣性評(píng)估的基礎(chǔ)上,采用Qiime 軟件進(jìn)行微生物分類學(xué)分析。
2 結(jié)果與討論
2.1 反應(yīng)器中混合液pH值變化
從圖1中可以看出,反應(yīng)開始時(shí),兩個(gè)反應(yīng)器的混合液都處于酸化狀態(tài),pH值為5.7左右。其后, AA組加入堿以后pH值就在6.5以上,反應(yīng)裝置不再酸化,其pH值隨加堿的變化而變化;而未加入堿的CG組,在前期出現(xiàn)波動(dòng),出現(xiàn)pH值上升現(xiàn)象,從第84天以后開始到試驗(yàn)結(jié)束第203天,pH值逐漸下降,直至穩(wěn)定在5.7左右。AA組出水pH值平均值為7.9,說明改進(jìn)策略能明顯抑制酸化。AA組改進(jìn)原因是因?yàn)橥饧拥膲A能中和硝化過程中產(chǎn)生的酸,使其pH值能維持在6.5以上。
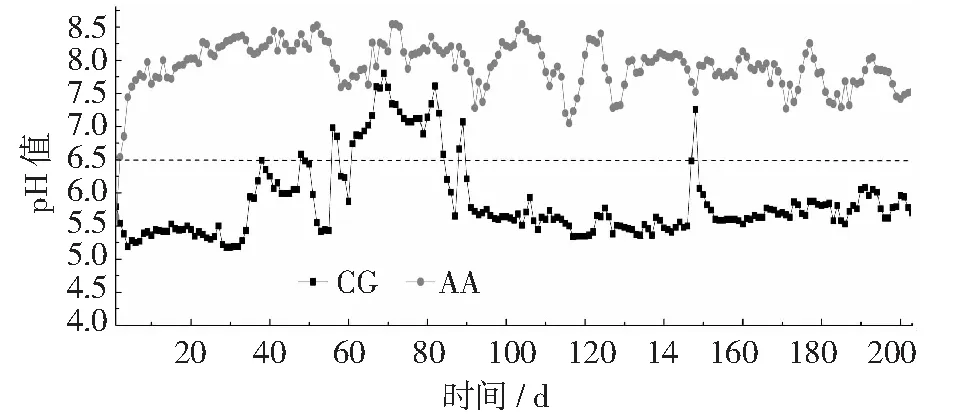
圖1 不同反應(yīng)器曝氣結(jié)束時(shí)混合液pH值
2.2 SBR對(duì)COD的去除
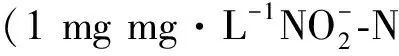
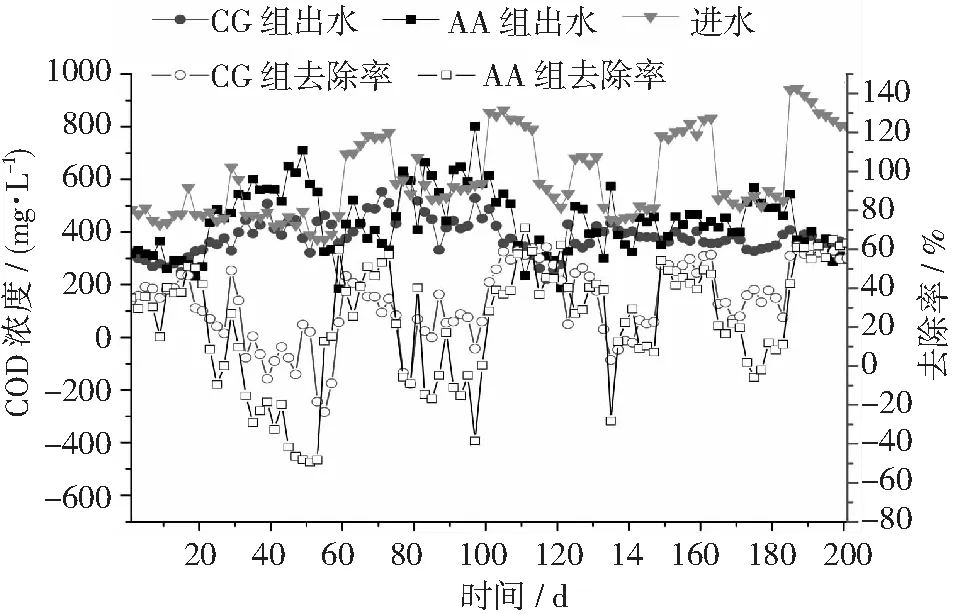
圖2 不同反應(yīng)器對(duì)豬場(chǎng)廢水厭氧消化液COD的去除
2.3 氮去除
2.3.1 氨氮去除

圖3 不同反應(yīng)器對(duì)豬場(chǎng)廢水厭氧消化液的去除
2.3.2 氨氮轉(zhuǎn)化物
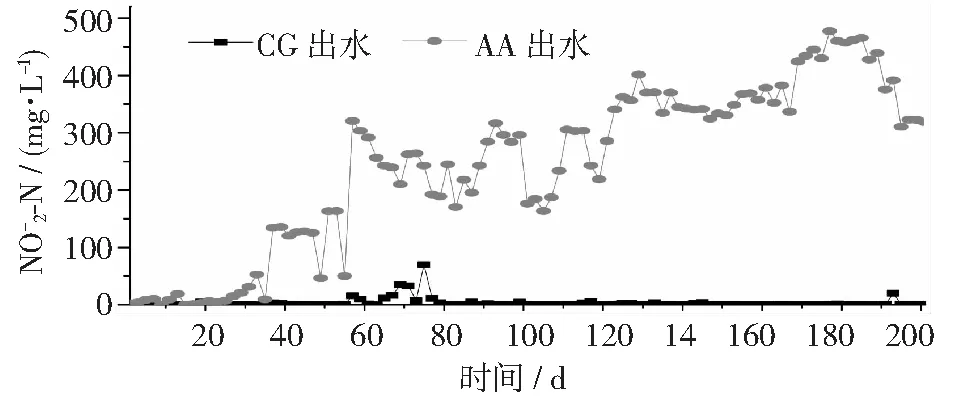
圖4 不同反應(yīng)器中濃度變化情況
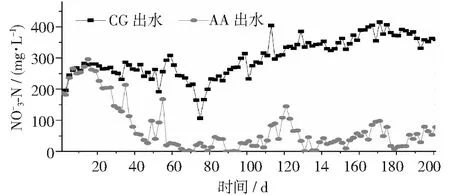
圖5 不同反應(yīng)器中濃度變化情況
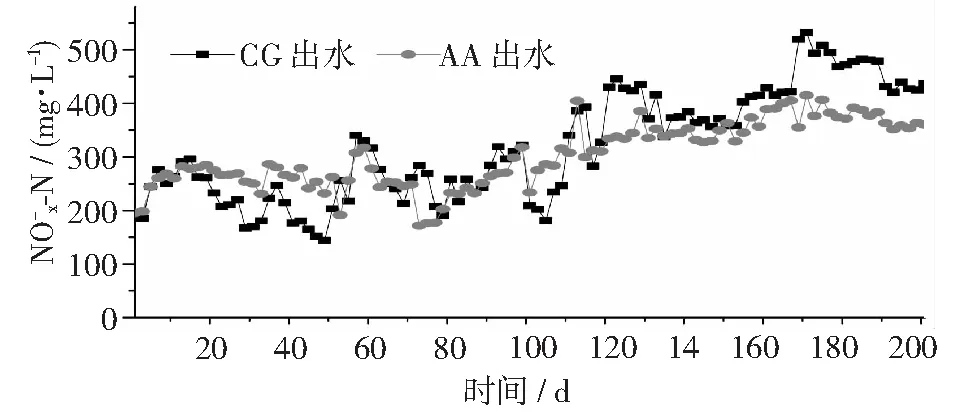
圖6 不同反應(yīng)器中濃度變化情況
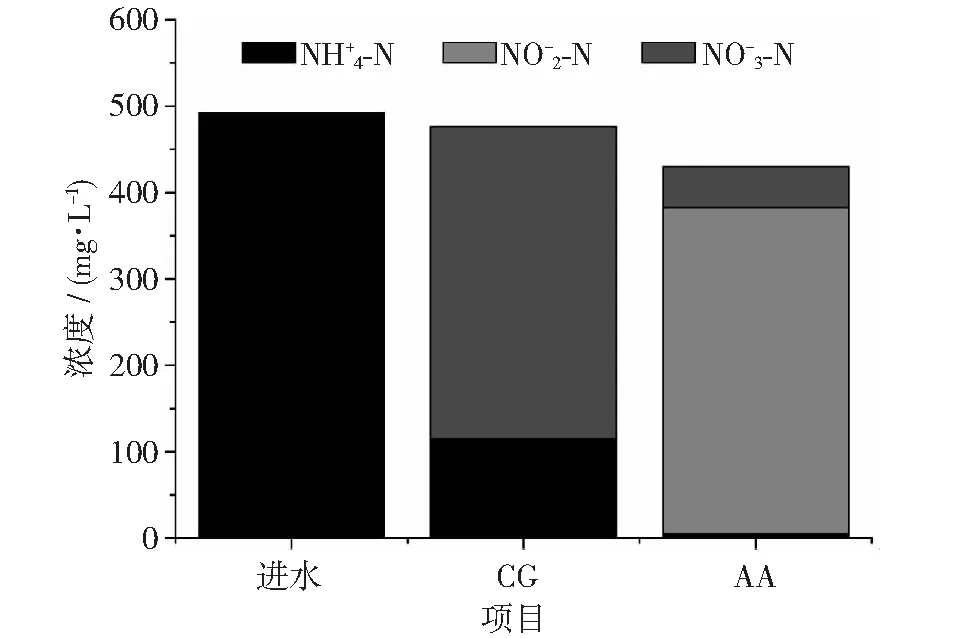
圖7 進(jìn)水和129~201天期間CG和AA組出水中和濃度變化
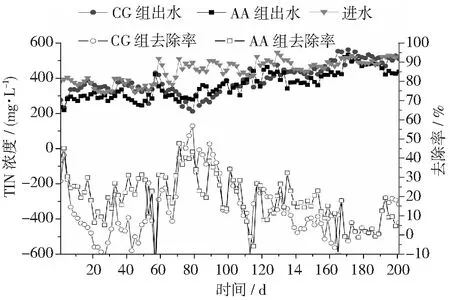
圖8 不同反應(yīng)器對(duì)豬場(chǎng)廢水厭氧消化液的去除
2.4 總磷去除
圖9顯示了SBR對(duì)豬場(chǎng)廢水厭氧消化液TP去除效果。從圖6可知,在試驗(yàn)前期(第19周前),進(jìn)水(厭氧消化液)TP濃度為45.2 mg·L-1,CG和AA組出水TP濃度分別為58.2 mg·L-1,48.9 mg·L-1和23.8 mg·L-1,對(duì)應(yīng)去除率分別為-36.1%和-19.6%。在穩(wěn)定期(第19~28周),進(jìn)水(厭氧消化液)TP濃度為37.9 mg·L-1,CG和AA組出水TP濃度分別為43.6 mg·L-1和38.3 mg·L-1,對(duì)應(yīng)去除率分別為-19.4%和-7.48%。通過對(duì)比發(fā)現(xiàn),穩(wěn)定期間酸化改進(jìn)AA組,可以提高11.9%的TP去除率。出現(xiàn)AA組對(duì)TP去除率為負(fù)數(shù)的原因是在反應(yīng)器中前期積累的大量的TP而沒有排除,造成后期出水濃度比進(jìn)水濃度高。傳統(tǒng)的生物除磷理論[26~27]認(rèn)為,生物除磷主要通過聚磷菌(Poly-P Accumulating Organisms, PAOs)在好氧條件下過量的吸磷,在厭氧條件下,水解細(xì)胞原生質(zhì)中聚合磷酸鹽(poly-P)從而釋放磷;通過排除富含磷的污泥,以此達(dá)到除磷的目的。因?yàn)槿コ齌P是通過排除富含微生物的剩余污泥[28],而運(yùn)行203 d期間沒有排富含磷的污泥,導(dǎo)致污泥老化,也是磷去除效果差的又一原因。因此,在試驗(yàn)運(yùn)行期間沒有進(jìn)行排泥和污泥老化解體是筆者試驗(yàn)除磷效果差的根本原因。
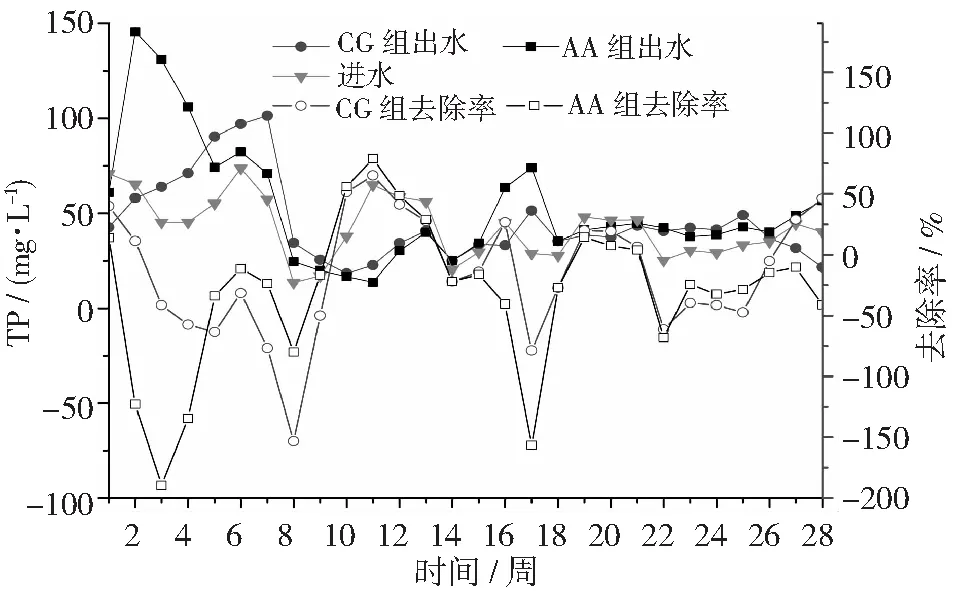
圖9 不同反應(yīng)器對(duì)豬場(chǎng)廢水厭氧消化液TP的去除
2.5 微生物菌群結(jié)構(gòu)的變化
利用Hiseq高通量測(cè)序平臺(tái)對(duì)對(duì)照組和改進(jìn)組污泥中微生物多樣性進(jìn)行了分析。高達(dá)99.9%以上的覆蓋率表明,測(cè)序結(jié)果能真實(shí)反映樣品中的菌群分布情況。CG組和AA組得到相同的56676和57563條有效序列,平均長(zhǎng)度為分別為420和420 bp,在99%的相似水平上可聚類產(chǎn)生1262和1286個(gè)OTU。不同污泥樣品的具體細(xì)菌群落多樣性指數(shù)如表2所示。由ACE, Chao, Simpson和Shannon指數(shù)分析可知,加堿的AA組中污泥細(xì)菌群落的豐富度和多樣性高于未加堿的CG組,同時(shí)也增加了細(xì)菌群落的均一性。對(duì)測(cè)序樣品得到的序列進(jìn)行比對(duì)分析,兩組樣品在生物分類學(xué)門的水平上進(jìn)行分類。由表1可知變形菌門 (Proteobacteria)和擬桿菌門(Bacteroidetes)是兩個(gè)反應(yīng)器共有的優(yōu)勢(shì)菌門,CG組相對(duì)豐度為27.4%和26.2%,AA組相對(duì)豐度為35.2%和31.2%。與未加堿CG組相比,加堿的AA組使變形菌門 (Proteobacteria)和擬桿菌門(Bacteroidetes)的相對(duì)豐度有所增加,而Acidobacteria(酸桿菌門)的相對(duì)豐度急劇減小,從相對(duì)豐度28.9%減少到4.78%。
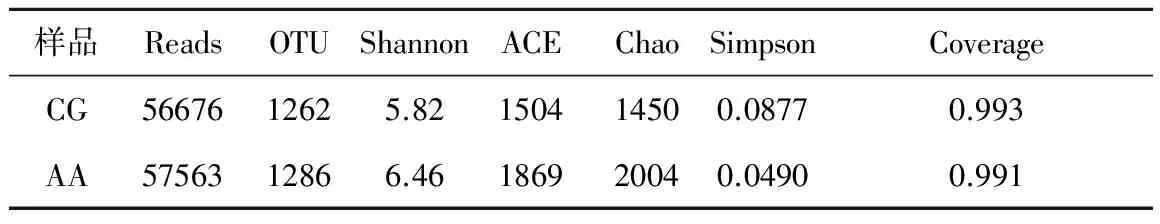
表1 樣本微生物多樣性指數(shù)
注:表中Chao和ACE指數(shù)常用來表征菌群的豐富度,數(shù)值越大,表示樣品中群落結(jié)構(gòu)越豐富。Shannon和Simpson指數(shù)常用來估算樣本中微生物的多樣性,Shannon值越大,其說明群落多樣性越高;而Simpson指數(shù)值越大,說明群落多樣性越低,均一性相對(duì)越差。
表2是CG和AA組污泥中由相對(duì)豐度所有大于1%的屬組成,共有22個(gè)屬。在CG組中,主要是好氧異養(yǎng)微生物,如Blastocatella[29],Saprospiraceae和Chitinophagacea[30-31]。其中也包含一些自養(yǎng)細(xì)菌如硝化細(xì)菌 (Nitrospira; NOB) 和Nitrosomonas(ammonium-oxidizing bacteria, AOB)[31], 也包括一些異養(yǎng)脫氮菌如Thermomona[30]和自養(yǎng)脫氮菌 (Limnobacter)[32]。 在AA組污泥中,主要不同于CG組的微生物為具有聚磷作用PHOS-HE51(17.34%)和PHOS-HE36(1.78%)[33]和好氧異養(yǎng)型的OPB35_soil_group_norank[34]和OM1clades[35]。 與CG組微生物相對(duì)豐度相比, AA組中的主要功能Nitrosomonas(AOB)的相對(duì)豐度從 0.65% 增加到5.75%, 而Nitrospira(NOB) 從 1.27% 下降到 0.01%。氨氧化細(xì)菌的富集和亞硝酸鹽細(xì)菌的下降能很好解釋在AA組出現(xiàn)亞硝酸鹽的積累。

表2 對(duì)照組和IS組污泥中相對(duì)豐度>1%的屬 (%)
注:每組加粗為反應(yīng)器前三主要菌屬
3 結(jié)論
(2)不同于對(duì)照(CG)組,加堿(AA)組形成了亞硝酸積累,亞硝酸鹽積累率(NAR)為88.8%。
(3)加堿(AA)組的氨氧化細(xì)菌(Nitrosomonas)豐度(AOB)上升,亞硝酸氧化菌(NOB)豐度下降,有利于實(shí)現(xiàn)短程硝化。
[1] L Deng, P Zheng, Z Chen, Q Mahmood.organic material, nitrogen and phosphorus[J].Bioresource Technology, 2008 (99):3136-3145.
[2] D Yang, L Deng, D Zheng, L Wang, Y Liu.Separation of swine wastewater into different concentration fractions and its contribution to combined anaerobic-aerobic process[J].Journal of Environmental Management, 2016 (168):87-93.
[3] T Jin, T Zhang, Q Yan.Characterization and quantification of ammonia-oxidizing archaea (AOA) and bacteria (AOB) in a nitrogen-removing reactor using T-RFLP and qPCR[J].Appl Microbiol Biot, 2010 (87):1167-1176.
[4] B Wett, W Rauch.The role of inorganic carbon limitation in biological nitrogen removal of extremely ammonia concentrated wastewater[J].Water Research, 2003 (37):1100-1110.
[5] B Sinha, A P Annachhatre.Partial nitrification—operational parameters and microorganisms involved[J].Reviews in Environmental Science and Bio/Technology, 2007 (6):285-313.
[6] M Muβmann, I Brito, A Pitcher, J S Sinninghe Damsté, R Hatzenpichler, A Richter, J L Nielsen, P H Nielsen, A Müller, H Daims, M Wagner, I M Head.Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers[J].Proc Natl Acad Sci, 2011 (108): 16771-16776.
[7] S Park, W Bae, J Chung, S C Baek.Empirical model of the pH dependence of the maximum specific nitrification rate[J].Process Biochemistry, 2007 (42):1671-1676.
[8] Metcalf I,Eddy G,Tchobanoglous F,Burton H D Stensel.Wastewater Engineering: Treatment and Reuse, 4 ed[M].McGraw-Hill Education, 2002.
[9] R A Morris.Investigation of the Optimal Dissolved CO2Concentration and pH Combination for the Growth of Nitrifying Bacteria[J].University of South Florida, 2011.
[10] A Gieseke, S Tarre, M Green, D de Beer.Nitrification in a biofilm at low pH values: role of in situ microenvironments and acid tolerance[J].Applied and Environmental Microbiology, 2006 (72):4283-4292.
[11] S Tarre, M Green.High-rate nitrification at low pH in suspended-and attached-biomass reactors[J].Applied and Environmental Microbiology, 2004(70): 6481-6487.
[12] C Grunditz, G Dalhammar.Development of nitrification inhibition assays using pure cultures of nitrosomonas and nitrobacter[J].Water Research, 2001 (35): 433-440.
[13] S A Burton, J I Prosser.Autotrophic ammonia oxidation at low pH through urea hydrolysis[J].Applied and environmental microbiology, 2001(67):2952-2957.
[14] 王 伸, 鄧良偉, 徐 則, 鄭 丹, 王 蘭, 王 霜.pH值對(duì)好氧處理及污泥性能的影響[J].中國(guó)沼氣, 2016 (34):22-26.
[15] 楊 虹, 李道棠.集約化養(yǎng)豬場(chǎng)沖欄水的達(dá)標(biāo)處理[J].上海交通大學(xué)學(xué)報(bào), 2000 (34):558-560.
[16] 胡啟智, 朱凰榕, 王 軍, 歐陽(yáng)春飛.豬場(chǎng)廢水的石灰處理研究[J].安徽農(nóng)業(yè)科學(xué), 2012 (40): 8551-8552.
[17] 方炳南, 顧欣欣, 朱 亮.常規(guī)SBR工藝對(duì)豬場(chǎng)沼液的處理性能研究[J].中國(guó)沼氣, 2012 (30):27-30.
[18] 王 新, 倪晉仁, 翟風(fēng)敏.豬場(chǎng)穩(wěn)定塘廢水的IBAF脫氮影響因素研究[J].應(yīng)用基礎(chǔ)與工程科學(xué)學(xué)報(bào), 2006 (5):10-15.
[19] T Yamamoto, K Takaki, T Koyama, K Furukawa.Long-term stability of partial nitritation of swine wastewater digester liquor and its subsequent treatment by Anammox[J].Bioresource Technology, 2008 (99):6419-6425.
[20] G Bortone, S Gemelli, A Rambaldi, A Tilche.Nitrification, denitrification and biological phosphate removal in sequencing batch reactors treating piggery wastewater[J].Water Science Technology, 1992 (26):977-985.
[21] B Boiran, Y Couton, J Germon.Nitrification and denitrification of liquid lagoon piggery waste in a biofilm infiltration-percolation aerated system (BIPAS) reactor[J].Bioresource technology, 1996(55):63-77.
[22] W Bae, S Baek, J Chung, Y Lee.Optimal operational factors for nitrite accumulation in batch reactors[J].Biodegradation, 2001 (12):359-366.
[23] G BITTON.Wastewater Microbiology, 3ra.edic [M].New Jersey,2005.
[24] M H Gerardi.Nitrification and denitrification in the activated sludge process [M].New York:Wiley Interscience,2003.
[25] S E Jorgensen, B Fath.Encyclopedia of Ecology [M].Elsevier, 2008.
[26] G L Leung.N Tam.Operation strategy of a sequencing batch reactor for simultaneous removal of wastewater organic matter and nutrients[J].Resources, Conservation and Recycling, 1994 (11):209-223.
[27] D T Sponza, H Atalay.Influence of nitrate and COD on phosphorus, nitrogen and dinitrotoluene (DNT) removals under batch anaerobic and anoxic conditions[J].Anaerobe, 2004 (10):287-293.
[28] S Won, C Ra.Biological nitrogen removal with a real-time control strategy using moving slope changes of pH (mV)-and ORP-time profiles[J].Water Research, 2011 (45):171-178.
[29] M Tank, D A Bryant.Chloracidobacterium thermophilum gen nov sp nov an anoxygenic microaerophilic chlorophotoheterotrophic acidobacterium[J].International Journal of Systematic Evolutionary Microbiology, 2015 (65):1426-1433.
[30] Y Yue, J Liu, B Ma, L Ye, W Bo, Y Peng.Improving municipal wastewater nitrogen and phosphorous removal by feeding sludge fermentation products to sequencing batch reactor (SBR) [J].Bioresource Technology, 2016 (222):326-334.
[31] J Meng, J Li, J Li, K Sun, P Antwi, K Deng, C Wang, G Buelna.Efficiency and bacterial populations related to pollutant removal in an upflow microaerobic sludge reactor treating manure-free piggery wastewater with low COD/TN ratio[J].Bioresource Technology, 2016 (201):166-173.
[32] S Deng, D Li, X Yang, W Xing, J Li, Q Zhang.Biological denitrification process based on the Fe(0)-carbon micro-electrolysis for simultaneous ammonia and nitrate removal from low organic carbon water under a microaerobic condition[J].Bioresource Technology, 2016(219):677-686.
[33] S A Baldwin, M Khoshnoodi, M Rezadehbashi, M Taupp, S Hallam, A Mattes, H Sanei.The microbial community of a passive biochemical reactor treating arsenic, zinc, and sulfate-rich seepage[J].Frontiers in Bioengineering Biotechnology, 2015(3).
[34] P F Dunfield, A Yuryev, P Senin, A V Smirnova, M B Stott, S Hou, B Ly, J H Saw, Z Zhou, Y Ren.Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia[J].Nature, 2007 (450):879-882.
[35] R O, S J, J MSM, L C.Metagenomic potential for and diversity of N-cycle driving microorganisms in the Bothnian Sea sediment[J].Microbiology Open, 2017.
AlleviatingAcidificationofAerobicPost-treatmentofDigestedPiggeryWastewaterbyAddingAlkaliandItsInfluenceonMicrobialCommunity
WANGShen1,2,DENGLiang-wei1,2,JIANGYi-qi1,2,WANGShuang1,2,XUZe1,2,ZHENGDan1,2
( 1.BiogasInstituteofMinistryofAgriculture,Chengdu610041,China; 2.LaboratoryofDevelopmentandApplicationofRuralRenewableEnergy,MinistryofAgriculture,Chengdu610041,China)
Digested effluent; SBR; shortcut nitrification; pH; Adding alkali
2017-10-09
項(xiàng)目來源: 國(guó)家生豬技術(shù)產(chǎn)業(yè)體系(CARS-36-10B); 國(guó)家自然科學(xué)基金(31572450)
王 伸(1990-),男,安徽亳州人,在讀碩士,主要研究方向?yàn)檗r(nóng)村廢棄物處理技術(shù),E-mail:ws55185366@163.com
鄧良偉,E-mail:dengliangwei@caas.cn
S216.4; X703
A
1000-1166(2017)06-0003-07

