Synthesis, Crystal Structure and Photoluminescent Property of a New Zn(II) Complex Based on 3,4-Bis(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole①
WU Hun-Ping LI Bing TIAN Xio-Yn JIN Xio-Dong LIANG Hong-Jio QIU Jing-Ru MENG Qing-Feng
?
Synthesis, Crystal Structure and Photoluminescent Property of a New Zn(II) Complex Based on 3,4-Bis(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole①
WU Huan-PingaLI Binga②TIAN Xiao-YanaJIN Xiao-DongaLIANG Hong-JiaoaQIU Jing-RuaMENG Qing-Fengb
a(750021)b(810008)
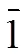
complex, crystal structure, 2,2?,4??-tpt, photoluminescent;
1 INTRODUCTION
It has been well established that polymeric coor- dination complexes with metal ions and conjugated ligands may be regarded as promising candidates for potential photoluminescent materials[1-5]. In this connection, Zn(II) ion with the adjustable coordina- tion numbers and10electronic configuration has been applied in preparing coordination supramole- cular systems with interesting structures and promising properties[6-9].
Currently, triazole derivatives have been widely used in the construction of metal complexes[10-13]. As one of the derivatives of triazole, tripyridyltriazole (tpt) can be considered as excellent building blocks for their multiple binding sites and diverse con- formations upon complexation[14-16]. Furthermore, the prototropy and conjugation between the 1H-1,2,4-triazole and pyridyl groups alter the electron density in different sections of the molecules, thus making the ligand more flexible[17, 18]. So, we have chosen 3,4-bis(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole as an organic ligand, which can be regarded as an excellent building block for the construction of new coordination compounds. In addition, terephthalic acid (H2tpa) has been extensively explored to pro- duce robust crystalline materials due to their exclu- sive bridging abilities and versatile coordination modes[19-21]. Thus, it is usually used as an auxiliary ligand in the construction of metal organic frame- works[22, 23].
As the further investigation of tpt[24], a new Zn(II) coordination polymer [Zn1.5(2,2?,4??-tpt)(tpa)2]nbased on 2,2?,4??-tpt (3,4-bis(2-pyridyl)-5-(4-pyri- dyl)-1,2,4-triazole) and H2tpa has been structurally established by single-crystal X-ray diffraction analysis, and characterized by EA, IR, TGA and XPRD. The luminescent property of complex 1 is also studied, which shows it can be a good candidate for potential photoactive materials.
2 EXPERIMENTAL
2. 1 Reagents and instruments
All chemicals were commercially available and used as purchased. Elemental analyses (C, H and N) were performed on a Vario EL III analyzer. Infrared spectra were obtained from KBr pellets on a BEQ VZNDX 550 FTIR instrument within the 400~4000 cm-1region. Thermogravimetric analysis was carried out on a TA Instruments NETZSCH STA 449 C simultaneous TGA at a heating rate of 10 ℃·min-1under hydrostatic air. The luminescent spectra for the powdered solid samples were recorded at room temperature on an F-7000 FL Spectrophotometer.
2. 2 Synthesis of the ligand
2,2?,4??-tpt was synthesized according to refe- rence[25]. A mixture of 2-aminopyridine (4.655 g, 0.05 mol), sulfur powder (4.81 g, 0.15 mol) and sodiumsulfide nonahydrate (0.24 g, 0.01 mol) in 2-methyl pyridine (30 mL) was refluxed for 48 h. After cooling and removal of all volatiles in vacuo, the dark solid residue was taken up in 2 mol/L aqueous sodium hydroxide (100 mL), and the mixture was filtered. The filtrate was diluted with water (200 mL) and acidified to pH = 5 by dropwise adding hydrochloric acid. The resulting yellow precipitate was filtered off and washed thoroughly with water. Then, a mixture of N-(2-pyridyl)py- ridine-4-thio formamide (2.15 g, 10.0 mmol) and 4-pyridine formyl hydrazine (1.645 g, 12.0 mmol) in n-butyl alcohol (40 mL) was refluxed for 24 h. On cooling, the product crystallized from the orange reaction mixture. It was filtered off, washed with ethanol and dried in vacuo to give 2.85 g (52%) as fine colourless needles (Scheme 1). m.p. 230.5~233.5 ℃. C17H12N6: Calcd. (%): C, 67.99; H, 4.03; N, 27.98. Found (%): C, 68.43; H, 3.51; N, 28.06. IR (KBr, cm-1): 1589s, 1568m, 1471m, 1454m, 1427m, 1342w, 1327w, 1284m, 1242w, 1176m, 1149w, 1099m, 1047m, 1020m, 993m, 929w, 817s, 798s, 754s, 711s, 608s.1H NMR (400 MHz, CDCl3): 8.59 (dd, J = 1.9, 0.8Hz, 1H), 8.58 (dd, J = 3.7, 1.2Hz, 1H), 8.56 (d, J = 1.7Hz, 1H), 8.31 (dt, J = 8.0, 1.0Hz, 1H), 8.20 (ddd, J = 4.8, 1.7, 0.9Hz, 1H), 7.85 (td, J = 7.7, 1.9 Hz, 1H), 7.80 (td, J = 7.8, 1.8Hz 1H), 7.48 (ddd, J = 7.5, 4.9, 1.0Hz, 1H), 7.34 (d, J = 1.7 Hz, 1H), 7.33 (d, J = 1.7 Hz, 1H), 7.29 (dt, J = 7.9, 0.8 Hz, 1H), 7.22 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H).
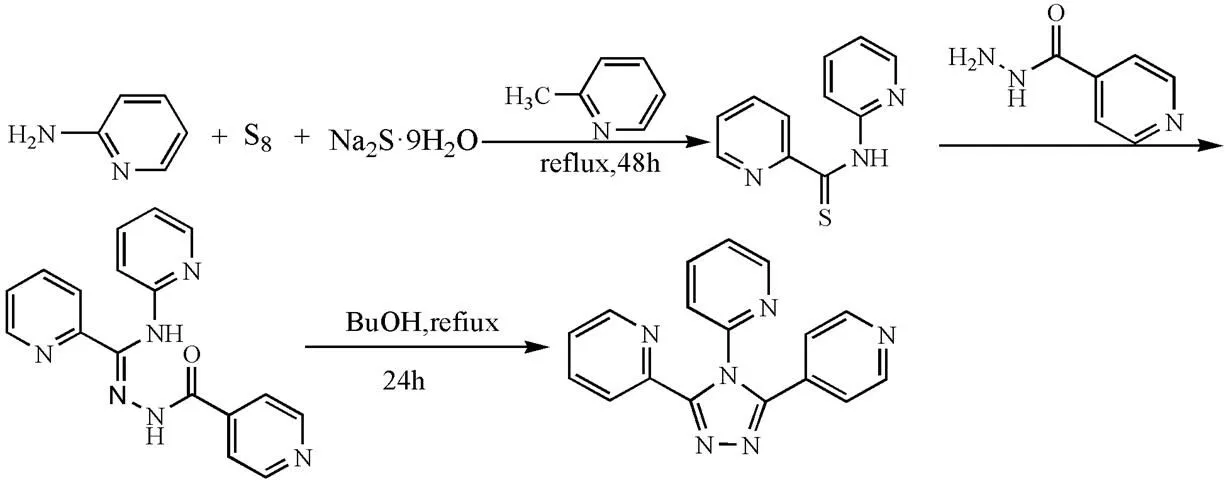
Scheme 1. Synthetic route of the 2,2?,4??-tpt ligand
2. 3 Synthesis of compound 1
Compound 1 was prepared as follows.A water solution (5 mL) of Zn(NO3)2·6H2O (29.1 mg, 0.1 mmol) and H2tpa (21.0 mg, 0.1 mmol) was added to a solution of 2,2?,4??-tpt (15.0 mg, 0.05 mmol) in CH3OH (10 mL). After ca. 30 min of vigorous mixing, the resulting solution was filtered and left to stand under ambient conditions. Colorless block crystals of 1 were obtained after10 days in 60% yield (based on 2,2?,4??-tpt).C33H21Zn1.5N6O8: calcd.: C, 54.47; H, 2.91; N, 11.55%. Found: C, 54.62; H, 2.92; N, 11.59%. IR(KBr, cm-1): 1586s, 1578s, 1514m, 1464m, 1410s,1397m, 1217m, 1195m, 1152w, 1112w, 1005s,991m, 976w, 836s, 822m, 787m, 742m, 722m, 707m,632s, 610s, 523w.
2. 4 X-ray crystallography
All diffraction data of complex 1 were collected on a Bruker/Siemens Smart Apex II CCD diffrac- tometer with graphite-monochromated Moradia- tion (= 0.71073 ?) at 293(2) K. Cell parameters were retrieved using SMART software and refined using SAINTPLUS for all observed reflections. Data reduction and correction forand decay were performed using the SAINTPLUS software. Absorp- tion corrections were applied using SADABS. All structures were solved by direct methods using the SHELXS program of the SHELXTL-97 package and refined with SHELXL. For 1, a total of 25507 reflections were collected in the range of 3.10≤≤25.01°, of which 1966 were independent (int= 0.0648). The final= 0.0322 and= 0.0815 for observed reflections with> 2(),= 0.0359 and= 0.0841 for all data with (Δ)max= 0.812 and (Δ)min= 0.557 e·?-3.
3 RESULTS AND DISCUSSION
3. 1 Description of the structure
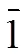
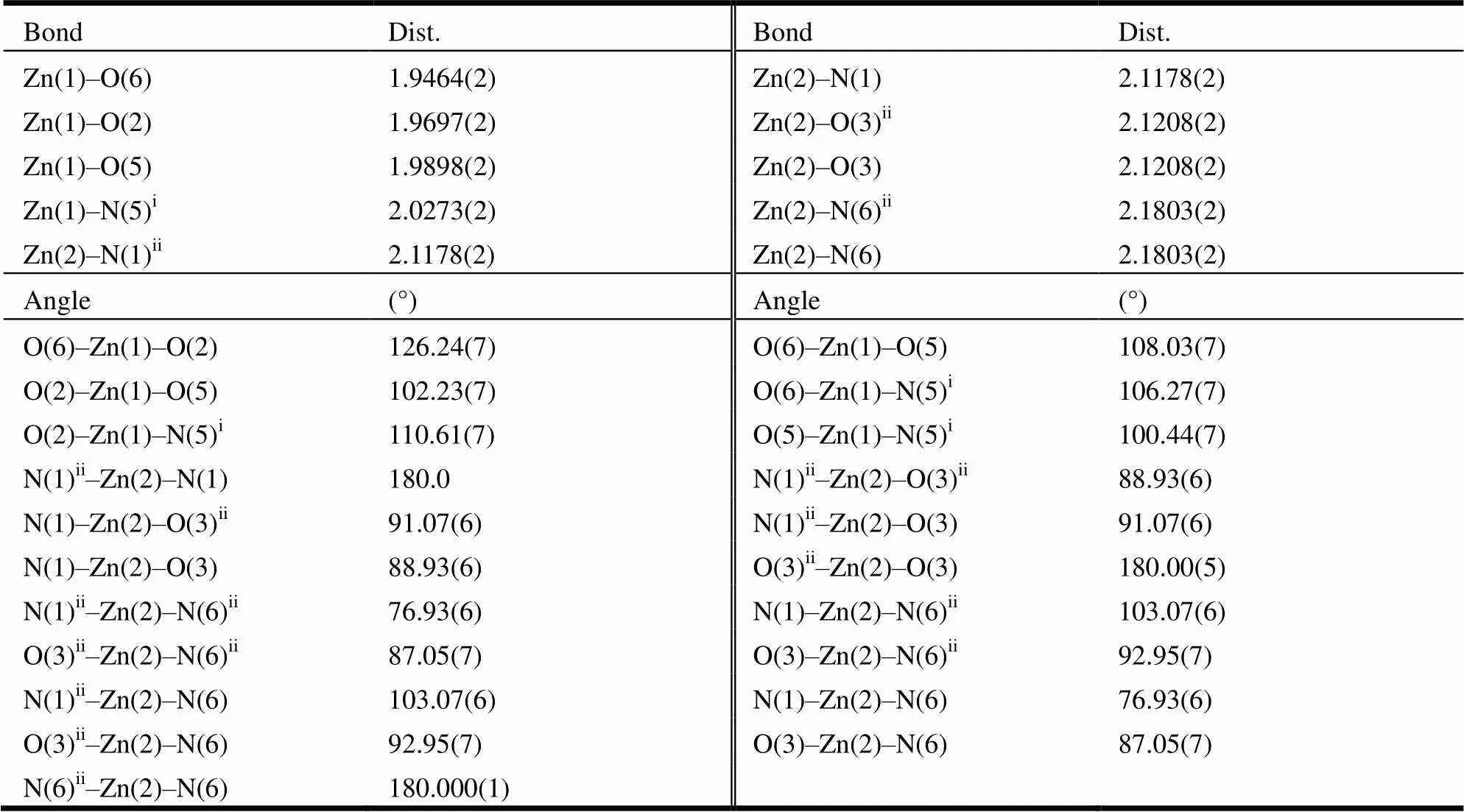
Table 1. Selected Bond Lengths and Bond Angles for Complex 1
Symmetry codes: i:+1,+1,+1; ii: –+1, –+1, –+1
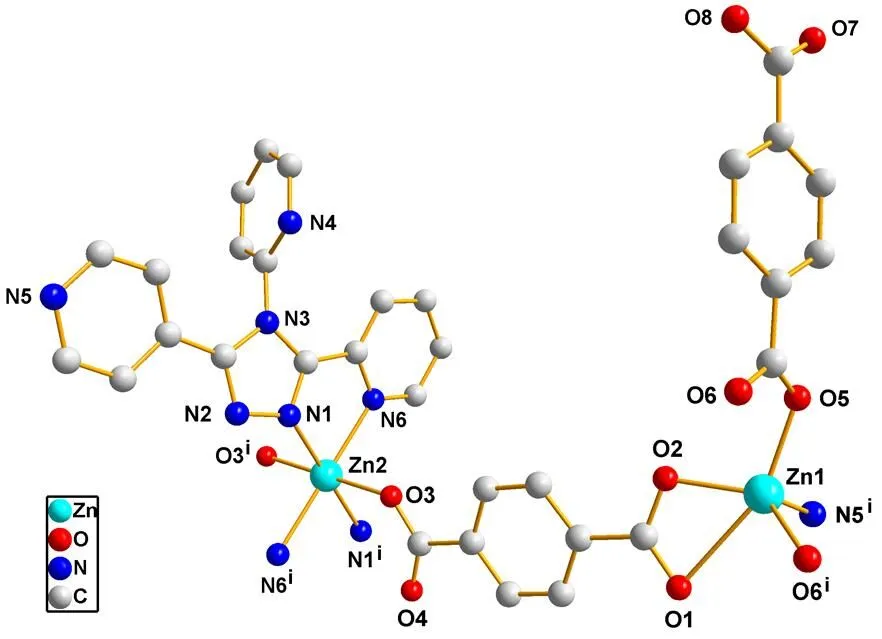
Fig. 1. Coordination environment of complex 1 (Hydrogen atoms are omitted for clarity)
In this structure, adjacent two Zn(Ⅱ) ions are chelating-bridged by chelating pyridyl-triazolyl segment and bridging 4-pyridyl groups of 2,2?,4??-tpt ligand to form a dimeric subunit; then the nei- ghboring dimeric subunits are doubly bridged by tpt2-(Scheme 2a) to generate a 1D chain extending with the Zn···Zn distance of 10.9401(3) ?. The structure is further crossed through bridging/chelating mono- dentate-bidentate (Scheme 2b) tpa2-ligands into a 2D network skeleton (Fig. 2). Furthermore, the hydrogen bonds O(8)–H(8)···O(2) (Table 2) arise out of interactions between two different H2tpa groups, expanding the 2D networks into a 3D supramolecular architecture (Fig. 3).
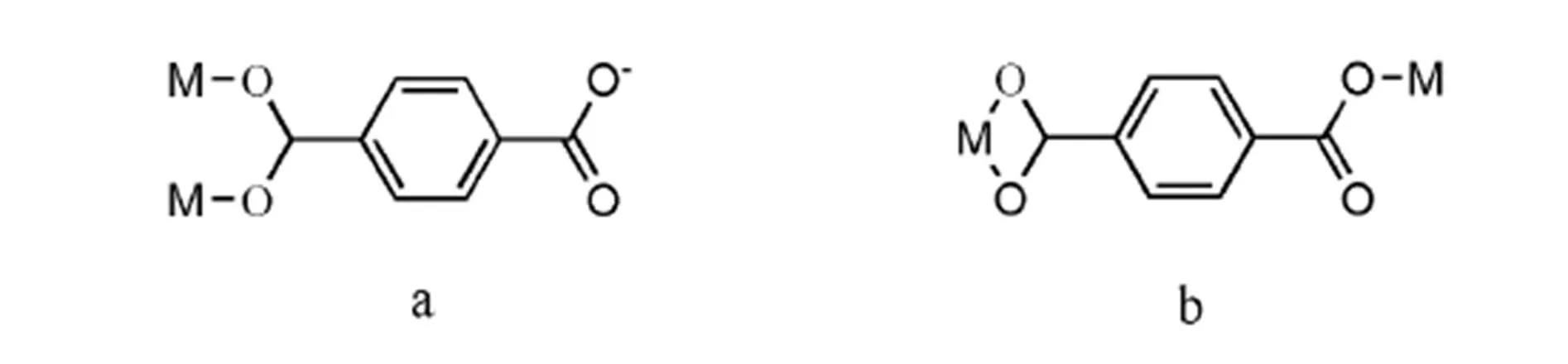
Scheme 2. Coordination modes of the terephthalic acid
Fig. 2. 2D structure diagram in complex 1
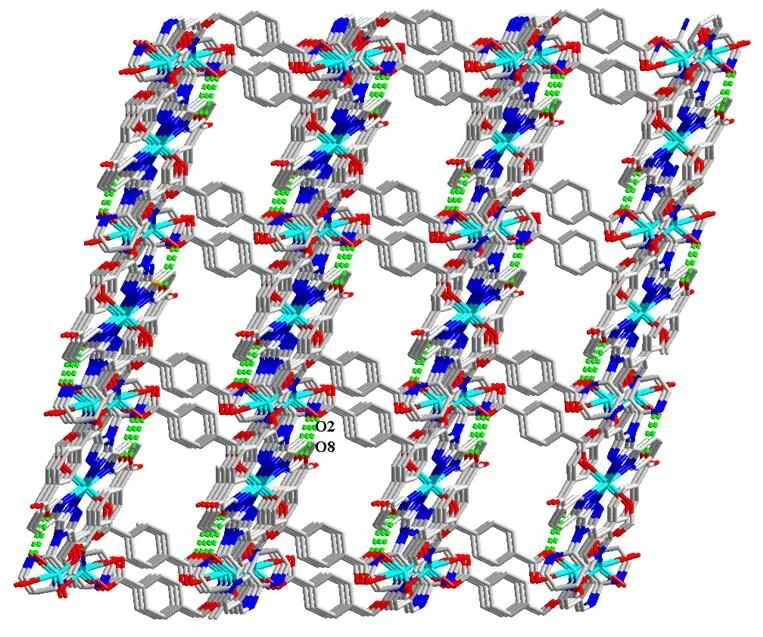
Fig. 3. A 3D framework directed by hydrogen bonding interaction (green dashed lines)

Table 2. Hydrogen Bond Lengths (?) and Bond Angles (°) for Complex 1
Symmetry code: i: –, –+2, –+2
3. 2 Thermal decomposition process of the complex
Thermogravimetric experiments were conducted to study the thermal stability of 1, which is an important parameter for metal-organic framework materials. As shown in Fig. 4, the TGA curve of 1 begins to decompose at 320 K, leading to the collapse of the framework, and releases all the ligands completely from 320 to 887 K with an exothermic peak at 345 K.
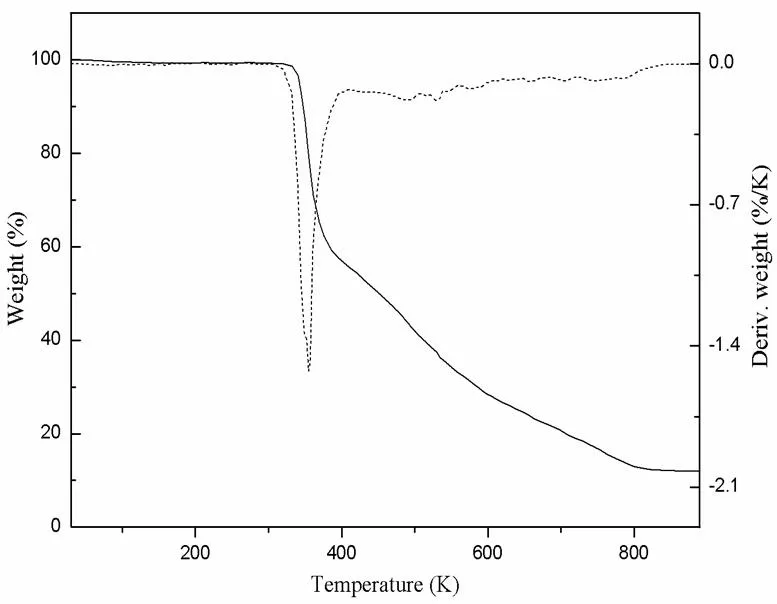
Fig. 4. TGA curves for 1
To confirm the stable framework of 1, the original and processed samples were characterized by X-ray powder diffraction (XRPD) at room temperature. As shown in Fig. 5, the processed sample resulted in a slightly broadened XRPD pattern with similar peak positions to that of the original one, which shows that the crystallinity of 1 is retained.
3. 3 Photoluminescent properties
Coordination compounds with10metal centers and aromatic organic tectons are generally con- sidered as potential hybrid-photoactive materials[26, 27]. The solid state photoluminescent behavior of 1 and the free ligands are investigated at room temperature (Fig. 6). Excitation at 300 nm leads to photolumi- nescencewith the emission maxima at approximately 482 nm for 1, and 490 nm for free ligand 2,2?,4??-tpt.The similar profile and location for both emission peaks reveal that the photoluminescent mechanism of 1 should be ascribed to the intraligand transitions of 2,2?,4??-tpt[28-30]. In contrast to the free ligand, a slight blue shift of 8 nmhas appeared in complex 1,which can be attributed to the metal-ligand coordination effect that will effectively reduce the energy loss[31]. The emission properties make it as potential photoactive material.
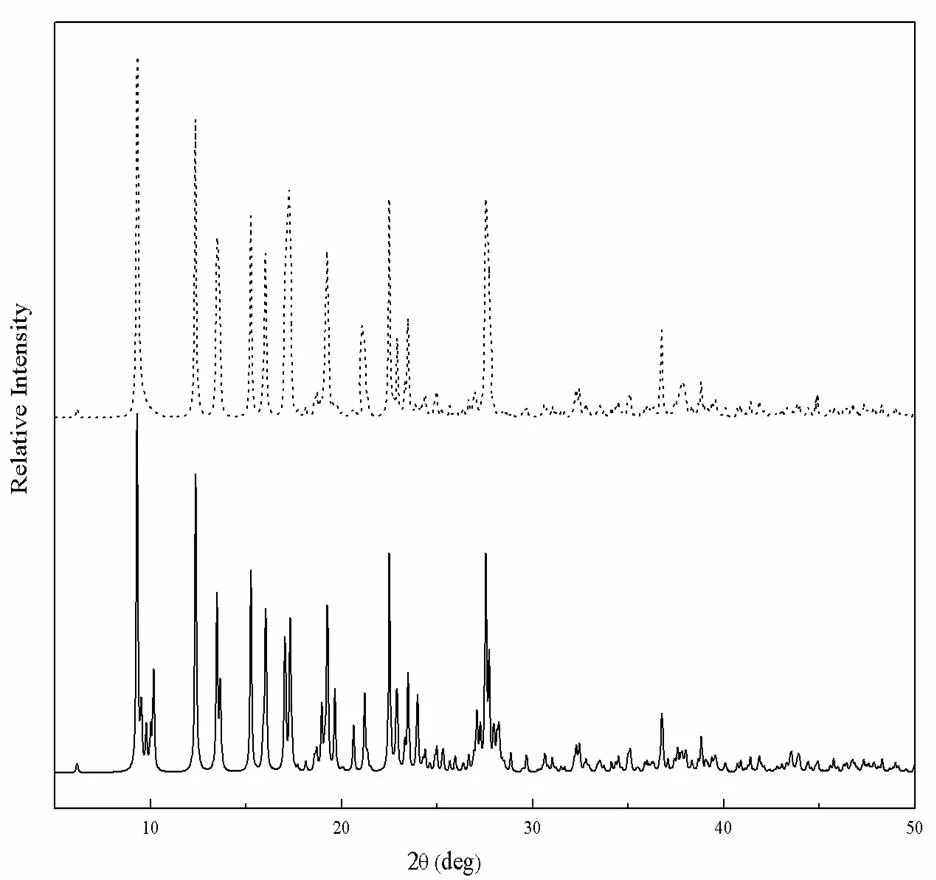
Fig. 5. Comparison of XRPD patterns of theoriginal sample and the processed sample:– , original sample; … , processed sample
Fig. 6. Photoluminescent spectrum of 1 and ligands in the solid state at room temperature
4 CONCLUSION
In summary, a new complex [Zn1.5(2,2?,4??- tpt)(tpa)2]nhas been successfully synthesized from mixed ligand systems of2,2?,4??-tpt and H2tpa. 2,2?,4??-tpt acting as a tridentate ligand connects Zn(II) atoms to form a dimeric motif, which is linked into a 2D framework by tpa2-linkers. Moreover, the title complex has good thermal stability and displays strong luminescence emission at room temperature, which indicates that the complex could be a good candidate for potential photoactive materials. Further investigation is currently being processed.
(1) Wei, G.; Shen, Y. F.; Li, Y. R.; Huang, X. C. Synthesis, crystal structure, and photoluminescent properties of ternary Cd(II)/triazolate/chloride system.. 2010, 49, 9191–9199.
(2) Feng, Q.; Yan, M. J.; Song, H. H.; Shi, S. K. Self-assembly of two chiral zinc coordination polymers based on N-benzoyl-l-glutamic acid: influence of different N-donor ancillary ligands on structural arrangement and photoluminescent properties.2014, 415, 75–80.
(3) Coban, M. B.; Erkarslan, U.; Oylumluoglu, G.; Aygun, M.; Kara, H. Hydrothermal synthesis, crystal structure and photoluminescent properties; 3D holmium(III) coordination polymer.2016, 447, 87–91.
(4) Liu, Y. Y.; Liu, B.; Yang, J.; Ma, J. F. Five new coordination polymers constructed from 1,4-bis(1H-imidazol-1-yl)butane and different carboxylates: syntheses, structures and photoluminescence.2013, 56, 96–101.
(5) Liu, B.; Feng, H. J.; Zhang, Z. H.; Xu, L.; Jiao, H. A new 3D Cd-triazolate framework obtained from in situ decarboxylication of 5-amino-3-carboxyl-1,2,4-triazole.2015, 1098, 240–245.
(6) Liu, J.; Zhang, H. B.; Tan, Y. X.; Wang, F.; Kang, Y.; Zhang, J. Structural diversity and photoluminescent properties of zinc benzotriazole-5-carboxylate coordination polymers.2014, 53, 1500–1506.
(7) Zhao, J.; Wang, X. L.; Shi, X.; Yang, Q. H.; Li, C. Synthesis, structure, and photoluminescent properties of metal-organic coordination polymers assembled with bithiophenedicarboxylic acid.2011, 3198–3205.
(8) Wang, J.; Lin, Z. J.; Ou, Y. C.; Yang, N. L.; Zhang, Y. H.; Tong, M. L. Hydrothermal synthesis, structures, and photoluminescent properties of benzenepentacarboxylate bridged networks incorporating zinc(II)?hydroxide clusters or zinc(II)-carboxylate layers.2008, 47, 190–199.
(9) Li, B.; Chen, S. P.; Xie, G.; Yang, Q.; Gao, S. L. Benzenedicarboxylate-modulated zinc(II)-3,5-bis(3-pyridyl)-1H-1,2,4-triazole frameworks: syntheses, structures and luminescent properties.. 2014, 67, 2028–2038.
(10) Li, C. P.; Chen, J.; Guo, W.; Du, M. Anion-directed assembly and crystal trans-formation of Ag(I) coordination polymers with a versatile tripyridyltriazole ligand 3,4-bis(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole.2015, 223, 95–103.
(11) Guo, W.; Yang, Y. Y.; Du, M. Anion-directed assembly of two fluorescent Cd(II) coordination polymers with a versatile multidentate N-donor building block 3-(2-pyridyl)-4,5-bis(4-pyridyl)-1,2,4-triazole.2010, 13, 863–866.
(12) Yang, Y. Y.; Guo, W.; Du, M. Solvent-controlled assemblies, structures, and properties of two Hg(II) coordination polymers with a multidentate N-donor tecton 3,4-bis(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole.2010, 13, 1195–1198.
(13) Wang, H. H.; Shi, W. J.; Hou, L.; Li, G. P.; Zhu, Z. H.; Wang, Y. Y. A cationic MOF with high uptake and selectivity for CO2due to multiple CO2-philic Sites.2015, 21, 16525–16531.
(14) Ahmad, M.; Hassan, H. M.; Ali, M.; Peter, M. Sonochemical synthesis and characterization of new one-dimensional manganese(II) coordination polymer nanostructures.. 2015, 24, 140–145.
(15) Wang, X.; Guo, Y. M. Diverse structures and physicochemical properties of four zinc-tripyridyltriazole coordination polymers regulated by counter-ions.. 2016, 69, 33–40.
(16) Guo, J. H.; Guo, W.; Wang, X.; Du, M. Distinct 1D Cd(II) coordination polymers constructed by three isomeric tripyridyltriazole building blocks and thiocyanate anions.. 2012, 22, 77–81.
(17) Li, B.; Shen, D.; Chen, X. Y.; Li, T.; Ren, J. L.; Hu, Q. L.; Liu, W. Y. A new 1-D energetic complex [Cu(2,3?-bpt)2·H2O]n: synthesis, structure, and catalytic thermal decomposition for ammonium perchlorate.2014, 67, 2028–2038.
(18) Jin, X. D.; Li, B.; Gao, H.; Zhang, X.; Liu, W. Y. Synthesis, crystal structure, fluorescent property and DFT calculations of a new Zn(II) complex based on 3-(2-pyridyl)-5-(4-pyridyl)-1H-1,2,4-triazole.2016, 7, 1129–1136.
(19) Ye, B. H.; Tong, M. L.; Chen, X. M. Metal-organic molecular architectures with 2,2-bipyridyl-like and carboxylate ligands.. 2005, 249, 545–565.
(20) Yan, L.; Liu, W. Synthesis, crystal structure and luminescence study of a new two-dimensional Mn(II) coordination polymer.. 2016, 11, 1770–1776.
(21) Marmier, M.; Wise, M. D.; Holstein, J. J.; Pattison, P.; Schenk, K.; Solari, E.; Scopelliti, R.; Severin, K. Carboxylic acid functionalized clathrochelate complexes: large, robust, and easy-to-access metalloligands.2016, 55, 4006–4015.
(22) Chen, F.; Wang, C. Z.; Li, Z. J.; Lan, J. H.; Ji, Y. Q.; Chai, Z. F. New three-fold interpenetrated uranyl organic framework constructed by terephthalic acid and imidazole derivative.2015, 54, 3829–3834.
(23) Dong, W. K.; Ma, J. C.; Zhu, L. C.; Sun, Y. X.; Akogun, S. F.; Zhang, Y. A series of heteromultinuclear zinc(II)?lanthanide(III) complexes based on 3-MeOsalamo: syntheses, structural characterizations, and luminescent properties.2016, 16, 6903–6914.
(24) Li, B.; Han, J.; Yang, Q. F.; Tian, X. Y.; Chen, X. Y. A new energetic complex [Co(2,4,3-tpt)2(H2O)2]·2NO3: synthesis, structure, and catalytic thermal decomposition for ammonium perchlorate.2015, 641, 2371–2375.
(25) Klingele, M. H.; Brooker, S. From N-substituted thioamides to symmetrical and unsymmetrical 3,4,5-trisubstituted 4H-1,2,4-triazoles: synthesis and characterization of new chelating ligands.2004, 16, 3422–3434.
(26) Li, T.; Meng, Q. F.; Li, B.; Jin, X. D.; Gao, H.; Chen, X. Y.; Song, W. M.; Liu, W. Y. Assembly of a new fluorescent Cd(II) coordination compound with a versatile multidentate N-donor building block 3-(2-pyridyl)-5-(3?-pyridyl)-1,2,4-triazole.. 2015, 10, 1525–1532.
(27) Du, L. Y.; Shi, W. J.; Hou, L.; Wang, Y. Y.; Shi, Q. Z.; Zhu, Z. H. Solvent or temperature induced diverse coordination polymers of silver(I) sulfate and bipyrazole systems: syntheses, crystal structures, luminescence, and sorption properties.. 2013, 52, 14018?14027.
(28) Li, G. P.; Liu, G.; Li, Y. Z.; Hou, L.; Wang, Y. Y.; Zhu, Z. H. Uncommon pyrazoyl-carboxyl bifunctional ligand-based microporous lanthanide systems: sorption and luminescent sensing properties.2016, 55, 3952?3959.
(29) Liu, B.; Wu, W. P.; Hou, L.; Wang, Y. Y. Four uncommon nanocage-based Ln-MOFs: highly selective luminescent sensing for Cu2+ions and selective CO2capture.2014, 50, 8731?8734.
(30) Yang, Y. Y.; Guo, W.; Du, M. Solvent-controlled assemblies, structures, and properties of two Hg(II) coordination polymers with a multidentate N-donor tecton 3,4-bis(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole.. 2010, 13, 1195?1198.
(31) Allendorf, M. D.; Bauer, C. A.; Bhakta, R. K.; Houk, R. J. T. Luminescent metal-organic frameworks.. 2009,38, 1330?1352.
10 May 2017;
31 August 2017 (CCDC 1543223)
10.14102/j.cnki.0254-5861.2011-1717
① Supported by the Natural Science Foundation of Ningxia (No. NZ15006)and theResearch Project of Ningxia Colleges and Universities (No.NGY2017004)
②. E-mail: nxdaxue@126.com
- 結(jié)構(gòu)化學(xué)的其它文章
- Transitional Area of Ce4+ to Ce3+ in SmxCayCe1-x-yO2-δ with Various Doping and Oxygen Vacancy Concentrations: A GGA + U Study①
- A New Dinuclear Zinc Polymer Based on 3-Methoxy-2-hydroxybenzaldehyde:Synthesis, Structure, Spectral Characterization and Hirshfeld Surface Analysis①
- Fabrication of WO3/TiO2 Heterostructures for Efficiently Photocatalytic Gaseous Hydrocarbons Degradation: Origin of Photoactivity and Revisit the Role of WO3 Decoration①
- Two Copper Complexes Based on Pyrazole- 3-carboxylic Acid as Heterogeneous Catalysts for Highly Selective Oxidation of Alkylbenzenes①
- Synthesis, Crystal Structure and Cytotoxic Activities of Oxazolidin-2-one Derivatives①
- Crystal Structures, Thermal Behaviors and Biological Activities of Acylhydrazone Compounds Containing Pyrazine Rings and Halogen Atoms①

