Towards frequency adaptation for delayed feedback deep brain stimulations
In neurodegenerative disorders such as Parkinson’s disease(PD), deep brain stimulation (DBS) is a desirable approach when the medication is less effective for treating the symptoms. DBS incorporates transferring electrical pulses to a specific tissue of the central nervous system, obtaining therapeutic results by modulating the neuronal activity of that region. DBS has certain advantages such as reversibility and adjustability features over medication, since the neuronal firing patterns can be recorded and used to alter the parameters of the DBS signal (Benabid et al., 2009). One of the DBS indications is its ability to suppress the abnormal neuronal activity to treat symptoms like tremor, akinesia and dystonia. Although the mechanism of DBS is not fully understood, the inhibition of neurons, entrainment of bursting neurons and activation of axons has been associated with DBS therapy (Chiken and Nambu, 2016). Electric fields induced by DBS generally disrupt any abnormal information flow coming from the cortex to the basal ganglia neurons. DBS signals also increase and regularize the neuronal firing rates by direct activation of the axons of the stimulated neuron. This regularization of neuronal firing rate prohibits the oscillatory and bursting abnormalities of the basal ganglia neurons, leading to highly therapeutic results in PD. The therapeutic effects of DBS are enhanced once it is used in a closed loop paradigm. The cortical and pallidal discharge patterns of neurons are more improved by closed loop DBS rather than traditional open loop stimulations (Rosin et al., 2011). DBS is mainly targeted at subthalamic nucleus (STN) or globus pallidus externa (GPe) cells to disrupt the thalamo-cortical synchronizations seen in PD. Therefore,the local field potential (LFP) recorded from a population of the STN cells is often used as the feedback variable for DBS parametrization. Retrospective studies mainly focused on adjusting the stimulation amplitude based on the recorded LFP (Popovych et al., 2017). However, adapting the frequency of stimulation might provide superior results in desynchronizing the coupling patterns of STN-GPe. In addition, high frequency stimulation (HFS)typically used in DBS, signi ficantly increases the device battery usage. In contrast, adapting the frequency of stimulation to a protocol where HFS is only used when high desynchronization is needed, can expand the battery lifespan and reduces the necessity of costly battery replacement surgeries (Lyons et al., 2004).
Neuronal synchrony:Due to the coupling dynamics of STN and GPe neurons, a synchronous burst firing is seen in the STN cells. This synchronized dynamic re flects a rhythmic activity in the STN neurons, which is observable from the LFP recordings and can be used to adjust the stimulation parameters. LFP or the power spectral density of the LFP signal as the control variables are correlated with tremor and alteration of motor symptoms in PD. Moreover, in flections of LFP by the DBS signal are recordable from the same DBS electrode (Priori et al., 2013).
Closed loop protocols:There are two approaches for closed loop DBS where both can optimize the stimulus signal to maintain a desired efficiency in terms of desynchronization as neuronal activities fluctuate. This is in contrast with open loop stimulation where a fixed HFS pulse train is applied to a target within the basal ganglia and in some cases it causes tissue damage rather than alleviating the symptoms. The first approach for closed loop DBS defines a relationship between the measured output and the input stimulus. Since the stimulus is a function of the output (LFP) recordings only, other parameters such as the global interaction of cells with other regions of the brain are neglected.However, in delayed feedback closed loop DBS methods, the input stimulus is updated after the output recordings were put in a decision state. The decision state is where we de fine how to adjust the input stimuli according to the measured LFP signal for better therapeutic results. In this state, often one or a couple of parameters of the input stimulus are modi fied considering more general features from the LFP signal such as power density of the recorded output and the oscillation frequencies.
Delayed feedback:To date, most of the delayed feedback algorithms focus on updating the amplitude of the stimulation signal according to the measured LFP. It has been shown that the power spectral density of the LFP signal can be used in a phase response curve (PRC) measure in order to deliver the stimulus signal at optimum frequencies (Holt et al., 2016). In this method, using the subthreshold amplitudes for stimulation provided more compelling reduction of pathological oscillations. However, stimulation with a burst of subthreshold amplitude increases the amount of energy consumed by the DBS device. In recent studies, the amplitude of the DBS signal was adjusted based on the damped filtered LFP signal and a gap was inserted between the phases of each DBS pulse. By this pulsatile feedback, the stimulation amplitude would have a linear relation with the filtered LFP. The advantage of this method is its ability to increase the battery lifespan while providing an adequate desynchronization (Popovych et al., 2017). Moving from adjusting the amplitude in feedback loops towards frequency adaptation might contribute to superior tradeoff between the desynchronization performance and the battery lifetime.
Frequency of stimulations:It is of great concern to somehow control the frequency of stimulation in a delayed feedback manner as various stimulus frequencies often have different therapeutic outcomes. While HFS has shown to improve the tremor and rigidity symptoms of PD, it fails in enhancing the axial symptoms such as gait dysfunction, swallowing and speech problems (Brozova et al., 2009). Hence, low frequency stimulation(LFS) is more promising for axial treatments in PD. To address both axial and appendicular symptoms of PD, we must design innovative stimulation protocols. Since high and low frequency stimulations account for treatments of different symptoms, the developing protocols must embody both LFS and HFS. Recently,a new stimulation paradigm focused on delivering fixed or random various frequencies assuming each frequency is benign for an exact symptom in PD (Jia et al., 2017). In this paradigm, LFS(60–80 Hz) is assigned for axial symptoms while HFS (> 100 Hz)is used for cardinal symptoms such as tremor and bradykinesia.The sequence of frequencies were then selected in random orders with fixed durations such as HFS-LFS-LFS-HFS. This approach might have clinical bene fits in terms of expediting abnormalities at speci fic frequencies. However, lack of a feedback control, results in drawbacks in the desynchronization efficiency.
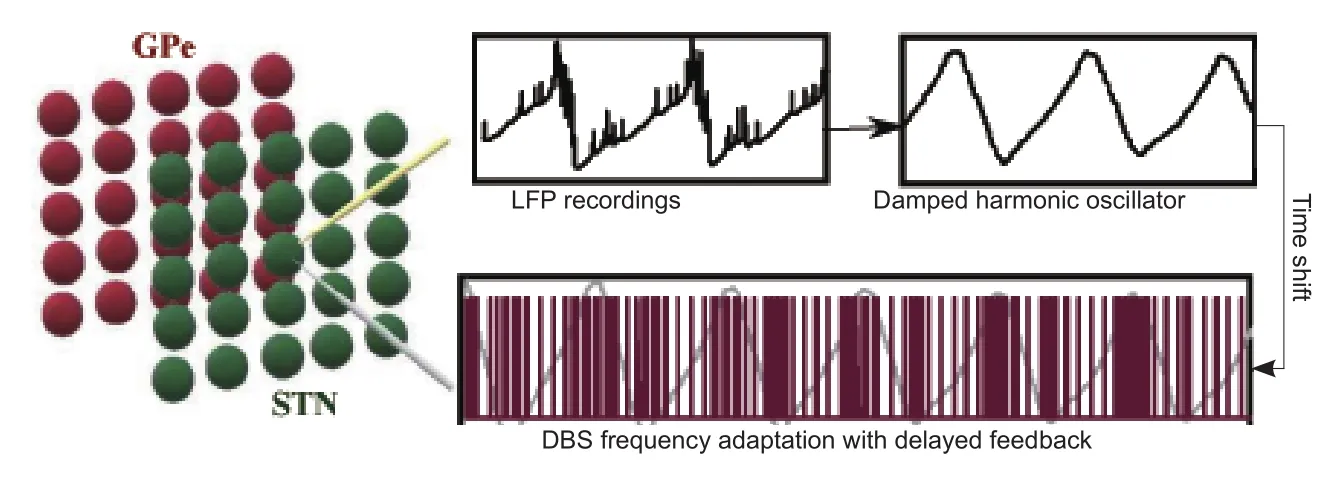
Figure 1 Frequency adaptation paradigm in a STN-GPe Oscillator.
Frequency adaptation:We propose new delayed feedback paradigms to adapt the frequency of stimulations. First of all, the control variable for adjusting the input stimulation signal must be defined to capture the synchronization of the STN neurons seen in PD. For this, we use the LFP signal filtered by a damped harmonic oscillator. This can suppress the need for a constant frequency stimulation and provide a reliable control variable for desynchronization (Tukhlina et al., 2007). The control variable must be time shifted to compensate for the instability often happening due to the delayed feedback (Figure 1). The outcome of the control variable is then used to alter the stimulation signal either by linear or nonlinear techniques. Linear delayed feedback provides great desynchronization of oscillatory activities seen in PD. However, in some cases it will increment the synchronization due to various spiking frequencies of the neuronal populations. On the other hand, nonlinear delayed feedbacks grant robust desynchronization by saturation mechanisms in order to suppress the amplitude of oscillations (Popovych et al., 2017).The nonlinear delayed feedback does not require time consuming calibration and cannot reinforce synchronization which is critical in the DBS procedure. This nonlinear transform is then applied on the control variable in order to adjust the parameters of the input stimulus signal. We suggest modifying the frequency parameters of the DBS signal in contrast to the amplitude adjustment protocols studied before (Dovzhenok et al., 2013;Popovych et al., 2017). The frequency is adapted according to the amplitude of the control variable once it is transformed through the nonlinear block. This frequency adaptation simply sends HFS when there is a boost in synchronization of the STN neurons and reduces to LFS as soon as synchronization disappears. Adaptive frequency stimulation can also perform with lower amounts of stimulus amplitude after a couple of stimulation cycles which allows for less battery consumption and lower risk of tissue damage.Figure 1depicts the entire process from LFP recordings of the STN population to de fining the feedback protocol and finally adapting the frequency of the DBS signal.
Discussion:The clinical indications for DBS therapy include controlling symptoms such as tremor, dystonia, movement disorders, depression, epilepsy, chronic pain and amputation. On the other hand contraindications of DBS therapy include dementia and uncontrolled psychiatric diseases with chances of comorbid conditions. Other contraindications and complications include hardware discomfort, loss of effect, the necessity of frequently undergoing MRI procedure, having cardiac pacemakers and risk of not showing promising results in the test stimulations. Adapting the frequency of stimulation in a delayed feedback paradigm shows promising performance in desynchronization and energy efficiency compared to amplitude adjustment techniques. There are some limitations to delayed feedback protocols in general that are worth investigation in the future. All delayed feedback algorithms focus on destabilizing the synchronous state. However,the mechanism by which the neuronal population is pushed back into synchronization is not fully addressed by delayed feedback methods. Another future direction for DBS therapy is to investigate whether the LFP power spectrum can be a biomarker for PD,and if so, if it is observable consistently for all patients. Moreover the interactions of various oscillations might direct researchers to a better understanding of the synchronization mechanism and eventually navigate the field into the development of more robust feedback and stimulation therapy methods.
A network of STN and GPe cells generates coupled oscillation due to PD. These oscillations are measured through LFPs (yellow electrode) and filtered by a damped harmonic oscillator defining the feedback control variable. The frequency of stimulation is then adapted based on the amplitude of oscillations. High and low amplitudes of the filtered and shifted LFP require HFS and LFS, respectively. The new stimulation signal with adapted frequency is then applied to the centric point of the STN population (grey electrode)for better desynchronization. The HFS-LFS mixture in DBS signal attains lower battery usage. STN: Subthalamic nucleus; GPe: globus pallidus externa; LFP: local field potential; HFS: high frequency stimulation; LFS:low frequency stimulation; PD: Parkinson’s disease.
Mohammad Daneshzand*, Miad Faezipour, Buket D. Barkana
D-BEST Lab, Departments of Computer Science and Engineering and Biomedical Engineering, University of Bridgeport, Bridgeport,CT, USA (Daneshzand M, Faezipour M)
Department of Electrical Engineering, University of Bridgeport,Bridgeport, CT, USA (Barkana BD)
orcid:0000-0002-4237-5535 (Mohammad Daneshzand)
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewers:Theodore A Henderson, Neuro-Luminance Brain Health Centers, Inc., USA; Fabricio Ferreira de Oliveira, Universidade Federal de Sao Paulo, Brazil.
Benabid AL, Chabardes S, Mitrofanis J, Pollak P (2009) Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease.Lancet Neurol 8:67-81.
Brozova H, Barnaure I, Alterman RL, Tagliati M (2009) STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 72:770.
Chiken S, Nambu A (2016) Mechanism of deep brain stimulation: inhibition, excitation, or disruption? Neuroscientist 22:313-322.
Dovzhenok A, Park C, Worth RM, Rubchinsky LL (2013) Failure of delayed feedback deep brain stimulation for intermittent pathological synchronization in Parkinson’s disease. PLoS One 8:e58264.
Holt AB, Wilson D, Shinn M, Moehlis J, Netoff TI (2016) Phasic burst stimulation: a closed-loop approach to tuning deep brain stimulation parameters for Parkinson’s disease. PLoS Comput Biol 12:e1005011.
Jia F, Hu W, Zhang J, Wagle Shukla A, Almeida L, Meng FG, Okun MS, Li L (2017) Variable frequency stimulation of subthalamic nucleus in Parkinson’s disease: Rationale and hypothesis. Parkinsonism Relat Disord 39:27-30.
Lyons KE, Wilkinson SB, Overman J, Pahwa R (2004) Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology 63:612-616.
Popovych OV, Lysyansky B, Tass PA (2017) Closed-loop deep brain stimulation by pulsatile delayed feedback with increased gap between pulse phases. Sci Rep 7:1033.
Priori A, Foffani G, Rossi L, Marceglia S (2013) Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol 245:77-86.
Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H (2011) Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72:370-384.
Tukhlina N, Rosenblum M, Pikovsky A, Kurths J (2007) Feedback suppression of neural synchrony by vanishing stimulation. Phys Rev E Stat Nonlin Soft Matter Phys 75:011918.
- 中國神經再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Optic radiation injury in patients with aneurismal subarachnoid hemorrhage: a preliminary diffusion tensor imaging report
- Regulatory role of calpain in neuronal death
- The ROCK pathway inhibitor Y-27632 mitigates hypoxia and oxidative stress-induced injury to retinal Müller cells
- Combined acupuncture and HuangDiSan treatment affects behavior and synaptophysin levels in the hippocampus of senescence-accelerated mouse prone 8 after neural stem cell transplantation
- Endoplasmic reticulum stress transducer old astrocyte speci fically induced substance contributes to astrogliosis after spinal cord injury

