The extraction of potassium from K-feldspar ore by low temperature molten salt method☆
Zhao Wang ,Quanyou Zhang *,Ying Yao Yongzhong Jia Bingjun Xie
1 Key Laboratory of Comprehensive and Highly Ef ficient Utilization of Salt Lake Resources,Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,Xining 810008,China
2 Qinghai Engineering and Technology Research Center of Comprehensive Utilization of Salt Lake Resources,Xining 810008,China
3 University of Chinese Academy of Sciences,Beijing 100049,China
4 Mangya Xingyuan Potash Corporation Ltd.,Haixi 817500,China
1.Introduction
As is known to us,potassium(K)is one of three essential nutritional elements for plant growth and development,together with nitrogen(N)and phosphorus(P).Specifically,K plays an important role in adjusting the electrochemical potential across the cell membrane[1],and its use in crops can not only improve the absorption of water by roots,but also boost photosynthesis which is good for producing organic substances[2–4].What is more,it can increase agriculture yields and guarantee food security.Based on above,the world produces a great number of K-fertilizers every year,and most of K-fertilizers are made from water-soluble potassium resources.However,the watersoluble potassium resources in China can only reach up to 2.20%(about 2.1× 108t K2O)of that around the world[5–7].In fact,there are over 90%K-fertilizers of all over the world produced and dominated by three countries:Canada,Russia and Belarus[8].That means many countries have to rely on imports for enough K-fertilizers.According to the related literature reports[9–11],nearly 50%of the total K-fertilizers that are used for agriculture in China need to import from other countries.But on the other hand,the water-insoluble K-feldspar resources that China possesses are over 2.0×1010t K2O[12],which is extremely abundant.Therefore,in order to make full good use of large existing K-feldspar resources and relieve the current situation short of K-fertilizer,many researchers have explored all kinds of efficient ways to extract potassium from K-feldspar resources.According to the existing literature reports,there are three sorts of methods to extract potassium from K-feldspar,and they are dry processes,wet processes and microbiological methods,respectively.The dry processes are making use of different kinds of additives including a series of calcium-and sodium-containing salts to react with K-feldspar at higher temperatures according to corresponding mass ratios,which can turn the insoluble K-feldspar into water-soluble potassium salts[13],thus making potassium extraction available.Yuan et al.[14]adopted the CaCl2calcination route to extract potassium from K-feldspar,and found that the potassium extraction ratio can reach 91%when CaCl2was reacted with K-feldspar under 900°C for 41 min with a mass ratio of 1.15:1.Nayak et al.[15]chose the mixture of K-feldspar,calcium sulfate and calcium carbonate for reaction under 900°C with a mass ratio of 1:0.75:3,and the solubility of K2O was beyond 80%.Hu et al.[16]studied on the decomposition of K-feldspar with composite additive composed of CaCl2and NaCl for potassium extraction,and found that the potassium extraction ratio reached 93.65%.Obviously,the dry processes have higher energy consumption,produce more useless slags and have a strict requirement for equipment during operation.
The wet processes include hydrothermal alkaline method[17–19]and acid decomposition method[20–22],which can achieve the goal of a high potassium extraction ratio at mild conditions.Wang et al.[23]used high concentration NaOH sub-molten salt to decompose K-feldspar powder,and the result shown that the dissolution rate of potassium ion was over 98%.Meng et al.[24]employed the wet process to extract potassium by reaction among potassium feldspar,phosphorite and sulfuric acid in a hydrothermal reactor,and found that the potassium extraction ratio reached up to 74.1%.However,there are a lot of wastes produced during the wet processes,such as waste gases,waste liquids and waste residues,which can lead to a lot of challenges to the environment.
As for microbiological methods,which are making use of microorganisms thathave specialfunctions to decompose K-feldsparand achieve the aim of extracting potassium.Bhattacharya et al.[25]reported potassium extraction process from K-feldspar through potassium solubilization in the halophilic Acinetobacter soli,and found thatmaximum solubilized potassium can reach up to 68%.Undoubtedly,the microbiological methods are the cleanest way among above ways,but it will take much time and energy to develop and select a certain kind of microorganism that has a good ability to decompose K-feldspar;however,the method cannot avoid the flaws of higher cost and lower potassium extraction ratio.
Hence,in this paper,we adopted low temperature molten salt method to extractpotassium from K-feldspar,which is environmentally friendly and has lower energy consumption.The low temperature molten salt is a new kind of green solvent that has a low melting point,high boiling point,good thermal stability and no harm to the environment.Besides,it is in liquid state when the temperature is below 300°C and convenient to recycle.In particular,it has a more excellent dissolution characteristic in comparison with the other solvents,such as water and organic solvents.What is more,there are fewer reports about this method used for extracting potassium from K-feldspar.In our work,we explored the optimum reaction condition for this method and analyzed the effects of some important factors on the potassium extraction ratio.At the same time,the possible mechanism of potassium extraction for this method was also proposed.
本文使用IBM SPSS軟件對(duì)數(shù)據(jù)進(jìn)行降維處理,在進(jìn)行因子分析之前先對(duì)調(diào)研量表的數(shù)據(jù)進(jìn)行信度檢驗(yàn)。內(nèi)在信度值表示每個(gè)量表是否測(cè)量單一概念,即組成量表的題項(xiàng)之間的內(nèi)在一致性如何。如果內(nèi)在信度系數(shù)在0.8以上,則可以認(rèn)為該量表具有較高的內(nèi)在一致性。本文數(shù)據(jù)的克朗巴哈(Cronbach)系數(shù)為0.885,大于0.8,證明本文所設(shè)計(jì)的調(diào)查量表具有較高的內(nèi)在一致性,進(jìn)行內(nèi)容分析的可靠度比較高。
2.Experimental
2.1.Materials
The mixture of NaOH(14 g),NaNO3(10 g),K-feldspar(10 g)and H2O(5 g)were added into the same hydrothermal reactor for reaction under different temperatures but with the same reaction time that was 6 h.The reaction temperature was adjustable from 125 °C to 250 °C with an interval of 25°C,and the relationship between the temperature and K-extraction ratio was shown in Fig.6.From the Fig.6,the extraction ratio ofpotassium is ever-increasing,which is because the diffusion rates of reagents and products were improved when the temperature of reaction system was increased,but it cannot be neglected for the fact that the potassium extraction ratio has a rapid enhancement when the temperature is lower than 200°C,after that,the growth
3.平均每個(gè)孵化器有注冊(cè)企業(yè)44個(gè)。數(shù)據(jù)顯示,孵化器平均場(chǎng)地面積26755.08 m2,共孵化企業(yè)1108個(gè),平均每個(gè)孵化器孵化44個(gè)企業(yè)。其中,中科云智國(guó)家級(jí)科技企業(yè)孵化器場(chǎng)地面積最大,達(dá)到154000 m2,占所調(diào)查孵化器總面積的23%;蜂巢空間孵化企業(yè)數(shù)最多,達(dá)184個(gè),占所調(diào)查孵化器企業(yè)總數(shù)的16.6%。松山湖創(chuàng)新科技園孵化器的孵化效率最低,擁有50000 m2面積,在所調(diào)查的25個(gè)孵化器中,排名第5位,僅僅只有2家注冊(cè)企業(yè)。

Table 1 The chemical composition result of the K-feldspar ore
The information of other chemical regents involved in the experiments were as follows:Sodium hydroxide(NaOH,AR)was obtained from Tianjin Yongda Chemical Reagent Company Limited.Sodium nitrate(NaNO3,AR)was purchased from Xi'an Chemical Reagent Factory.Hexadecyl trimethyl ammonium bromide(CTAB,C19H42BrN,AR)and sodium tetraphenylboron(C24H20BNa,AR)were obtained from Tianjin Guangfu Fine Chemical Research Institute and Sinopharm Chemical Reagent Company Limited,respectively.In addition,there were other reagents used in the experiments,and they were deionized water,0.1 wt%bromophenol blue indicator and HCl-NaAc buffer solution with a pH of 3.3.
2.2.Methods
2.2.1.Experimental procedure
離退休黨支部書記、委員負(fù)責(zé)組織本支部的黨員活動(dòng),按時(shí)參加工作站的每季度的支委大會(huì),聽(tīng)取階段性工作安排,對(duì)黨員進(jìn)行走訪慰問(wèn),傳達(dá)支部活動(dòng)內(nèi)容,了解他們的思想和身體情況,及時(shí)掌握可能引發(fā)的不穩(wěn)定事端信息。
Based on the mass ratio of NaNO3and K-feldspar that was determined above,the mass of NaNO3and K-feldspar were both arranged to be 10 g.The mass ratio between H2O and K-feldspar was still 1:2;that was to say that the mass of H2O was 5 g,and different mass ratios between NaOH and K-feldspar were designed,respectively.In the same way,the above substances with given proportion were added into the same hydrothermal reactor for reaction,and the reaction temperature and time were set to 200°C and 6 h,separately.As shown in Fig.3,there is an ever-growing tendency for the extraction ratio of potassium when the mass ratio between NaOH and K-feldspar is inferior to 1.4,and the extraction ratio of potassium begins to reduce
2.2.2.The measurement of potassium ion content
The measurement of potassium ion content was based on sodium tetraphenylboron-quaternary ammonium salt(CTAB)volumetric method[16,26],and the specific processes were as follows.Firstly,about 10-g leaching liquid was put into a volumetric flask for dilution with a maximum volume of 250 ml,and 10 ml diluted solution from the volumetric flask was transferred into a 150-ml Erlenmeyer flask.Secondly,a drop of 0.1 wt%bromophenol blue indicator that was used for adjusting pH value of solution was added into the Erlenmeyer flask until the solution became yellow,then 10-ml HCl-NaAc buffer solution with a pH value being equal to 3.3 was placed into the Erlenmeyer flask as well.Then,1-wt%four benzene boron sodium solution with a volume of5 mlwas putinto the Erlenmeyer flask and letitstand for 10 min.After that,the solution in the Erlenmeyer flask was stirred fully after the addition of 15 drops of turpentine,and followed by the addition of two drops of0.1-wt%bromophenolblue indicator.Lastly,the solution wasgiven a titration by CTAB standard solution,and the potassium extraction ratio from the K-feldspar ore was calculated as follows.
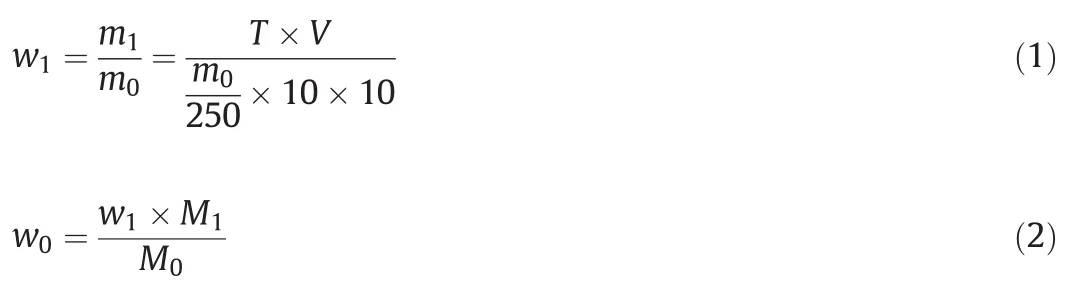
where the m0and m1represented the mass of leaching liquid that was used for titrimetric analysis and the mass of K+in the leaching liquid,respectively.T(mg?ml-1)and V(ml)represented the titer of the CTAB and titrant volume of CTAB,respectively.M0and M1denoted the mass of entire leaching liquid and the mass of K+in the K-feldspar ore,separately.w0and w1described the extraction ratio of K+from the K-feldspar ore and the mass percentage of K+in the leaching liquid that was used for titrimetric analysis,respectively.
綜上所述,腸道菌群的改變?cè)谂陨诚嚓P(guān)疾病的發(fā)生發(fā)展中發(fā)揮著重要作用,進(jìn)一步深入研究腸道菌群的作用,可以為女性生殖相關(guān)疾病的防治提供一種新的思路。
To have a better understanding of the structure and composition of the samples,the X-ray diffraction(XRD,X'Pert Pro,PANalytical,Netherlands)experiment was carried out,and the diffraction pattern was recorded from 5°to 80°with Cu Kαradiation(λ =0.154 nm)at a tube voltage of 40 kV and a tube current of 30 mA.
口語(yǔ)交際是一個(gè)生活性的概念,命題理所當(dāng)然應(yīng)該考慮到話題的生活性,并且要貼近被評(píng)價(jià)對(duì)象的生活,這樣才能準(zhǔn)確地評(píng)價(jià)學(xué)生的口語(yǔ)交際能力。本學(xué)期我班口語(yǔ)交際試題就是這樣一個(gè)貼近學(xué)生生活的題目:
2.2.4.SEM and EDS analysis
As presented in Fig.8 and Table 2,there exist three substances in K-feldspar raw ore;they are quartz,K-feldspar and albite,and their corresponding strongest peaks are observed at 2θ =26.6°,27.4°,and 27.9°,respectively.What is more,the corresponding semiquantitative compositions for them are 7%,74%and 20%,separately.In other words,the major component in raw ore is K-feldspar.On the other hand,there is only one substance named cancrinite in the leach residue of raw ore after reaction.According to the chemical formula of cancrinite,there exists no potassium element in it,which illustrates that most of potassium in K-feldspar is extracted from K-feldspar raw ore.In fact,there exists a possibility that very little potassium cannot be detected by the XRD.Therefore,the above results demonstrate that the low temperature molten salt method is practicable for extracting potassium from K-feldspar ore.
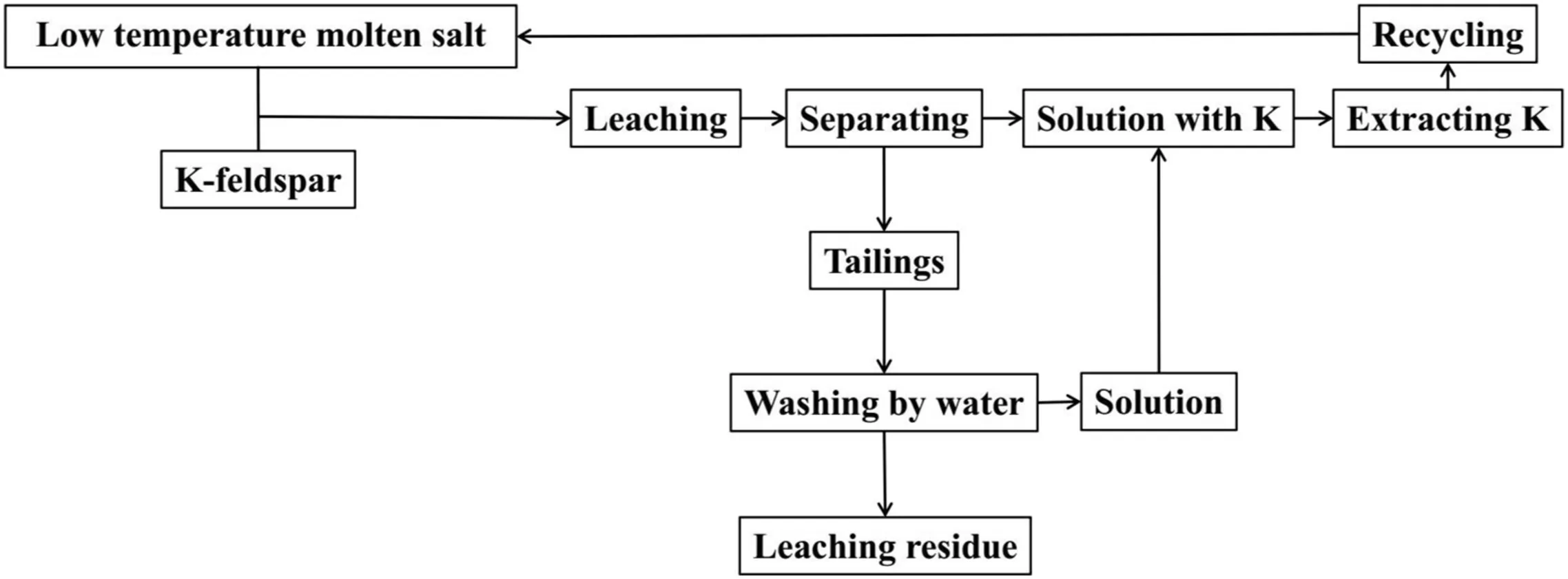
Fig.1.The flow chart of experimental procedure.
3.Results and Discussion
3.1.The effect of NaNO3/K-feldspar mass ratio on K-extraction ratio
The mass of K-feldspar ore powders was set a fixed level(10 g)for convenience of following experiments.The mass ratio of NaOH and K-feldspar was set as 1:1,and the mass ratio between H2O and K-feldspar was set as 1:2.In addition,a series of mass ratios of NaNO3and K-feldspar were prepared,separately.The above materials with a certain proportion were put into a hydrothermal reactor with a maximum volume of 50 ml for reaction 6 h under the temperature of 200°C.Seen from Fig.2,with the increase of mass ratio between NaNO3and K-feldspar,the extraction ratio of potassium shows a rising trend,which can be explained by the law of mass action that the rate of chemical reaction is proportional to the concentration of reactant.So,when the concentration was increased at initial stage,the rate of chemical reaction was improved,which boosted the potassium extraction ratio,and there is a highest value for extraction ratio of potassium when the mass ratio of NaNO3and K-feldspar is equal to 1:1.However,the potassium extraction ratio presents a downward trend when the mass ratio of NaNO3and K-feldspar is over 1:1,and it can be explained by the fact that a crowded liquid environment can affect the process of mass transfer;when the liquid/solid ratio decreased,the viscosity of the reaction system was enhanced,which hindered the previous process of mass transfer of reagents,thus making the reaction rate slower and the potassium extraction ratio decrease.Therefore,according to the above results,the mass ratio of NaNO3and K-feldspar was determined to be 1:1 for the following experiments.
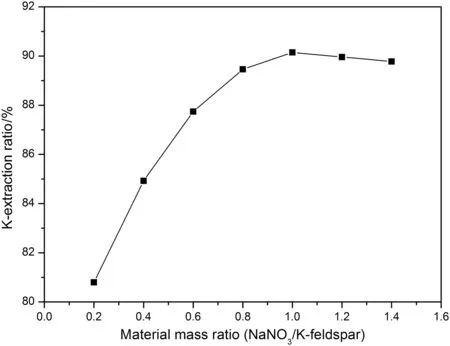
Fig.2.The effect of NaNO3/K-feldspar mass ratio on K-extraction ratio.
3.2.The effect of NaOH/K-feldspar mass ratio on K-extraction ratio
The specific experimental procedure was shown in Fig.1.Firstly,proper mass of NaOH,NaNO3,deionized water and ore powders of K-feldspar were measured and mixed uniformly in the hydrothermal reactor with a volume of 50 ml.Secondly,the hydrothermal reactor was put into dry oven for reaction with certain temperature and time.Furthermore,the sample after reaction was transformed into a 150-ml baker;then,100-ml deionized water was put into the baker to form homogeneous solution;after that,the baker was placed into a supersonic cleaner(KQ-300B,Kunshan Ultrasonic Instruments Company Limited,China)for 1-hour leaching.Lastly,the solution after ultrasonic treatment was filtered,and the separated filter cake was fully washed by deionized water until there was no soluble salt with K+;then,all filtrates including the washing liquid were used for an analysis experiment of K+.
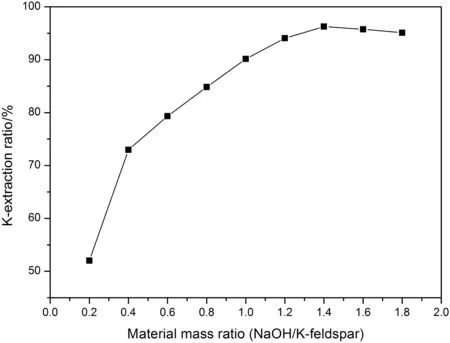
Fig.3.The effect of NaOH/K-feldspar mass ratio on K-extraction ratio.
The SEM images of the K-feldspar raw ore and the leach residue are presented in Fig.9.It is clear that the K-feldspar raw ore has a compact structure,while the leach residue after reaction is composed of a great number of tiny particles that have a diameter scope ranging from 2 μm to 5 μm.
2.2.3.XRD analysis
3.3.The effect of H2O/K-feldspar mass ratio on K-extraction ratio
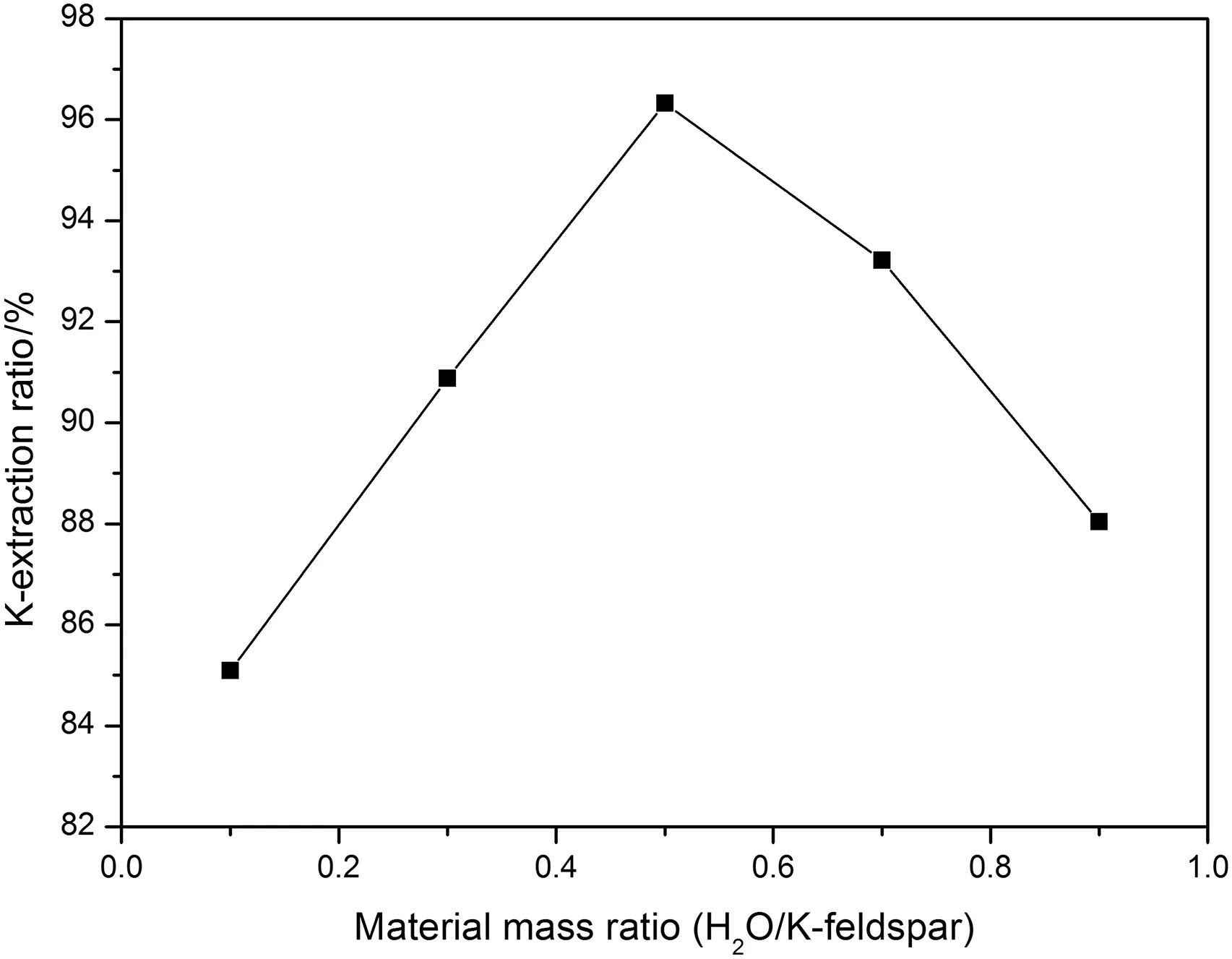
Fig.4.The effect of H2O/K-feldspar mass ratio on K-extraction ratio.
On the basis of above results,the mass ratio among NaOH,NaNO3and K-feldspar was set 1.4:1:1,so the corresponding mass of them were appointed 14 g,10 g,10 g,respectively.The mass ratios of H2O and K-feldspar were adjustable from 0.1 to 0.9 with an interval of 0.2.The above materials were mixed in the same hydrothermal reactor for reaction,and the reaction temperature and time were set to 200°C and 6 h,separately.From Fig.4,the extraction ratio of potassium begins increasing when the mass ratio of H2O and K-feldspar is within 0.5,and then decreases when the mass ratio of them is beyond 0.5.That is because a proper amount of water can guarantee a lasting reaction environment,with the increase of water,the ions concentration was rising atthe beginning stage,butlater the ion concentration gotreduced because ofdilution effect.As a result,the mass ofH2Oshould be setmass to 5 g;in other words,the mass ratio among NaOH,NaNO3,K-feldspar and H2Oshould be appointed to be 1.4:1:1:0.5 forthe nextexperiments.
3.4.The effect of particle size of ore on K-extraction ratio
According to the determined mass ratio among NaOH,NaNO3,K-feldspar and H2O,the corresponding mass of them were 14 g,10 g,10 g and 5 g,respectively.In order to investigate the effect of particle size on the extraction ratio of potassium,a series of K-feldspar ore powders with different particle size were selected and collected,and the particle size was ranging from 150 μm to 61 μm.The above substances with the above appointed proportion were put into the same hydrothermal reactor for reaction,and the reaction temperature and time were set to 200°C and 6 h,separately.As presented in Fig.5,the extraction rate of potassium is ever-increasing with the decrease of particle size ofthe ore,which is in accordance with the factthatthe tiny particles can provide large specific surface area that can enhance the contacting area among reagents,thus boosting mass transfer process and reaction rate;in other words,the extraction rate of potassium will be improved when the particle size of the ore powders is small enough.
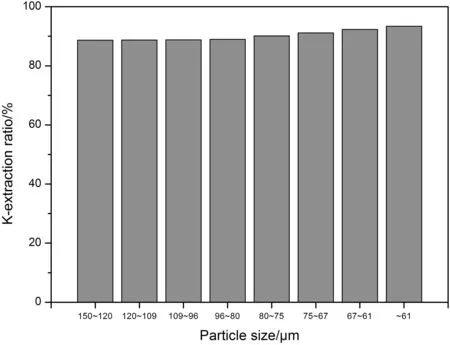
Fig.5.The effect of particle size of K-feldspar ore on K-extraction ratio.
3.5.The effect of temperature on K-extraction ratio
K-feldspar ore,derived from Wulan County,Qinghai province,China,was used as the raw material.The particle size distribution of ore powders after crushed was analyzed,and the result showed that the particle size of most ore powders was under 61 μm(240 mesh),which has a proportion of 71.18%among all ore powders.What is more,the chemical composition of the K-feldspar ore was analyzed by an inductive coupling plasma emission spectrograph(ICP-AES,IRIS Intrepid,Thermo Electron Corporation,USA)and the result was listed in Table 1.
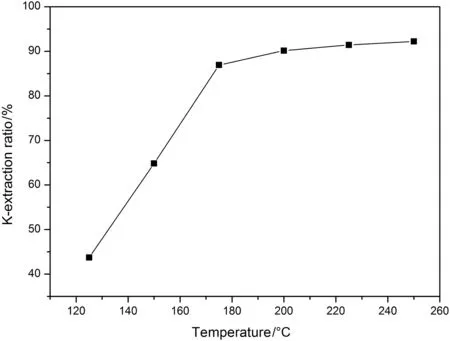
Fig.6.The effect of temperature on K-extraction ratio.
rate of potassium extraction ratio becomes very slow.That means the potassium extraction ratio nearly keeps constant,when this fact was considered together with energy saving,the reaction temperature should be set 200°C in a practical process.
另一方面,為量更大的角度,如110度角,教師引導(dǎo)學(xué)生在另一邊重新拼一個(gè)直角,以同樣的等分方法進(jìn)行均分。

Fig.7.The effect of reaction time on K-extraction ratio.
3.6.The effect of time on K-extraction ratio
According to the above results,the mixture of NaOH(14 g),NaNO3(10 g),K-feldspar(10 g)and H2O(5 g)were added into the same hydrothermal reactor for reaction with different reaction time,but all reactions were conducted under 200°C.Seen from Fig.7,the extraction ratio of potassium is ever-growing,which can be explained by the fact thatitneeds enough time to fully contactamong reagents and to destroy the structure of K-feldspar,but it is also noticed that the potassium extraction ratio rises fast when the reaction time is below 6 h,but the enhancement rate of potassium extraction ratio becomes slower and slower when the reaction time is over 6 h.So,from the perspective of energy saving,the suitable reaction time should be appointed to be 6 h.
現(xiàn)代木結(jié)構(gòu)建筑設(shè)計(jì)應(yīng)遵循模數(shù)協(xié)調(diào)原則,建立標(biāo)準(zhǔn)化結(jié)構(gòu)體系,優(yōu)化建筑空間尺寸[13]。項(xiàng)目建筑設(shè)計(jì)未嚴(yán)格遵循選材的模數(shù)要求,在項(xiàng)目圍護(hù)體系制作過(guò)程中,材料出現(xiàn)多次裁剪,造成了一定的浪費(fèi)。通過(guò)項(xiàng)目實(shí)踐深切體會(huì)到,模數(shù)化是建筑工業(yè)化的基礎(chǔ),實(shí)現(xiàn)預(yù)制構(gòu)件和內(nèi)裝部品的標(biāo)準(zhǔn)化、系列化和通用化[9]13,有利于組織生產(chǎn)、提高效率、降低成本。
In conclusion,taking all above results of condition experiments into consideration,the optimum reaction conditions for the low temperature molten salt method are as follows:mass ratio of NaNO3/K-feldspar 1:1,NaOH/K-feldspar 1.4:1,H2O/K-feldspar 0.5:1,particle size under 61 μm(240 mesh),reaction temperature of 200 °C,and reaction time of 6 h.
沈湖與河渠連接方案:規(guī)劃擴(kuò)挖沈湖處大治河、柴米河與蛇家壩干渠平行走向,三河共用兩堤。大治河、柴米河與沈湖相連通,過(guò)沈湖后兩條河道變?yōu)橐粭l向下游排水,在沈湖上游蛇家壩干渠處新建一節(jié)制閘,向沈湖補(bǔ)水。該方案基本不打亂原有灌排體系,實(shí)施矛盾小,且隨著雨污分流工程的實(shí)施,沈湖水質(zhì)將得到保證。
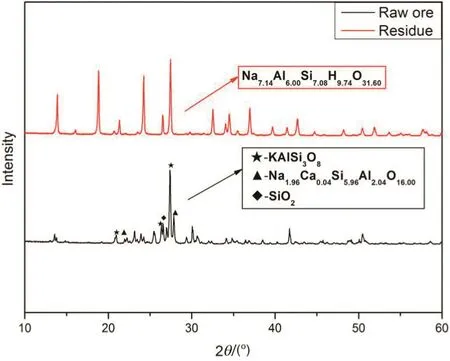
Fig.8.XRD patterns of K-feldspar raw ore and leach residue after reaction.
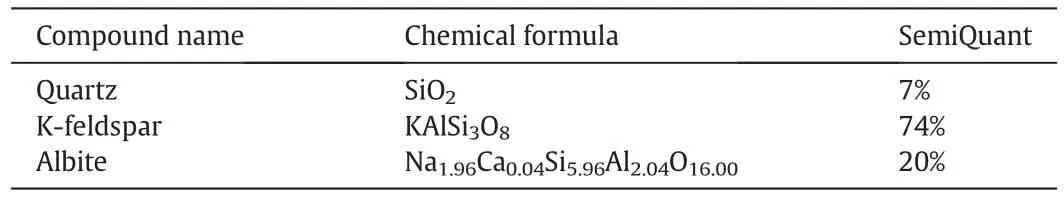
Table 2 The semiquantitative composition of K-feldspar raw ore
3.7.Crystal structure analysis of the raw ore powders and the residue after reaction
In order to investigate the changes of morphology and element content of samples,the field emission scanning electron microscopy(SEM,SU8010,Hitachi Corporation,Japan)and energy disperse spectroscopy(EDS,X-MAXN,OIMS,UK)were performed,respectively.
3.8.The mechanism of K-extraction based on the low temperature molten salt
with the further enhancement for the mass ratio between NaOH and K-feldspar.In conclusion,the mass ratio between NaOH and K-feldspar should be set 1.4:1 for the subsequent experiments.
Combining with the results of EDS tests that are shown in Figs.10 and 11,the content of sodium element on the surface of the K-feldspar raw ore is obviously lower than that of the leach residue after reaction,but the change of content of potassium element is just opposite to that of sodium element,which indicates that there exist an ion exchange between sodium and potassium during the reaction.As presented in Fig.12,the potassium ion lies in the interspace among Si-O or Al-O tetrahedron.The crush of ore reduced the particle size of the ore,which enhanced the amorphization and lattice distortion of the ore[27];in addition,with the induction of molten salt consisted of sodium hydroxide and sodium nitrate,the enhanced contact area because of liquid environment and the function of driving force that reaction temperature provided,all of these factors improved the reactivity of K-feldspar.With the reaction going on,the tetrahedralnetwork structure of Si(Al)-O was gradually destroyed,and the potassium ions were released fromthe structure.In orderto maintain electricalneutrality,the sodium ions begun to fill the position of potassium ions,which was regarded as an ion exchange process.Therefore,the potassium ions in K-feldspar crystal skeleton were exchanged with sodium ions supplied by the molten salt to form cancrinite as presented in XRD patterns.
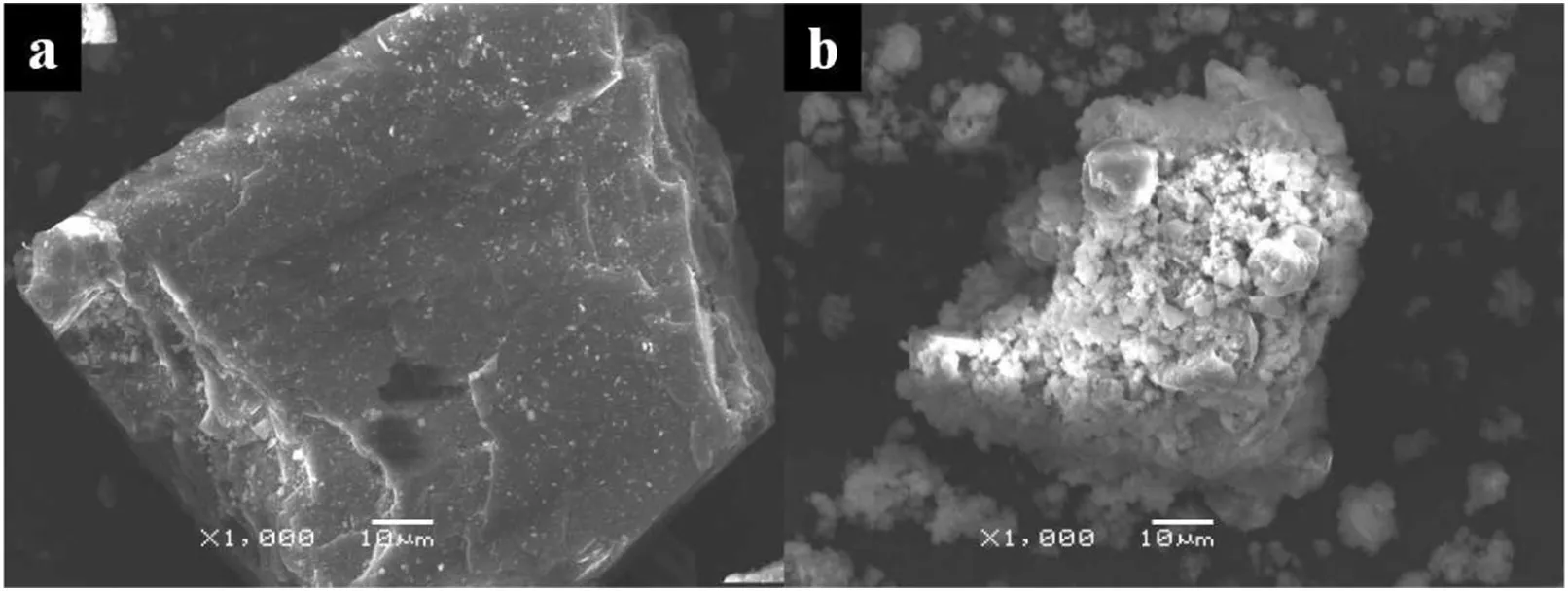
Fig.9.SEM images of(a)K-feldspar raw ore and(b)leach residue after reaction.
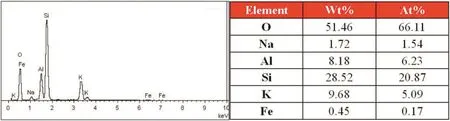
Fig.10.EDS graphs of the K-feldspar raw ore.
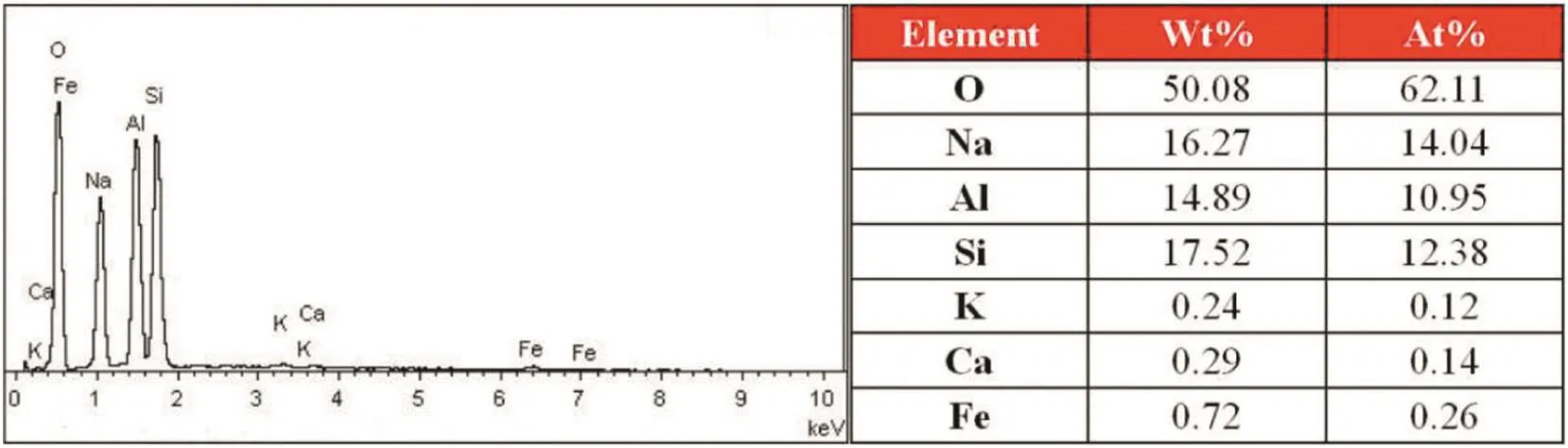
Fig.11.EDS graphs of the leach residue after reaction.
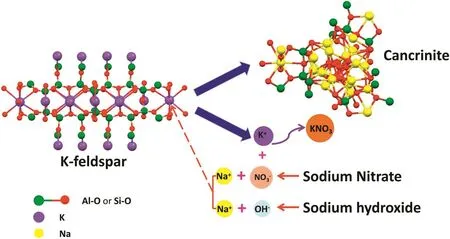
Fig.12.The schematic mechanism of potassium extraction.
4.Conclusions
In this paper,the process of extracting potassium from K-feldspar ore based on low temperature molten salt method was studied systematically.The factors that affect the extraction of potassium were investigated detailedly,and they were mass ratio of NaNO3/K-feldspar,NaOH/K-feldspar and H2O/K-feldspar,particle size ofK-feldsparore,temperature and time.Besides,the optimum condition for this process was determined according to a series of condition experiments,and it was as follows:mass ratio of NaNO3/K-feldspar 1:1,NaOH/K-feldspar 1.4:1,H2O/K-feldspar0.5:1,particle size under61μm(240 mesh),reaction temperature of200°C,and reaction time of6 h.On the other hand,the mechanism of K-extraction for this process was proposed and taken as ion exchange reaction between sodium ion and potassium ion.What was more,the extraction ratio of potassium can reach up to 96.25%under above conditions.Therefore,the low temperature molten salt method is a practicable way for extracting potassium from K-feldspar ore.
References
[1]D.Ciceri,D.A.C.Manning,A.Allanore,Historical and technical developments of potassium resources,Sci.Total Environ.502(2015)590–601.
[2]V.R?mheld,E.A.Kirkby,Research on potassium in agriculture:needs and prospects,Plant Soil 335(1)(2010)155–180.
[3]S.Pholsen,A.Suksri,Effects of phosphorus and potassium on growth,yield,and fodder quality of IS 23585 forage sorghum cultivar(Sorghum bicolor L.Moench),Pak.J.Biol.Sci.10(10)(2007)1604–1610.
[4]A.Tikkoo,S.S.Yadav,N.Kaushik,Effect of irrigation,nitrogen and potassium on seed yield and oil content of Jatropha curcas in coarse textured soils of northwest India,Soil Tillage Res.134(2013)142–146.
[5]R.H.Bao,Z.Y.Qi,2012 World potash supply and demand analysis,Yunnan Chem.Technol.40(6)(2013)20–24.
[6]Y.J.Li,Y.X.Sun,H.Y.Li,Z.Y.Qi,Distribution and development of potash resource in neighboring countries of China,Phosphate Compd.Fertil.28(4)(2013)12–15.
[7]The Ministry of Land and Resources Information Center,The World Mineral Resources Annual Review 2013,Geological Publishing House,Beijing,2013,259–266.
[8]X.Ma,J.Yang,H.W.Ma,C.J.Liu,Hydrothermal extraction of potassium from potassic quartz syenite and preparation of aluminum hydroxide,Int.J.Miner.Process.147(2016)10–17.
[9]Z.Y.Qi,S.Q.Duan,F.C.Liu,Z.Y.Mei,Production and supply of potash fertilizer in China in the recent years and its development forecast,Phosphate Compd.Fertil.27(6)(2012)1–3.
[10]China Inorganic Salt Industry Association Potash Branch,2012 China's Potash Industry Operation Report and Forecast in 2013,China Inorganic Salt Industry Association Potash Branch,Shanghai,2012,1–7.
[11]R.H.Bao,Z.Y.Qi,D.T.Zhou,Analysis and forecastofsupply and demand ofpotassium salt and potash fertilizer,Phosphate Compd.Fertil.28(2)(2013)1–5.
[12]S.Q.Su,J.Yang,H.W.Ma,P.Zhang,Evaluation on technology for potassium preparation from insoluble potash resources,Ind.Miner.Process.2(2014)46–51.
[13]K.Huang,X.W.Meng,G.L.Wang,Research progress of extracting potassium from potassium feldspar,Phosphate Compd.Fertil.26(5)(2011)16–19.
[14]B.Yuan,C.Li,B.Liang,L.Lü,H.R.Yue,H.Y.Sheng,L.P.Ye,H.P.Xie,Extraction of potassium from K-feldspar via the CaCl2calcination route,Chin.J.Chem.Eng.23(2015)1557–1564.
[15]R.Nayak,J.R.Rao,A.Suryanarayana,B.B.Nayak,Alkaline roasting as a route for extraction of potassium from feldspar,J.Sci.Ind.Res.56(1997)173.
[16]T.X.Hu,J.G.Yu,Experimental study on decomposition of K-feldspar with CaCl2and NaCl for extraction of potassium,Chin.J.Process.Eng.10(4)(2010)701–705.
[17]H.W.Ma,S.Q.Su,J.Yang,B.Y.Cai,M.T.Liu,W.G.Yao,H.Peng,Preparation of potassium sulfate from K-feldspar by hydrothermal alkaline method:reaction principle and process evaluation,J.Chem.Ind.Eng.65(6)(2014)2363–2371.
[18]Y.M.Nie,H.W.Ma,H.Liu,P.Zhang,M.Y.Qiu,L.Wang,Reactive mechanism of potassium feldspar dissolution under hydrothermal condition,J.Chin.Ceram.Soc.34(7)(2006)846–850.
[19]S.K.Liu,C.Han,J.M.Liu,H.Li,Experimental research on extracting potassium,silica and aluminum from potassium feldspar via hydrothermal chemical reaction,Acta Mineral.Sin.29(3)(2009)320–325.
[20]J.Shen,Summarization of comprehensive utilization of potash feldspar,Ind.Miner.Process.29(10)(2000)1–3.
[21]Q.J.Peng,The study of extracting potassium from the potash feldspar with the mixture of sulfuric acid and hydro fluoric acid,J.Jishou.Univ.17(2)(1996)62–65.
[22]L.C.Huang,X.Z.Han,Y.L.Lu,Z.B.Wang,W.Li,Study of decomposition of potassium feldspar by sulfuric acid,Anhui Chem.Ind.37(1)(2011)37–39.
[23]Y.Wang,J.H.Guo,J.F.Huang,H.D.Chen,H.M.Guan,J.L.Cao,Y.J.Tong,Decomposition of potassium feldspar by NaOH sub-molten salt method,Chin.J.Process.Eng.14(2)(2014)280–285.
[24]X.W.Meng,G.L.Wang,Study on extraction of potassium from potassium feldspar by wet process,Inorg.Chem.India 43(3)(2011)34–35.
[25]S.Bhattacharya,P.Bachani,D.Jain,S.K.Patidar,S.Mishra,Extraction of potassium from K-feldspar through potassium solubilization in the halophilic Acinetobacter soli(MTCC 5918)isolated from the experimental salt farm,Int.J.Miner.Process.152(2016)53–57.
[26]G.F.Rang,H.Z.Ma,R.Y.Meng,Y.G.Chen,Rapid determination of potassium content by sodium tetraphenylboron-quaternary ammonium salt volumetric method,J.Salt Lake Res.17(2)(2009)39–42.
[27]W.J.Shangguan,J.M.Song,H.R.Yue,S.Y.Tang,C.J.Liu,C.Li,B.Liang,H.P.Xie,An efficient milling-assisted technology for K-feldspar processing,industrial waste treatment and CO2mineralization,Chem.Eng.J.292(2016)255–263.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- Oil field produced water treatment in internal-loop airlift reactor using electrocoagulation/ flotation technique
- From pollutant to solution of wastewater pollution:Synthesis of activated carbon from textile sludge for dye adsorption
- 17α-Ethinylestradiol removal from water by magnetic ion exchange resin☆
- Transesteri fication of sun flower oil in microchannels with circular obstructions
- Decomposition behavior of CaSO4 during potassium extraction from a potash feldspar-CaSO4 binary system by calcination☆
