High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
Zhiguang Duan,Chenhui Zhu,Jingjing Shi,Daidi Fan*,Jianjun Deng,Rongzhan Fu,Rong Huang,Cuiying Fan
Shaanxi Key Laboratory of Degradable Biomedical Materials,School of Chemical Engineering,Northwest University,Taibai North Road 229,Xi'an 710069,China
Shanxi R&D Center of Biomaterials and Fermentation Engineering,School of Chemical Engineering,Northwest University,Taibai North Road 229,Xi'an 710069,China
Keywords:Ginsenoside Rb1 Ginsenoside Compound K Snailase Enzymolysis
ABSTRACT The rare ginsenoside Compound K(C-K)is attracting more attention because of its good physiological activity and urgent need.There are many pathways to obtain ginsenoside C-K,including chemical and biological methods.Among these,the conversion of PPD-type ginsenosides by enzymatic hydrolysis is a trend due to its high efficiency andmild conditions. For effectively extracting fromthe other panaxadiol saponins, the conversion process for ginsenoside C-K was investigated using snailases in this study. The univariate experimental designand response surface methodology were used to determine the optimal hydrolysis conditions for the conversion of ginsenoside Rb1 into ginsenoside C-K by snailases. The optimum conditions were as follows: pH 5.12, temperature 51 °C, ratio of snailase/substrate 0.21, and reaction time 48 h. On the basis of these parameters, the addition of 1.0mmol·L?1 ferric ionwas found to significantly improve the enzymolysis of snailases for the first time. With the above conditions, the maximumconversion rate reached 89.7%, suggesting that the process can obviously increase the yield of ginsenoside C-K. The bioassay tests indicated that the ginsenoside C-K showed anti-tumor activity in a series of tumor cell lines. Based on these results,we can conclude that the process of rare ginsenoside CK production by enzymolysis with snailase is feasible, efficient, and suitable for the industrial production and application.
1.Introduction
Ginsenosides are one of the main active ingredients of ginseng.The physiological activity of ginsenosides determines its edible and medicinal values.At present,more than 60 kinds of ginsenosides have been identified including ginsenoside Compound-K(C-K)[1].Ginsenoside C-K,not contained in native ginseng,is a degradation product of the other panaxadiol saponins like ginsenoside Rb1 in the human gut[2].
The rare ginsenoside C-K derived from Panax ginseng,is a kind of panaxadiol saponin[3].Compared to the parent ginsenosides,the metabolite ginsenoside C-K is more easily absorbed by the gut[3].To obtain the rare ginsenoside C-K,many methods of microbial or enzymatic conversion have been reported[4–6].The path of conversion is that the ginsenoside Rb1 and ginsenoside Rb2 can be hydrolyzed to ginsenoside F2 and ginsenoside C-K by intestinal bacteria[7–9].The conversion process of ginsenoside C-K from ginsenoside Rb1 and their structural formulas are shown in Fig.1.Ginsenoside C-K is capable of facilitating the apoptosis of a variety of tumor cells,such as lung cancer cells,gastric cancer cells,colorectal cancer cells,and multiple myeloma[10–12].In the heart transplant,ginsenoside C-K can increase the immune function and thus extend the survival time of transplantation. It also has the anti-inflammatory function and resistance to skin aging [10,13]. Owing to its beneficial effects in the aspects of anti-tumor, anti-aging, memory improvement, anti-inflammation and liver protection [14–17], highly efficient production and purification ofginsenoside C-K are of high importance.
The current methods for producing ginsenoside C-K are classified into chemical and biological methods. Considering that the former has many issues such as complex separation process and environmental pollution, biological conversion has received increasing attention, especially the use of some invertase [18,19]. For example, rare ginsenoside C-K can be prepared by enzymolysis of ginsenoside Rb1 by snailases, which is a mixed enzyme (including endoglucanase, exoglucanase, and β-glucosidase) and extracted from the crop and alimentary canal of the snail [20,21]. Snailase, which has a strong biological conversion ability, is able to selectively cut off the β-D-glucopyranoside bond of ginsenoside Rb1, resulting in the rare ginsenoside C-K [22]. In addition, Yu et al. investigated the conversion of rare ginsenoside C-K from ginsenoside Rb1 catalyzed by snailase immobilization onto microspheres [23].
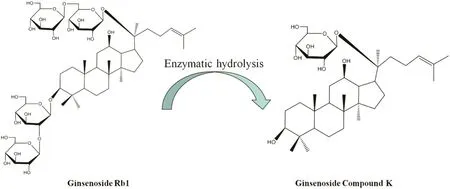
Fig.1.Illustrative conversion diagrams and structural formulas of ginsenoside Rb1 and ginsenoside C-K.
Although there are many reports about the production of ginsenoside CK, low conversion rate and yieldmay limit its large-scale production.With the aim to establish a more efficient conversion process for ginsenoside C-K, the hydrolysis of ginsenoside Rb1 catalyzed by snailase and the effects of different reaction conditions on ginsenoside C-K conversion rate were studied in this work. In order to further improve the catalytic efficiency of snailases, in this study, we investigated the influence ofmetal ions on the catalysis of snailase on the basis of optimized catalytic conditions. Thus, this study opens up a new possibility for the industrialization of ginsenoside C-K production from ginsenoside Rb1.
2.Materials and Methods
2.1.Reagents
70%ginsenoside Rb1 was purchased from Zhejiang Jinai Agricultural Biotechnology Co.Ltd.Ginsenoside C-Kwithapurity of98%was prepared byour laboratory.The snailase was obtained from Amresco/Sigma.Acetonitrile and methanolwere of HPLC grade.All theot herreagents were of an alytical grade.
2.2.Production of ginsenoside C-K from ginsenoside Rb1
100.0 mg of 70%ginsenoside Rb1 and snailase were added into 1.0 ml acetic acid–sodium acetate buffer,and centrifuged after reaction at 3000 r·min?1for 10 min.The precipitation namely transformed products containing ginsenoside C-K,were collected.
2.3.Analytical method
2.3.1.The standard curve of ginsenoside C-K
5mg of ginsenoside C-Kwasd is solved with5ml methanol to prepare store solutions with a concentration of 1 mg·ml?1.Then 200 μl,400 μl,600 μl,and 800 μl store solutions were diluted with methanol to a final volume of 1 ml,respectively.The working solutions(0.2 mg·ml?1,0.4 mg·ml?1,0.6 mg·ml?1,0.8 mg·ml?1,1.0 mg·ml?1)were analyzed by the HPLC system with acetonitrile–H2O as mobile phase at a flow rate of 1.5 ml·min?1,and detected at 203 nm.The regression equation was established from the values between the concentrations(X)of ginsenoside C-K and the peak area(Y).The regression equation is as follows:
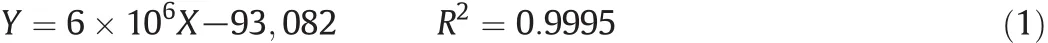
2.3.2.Calculation of conversion rate
After reaction,a certain amount of conversion products was weighed and dissolved in methanol followed by the ultraphonic treatment for 60 s.After centrifugation at 10000 r·min?1for 10 min,10 μl supernatant was injected into HPLC.The degree of conversion was calculated by the following equation:
C—mass concentration of ginsenosideC-K,V—volume of ginsenoside C-K,m—mass of ginsenoside Rb1,MW1—molecular weightof ginsenoside C-K,MW2—molecular weight of ginsenoside Rb1.
2.4.Univariate experiment for ginsenoside C-K
70% ginsenoside Rb1 and snailasewere dissolved in 1.0ml of glacial acetic acid–anhydrous sodium acetate buffer. The enzymatic hydrolysis conditions including pH value, temperature, ratio of snailase/substrate, and reaction time were tested. The effect of each factor on enzymatic hydrolysis was evaluated with the fixed other three factors. All the experiments were performed in triplicate. The final reaction products were vacuum dried and dissolved in methanol. Subsequently, the conversion rate of ginsenoside C-K was calculated to reflect the efficiency of enzymatic hydrolysis
2.5.Response surface optimization experiment design
On the basis of univariate experiments, the response surface analysis was used to optimize the preparation process of ginsenoside C-K.The temperature,pH value,and the ratio of snailase/substrate are main factors that affect the enzymatic hydrolysis.As a result,using the Box–Behnken design,the reaction pH value(X1),temperature(X2),and theratio of snailase/substrate(X3)as independent variables,were taken three levels,encoded by?1,0,and+1,respectively.The three factors and three level tests were performed with the conversion rate of ginsenoside C-K for response.The factors and levels were shown in Table 1.

Table 1 Factors and levels
2.6.The effect of metal ions on enzymatic hydrolysis
1 ml of 0.5 mmol·L?1of different kinds of metal ions(Cu2+,Ca2+,Mg2+,Fe3+,K+and Na+)wereadded tothehydrolysis system,respectively.The hydrolysis reaction was carried out under optimized conditions of reaction time,temperature,pH value of the system,and the ratio of snailase/substrate.After there action,the content of ginsenoside C-K was measured by HPLC to calculate the conversion rate.
2.7.Purification of ginsenoside C-K
The enzymatic hydrolysates were separated by preparative grade HPLC(Hanbon Sci.&Tech.).The preparation of ginsenoside C-K by HPLC used an acetonitrile(A)–water(B)gradient elution system with a C18column(320 mm×50 mm).The sample was injected into HPLC at a volume of 18 ml with a concentration of 20 mg·ml?1.The eluate wascollected at the corres ponding peak position.After vacuum distillation,the solvent was removed,and ginsenoside C-K can be obtained after freeze-dried treatment.Then the purity of freeze-dried product was calculate dusing the peak area normalization method in accordance with the detection results of HPLC.The operation conditions of HPLC are as follows:1)The operation condition of analytical HPLC:the mobile phase consisted of water(A)and acetonitrile(B)at ratios of(A:B)70:30(0–20 min),54:46(20–45 min),45:55(45–55 min),45:55(55–60 min),0:100(60–130 min),and 70:30(130–140 min)at a flow rate of 1.5 ml·min?1.Detection absorbance was 203 nm.2)The operation condition of preparative HPLC:the mobile phase consisted of water(A)and acetonitrile(B)at ratios of(A:B)50:50(0–20 min),30:70(20–25 min),and 30:70(25–50 min)at a flow rate of 60 ml·min?1.
2.8.Biological activity analysis of ginsenoside C-K
The ginsenoside C-K was dissolved in Roswell Park Memorial Institute-1640 culture media containing 0.1%DMSO with the final concentration of 0,10,20,30,40,50,and 60 μg·ml?1,respectively.Experimental cells(1 × 104/100 μl)including lung cancer(NCI-H460),human breast cancer(MCF-7),gastric cancer(SGC-7901),and colon cancer(CACO-2)cells were grown in a 96-well plate at 37°C,5%CO2for 24 h and then treated with various concentrations of ginsenoside C-K for48h.The MTT protocol was used to test cell viability as described previously[24].
2.9.Statistical analysis
All experiments were performed in triplicate.Data were analyzed in SPSS and the results were presented as means and standard deviations(SD),obtained by using Origins 8.5.A value of p<0.05 was considered statistically significant.
3.Results and Discussion
3.1.Single factor experimental results
3.1.1.Effect of pH value on enzymatic hydrolysis
The highest hydrolysis rate of ginsenoside Rb1 was obtained at the buffer pH value of 5.0 as shown in Fig.2,thus the optimal pH value for the hydrolysis of ginsenoside Rb1 was 5.0.
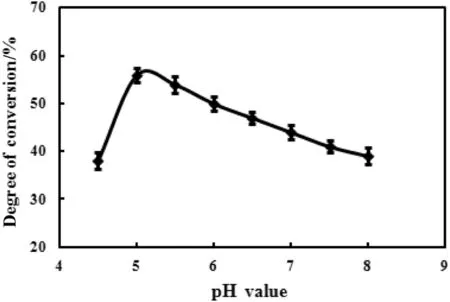
Fig.2.Effect of pH value on the enzymatic hydrolysis.Reaction was performed by adding 100 mg substrate,10 mg snailase,and 1 ml glacial acetic acid–anhydrous sodium acetate buffer(pH 4.5–8.0)at 37 °C for 24 h.
3.1.2.Effect of temperature on enzymatic hydrolysis
The effect of temperature on the conversion rate of ginsenoside Rb1 within a range of 20–80 °C was shown in Fig.3.It was found that the highest hydrolysis rate of the ginsenoside Rb1 was obtained at 50°C.Thus,the optimal temperature for the hydrolysis of ginsenoside Rb1 was 50°C.
3.1.3.Effect of snailase/substrate ratio on enzymatic hydrolysis
To investigate the effect of snailase/substrate ratio on enzymatic hydrolysis,the ratios were set to 0.01,0.1,0.2,0.4,0.6,0.8,and 1.0.As shown in Fig.4,the conversion rate of ginsenoside Rb1 gradually increased when the ratio of snailase/substrate was between 0.01 and 0.2,whereas the conversion rate was almost constant as the ratio continues to increase.It may be because the content of snailases has reached saturation at this time,and then excessive enzymes cannot improve the conversion of substrate.These results suggested that 0.2 was identified as the optimal ratio of snailase/substrate for the hydrolysis of ginsenoside Rb1.
3.1.4.Effect of reaction time on enzymatic hydrolysis
Different reaction times,12 h,24 h,36 h,48 h,60 h,and 72 h were employed,respectively,under the conditions of fixed pH value,hydrolysis reaction temperature,and ratio of snailase/substrate with the aim to investigate the Influence of reaction time on enzymatic hydrolysis.The conversion rate of ginsenoside C-K was calculated,and the results were shown in Fig.5.It indicated that the highest conversion rate was achieved at 48 h,prolonging reaction time may lead to an increase in the degradation of ginsenoside C-K.
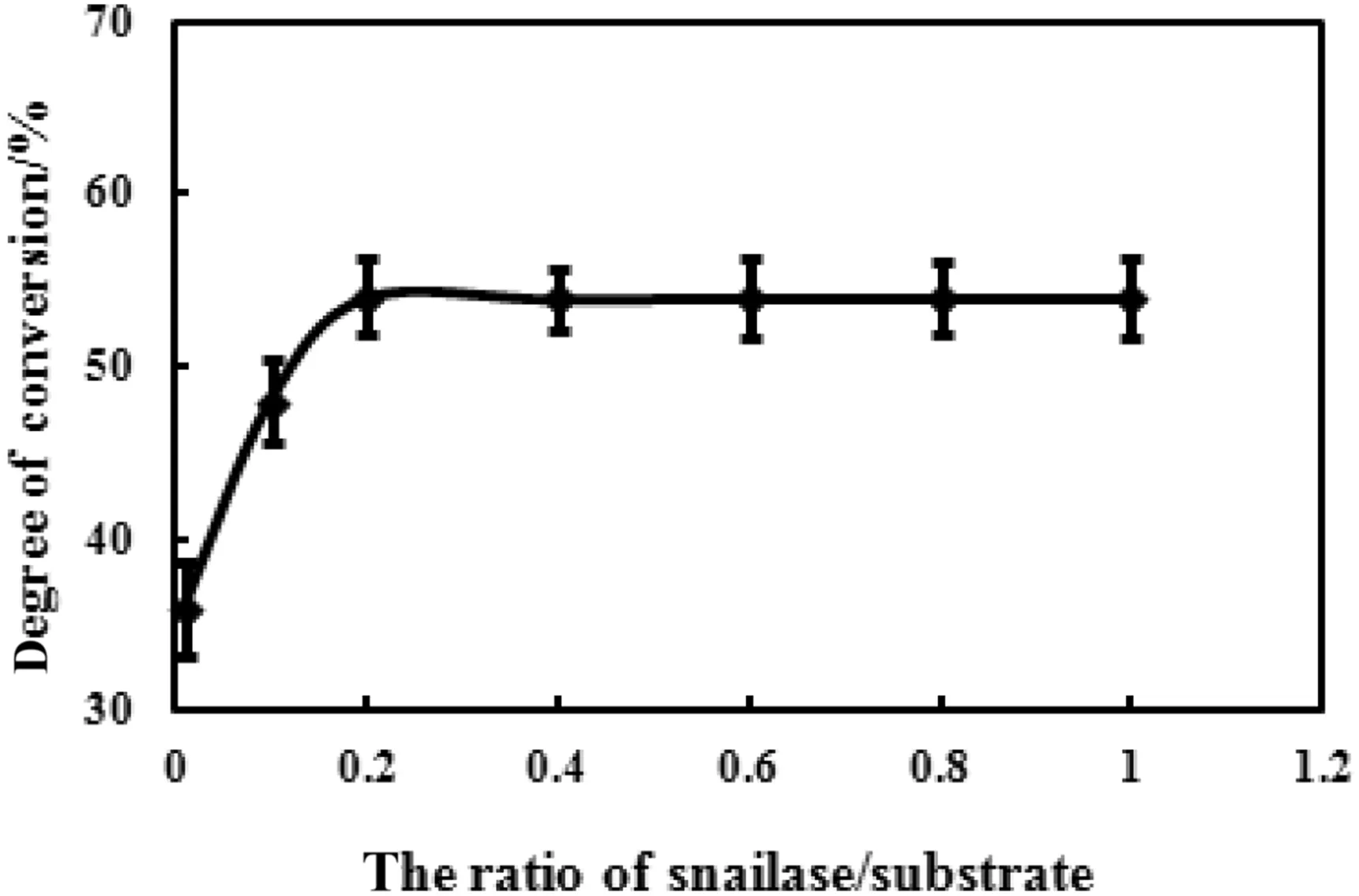
Fig.4.Effect of snailase/substrate ratio on the enzymatic hydrolysis.The snailase(1,10,20,40,60,80,100 mg)was added into reaction system containing 100 mg 70%Rb1,and 1 ml glacial acetic acid–sodium acetate anhydrous buffer(pH 6.0)at 37 °C for 24 h.

Fig.5.Effect of reaction time on the enzymatic hydrolysis.100 mg substrate,10 mg snailase,and 1 ml glacial acetic acid–sodium acetate anhydrous buffer(pH 6.0)were mixed in a 1.5 ml Eppendorf tube.Subsequently,the buffer was incubated in a water bath of 37°C at different reaction times.
3.2.Response surface analysis results
The results of the Box–Behnken design were shown in Table 2 and analyzed for response surface by Design-Expert.V8.0.6 software.Variance analysis and regression coefficient significance test results were shown in Table3.The established quadratic polynomial model has a significant difference(p<0.001),and the determination coefficient R2equals to 0.9865,indicating that the model is well fitted.From the results of Table 3,the p values of both reaction pH value(X1)and temperature(X2)were less than 0.01,demonstrating that they have a significant effect on the conversion rate of ginsenosideC-K.Thep values of X1X2,X2X3and X2X3were larger than 0.05,suggesting that the interactions between the various factors were not significant,which can also be observed from there sponse surface curve in Fig.6.The determination of optimum conditions for conversion of ginsenoside C-K by regression model were as follows:enzymolysis pH value 5.12,temperature 51.14°C,and the ratio of snailase/substrate 0.21.Thetheoretical conversion rate of ginsenoside C-K is 62.06%under these conditions.Considering the actual operation,the maximum conversion rate was 61.96%,which has a little difference compared to the theoretical value,indicating the process parameters from response surface method were of a certain practical value.

Table 2 Box–Behnken design and results
3.3.Effects of different metal ions on enzymatic hydrolysis
To further enhance the catalytic capacity of snailase,the hydrolysis reaction was carried out with the addition of 1 ml 0.5 mmol·L?1different metal ions(Cu2+,Fe3+,Mg2+,Ca2+,K+and Na+).After the enzymatic hydrolysis,the ginsenoside C-K content was determined by HPLC,and the conversion rate was calculated.Results were shown in Fig.7(a).From that,it was found that Fe3+is the optimal metal ion for the hydrolysis of ginsenoside Rb1 by snailases.Various metal ions in the body play different roles,Na+,K+and Ca2+exhibit ion pump-specific functions on the membrane transport,whereas other metal ions are more likely to play an important role in affecting enzyme function.For example,some metal ions can form complexes with enzyme proteins,and then lower the activation energy of the reaction,thus losing metal ions may lead to a loss of enzyme activity. In this study, the effect of ferric ion on the hydrolysis of ginsenoside Rb1 may be because ferric ion can regulate the catalytic activity of snailases. It has been reported that some metal ions play a role in the regulation of the enzyme, and the loss of these regulatory metals, the activity of the enzyme decreased [25].

Fig.6.The effects of two interaction factors on the conversion rate of rare ginsenoside C-K.
In order to determine the optimal concentration of Fe3+,0.01,0.1,0.5,1.0,1.5,2.0,2.5,and3.0mmol·L?1Fe3+were added into the reaction system.The results in Fig.7(b)indicated that 1.0 mmol·L?1is the optimal addition concentration of Fe3+,which can significantly improve the conversion rate of ginsenoside Rb1 to 89.7%.Generally,some enzymes play an effective role in the catalytic reaction with the participation of coenzymes,which are usually undertaken by metal ions or small molecules.This experiment con firms that metal ions,especially Fe3+,enable snailases to promote the conversion of ginsenoside Rb1 into ginsenoside C-K,thus significantly increasing the conversion rate of ginsenoside C-K.
3.4.Purification and purity analysis of ginsenoside C-K
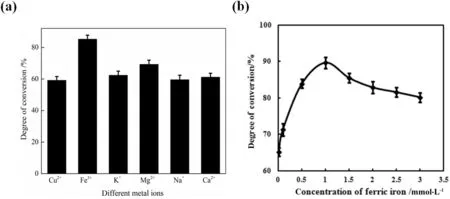
Fig.7.The effects of differentmetal ions on the catalysis of snailase on the basis of previous experiments. (a) The effect of differentmetal ions on ginsenoside Rb1 conversion rate. (b) The determination of optimal addition concentration of Fe3+.
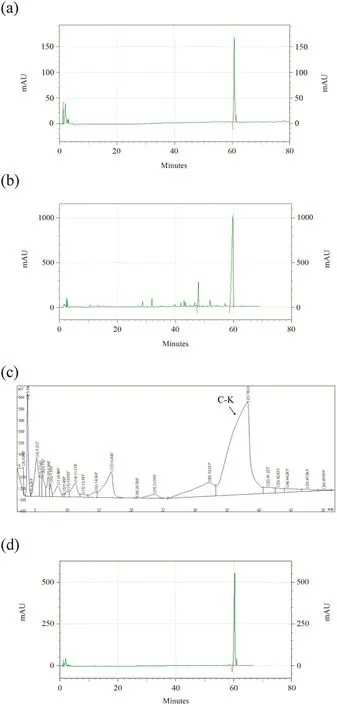
Fig.8.The results of HPLC.(a)Standards of ginsenoside C-K.(b)Analysis of ginsenoside Rb1 conversion products.(c)Purification of the transformed products by HPLC.(d)Analysis of ginsenoside C-K purity.
The transformed products of ginsenoside Rb1 under optimal conditions were shown in Fig. 8(b). Fromthat, it was found that the retention time of ginsenoside C-Kwas about 60 min comparedwith the standards [shown in Fig. 8(a)]. According to the peak area in the figure and the standard curve, the content of ginsenoside C-K in the products was about 90.3%.Then the ginsenoside C-K was isolated using preparative HPLC, resulting in a ginsenoside C-K purification of 98% as Fig. 8(c) and (d) (the arrow refers to ginsenoside C-K). The calculated maximum conversion rate was 89.7% based on Eq. (2). HPLC results showed that the conversion conditions used in this study for ginsenoside C-K can significantly improve the conversion rate to obtain high purity of ginsenoside C-K.
3.5.Biologic activity analysis
As shown in Fig.9(a),ginsenoside C-K has an inhibitory effect on lung cancer cells NCI-H460,the cell apoptosis increased with the extension of time.In addition,the ginsenoside C-K showed dose-dependent inhibition of a series of tumor cells including NCI-H460,MCF-7,SGC-7901,and CACO-2.The results from Fig.9(b)and(c)indicated that 97.1%of NCI-H460 cell inhibition was achieved when the concentration of ginsenoside C-K was 50 μg·ml?1,and ginsenoside C-K exerted the highest anti-tumor activity on NCI-H460 cell,the lowest on SGC-7901 cell.It was reported that ginsenoside C-K arrested the cells in the G1 phase,activated expression of caspases 8 and 9,and significantly induced cell apoptosis in colorectal cancer cells[26].The study has also demonstrated that ginsenoside C-K regulates distinct TLR4-mediated in flammatory responses as a functional ligand of glucocorticoid receptor,suggesting a novel therapy for Gram-negative septic shock[27].These results provide the evidence that ginsenoside C-K has significantly physiological activities in terms of disease prevention and therapy.
4.Conclusions
Ginsenoside C-K,as a rare ginsenoside,has low content in ginseng and high pharmacological activities[28–30].The production of rare ginsenoside C-K attracted great attention.In this study,snailases were utilized to catalyze the hydrolysis of ginsenoside Rb1 with the purpose of producing the ginsenoside C-K.
Besides the essential enzymatic conditions,this study innovatively investigated the effect of metal ions on the enzymatic hydrolysis by snailases,and successfully increased the conversion rate of ginsenoside Rb1 to 89.7%.It provides a theoretical basis and practical guiding significance for the industrialization of rare ginsenoside C-K production.Additionally,more efforts were need to utilize ginsenoside C-K in order to develop clinical therapeutic applications.
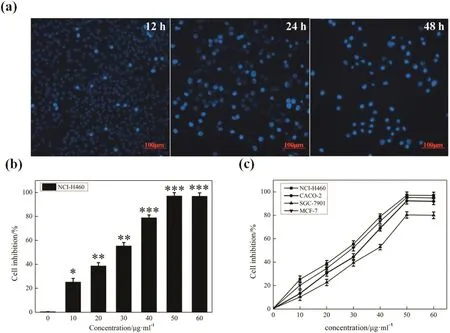
Fig.9.(a)Fluorescence microscope images of NCI-H460 cell morphology cultured with 50 μg·ml?1ginsenoside C-K at different times.(b)Anti-tumor activity comparison between different concentrations of ginsenoside C-K on NCI-H460 cell.(c)Anti-tumor activity of ginsenoside C-K on a series of tumor cell lines.
 Chinese Journal of Chemical Engineering2018年7期
Chinese Journal of Chemical Engineering2018年7期
- Chinese Journal of Chemical Engineering的其它文章
- Preparation of vapreotide-templated silver nanocages and their photothermal therapy efficacy☆
- DNA-assisted rational design of BaF2linear and erythrocyteshaped nanocrystals☆
- On the database-based strategy of candidate extractant generation for de-phenol process in coking wastewater treatment☆
- Hierarchical porous MgBO2(OH)microspheres:Hydrothermal synthesis,thermal decomposition,and application as adsorbents for Congo red removal☆
- Degradation analysis of A2/O combined with AgNO3+K2FeO4on coking wastewater
- Experimental study on the effects of drying methods on the stabilities of lignite☆
