兩個基于π-共軛二羧酸構筑的配位聚合物的晶體結構、磁性及光催化性能
翟麗軍 牛宇嵐 郝小艷 陳立杰 李國放 范黎明
(1太原工業學院化學與化工系,太原 030008)
(2中北大學理學院,太原 030051)
Coordination polymers(CPs),have attracted more and more attentions of chemists and materials scientist for their widely applications as functional materials[1-4].Generally speaking,the CPsareassembled from organic linkers and inorganic nodes[5-6].It is well known that the physicochemical properties of CPs are greatly depending on the nature of the organic ligands.Thus,the rational selection of organic linkers plays important roles in the design of targeting CPs[7-9].
Numberous organic ligands have been introduced into the construction ofCPs in the recent three decades.Among them,there was no doubt the polycarboxylates are stilldominant for their strong coordinating abilities,diverse coordinationmodes,stable backbones[10-11].It is noteworthy that theπ-conjugated polycarboxylates based CPsexhibits interesting single-chainmagnetand luminescent properties for their uniqueπ-conjugated system[12].At the same time,theπ-conjugated polycarboxylates based CPs are rarely reported up to now[13].Thus,the design ofπ-conjugated polycarboxylates based CPs ismeaningful.
Inspired by abovementioned points,we explored novel CPs based on the π-conjugated ligand of 3,3′-(1,3,6,8-tetraoxobenzol[lmn][3,8]-phenanthroline-2,7(1H,3H,6H,8H)diyl)-di-benzoic acid)(H2L,Scheme 1).Herein,we reported the structure and characterizations of two CPs,{[Mn2(L)2(H2O)5]·2H2O}n(1)and[Cd(L)(H2O)2]n(2),which displaying 2D sheetwith 1D{Mn3(COO)2}SBUs for 1,and 1D polymeric chain for 2,respectively.Expanded by hydrogen bonds,both two CPs show 3D supramolecular finally.Besides,the magnetic property of 1 as well as the photocatalytic activity of 2 have been investigated.
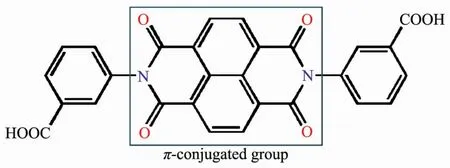
Scheme 1 Structure of H2L
1 Experimental
1.1 M aterials and methods
All the chemical reagents were purchased from Jinan Henghua Sci.&Technol.Co.,Ltd.without further purification.IR spectra were measured on a NEXUS 670 FTIR spectrometer.Elemental analyses were carried out on a CE instruments EA 1110 elemental analyzer.Thermogravimetric analyses(TGA)were performed with a heating rate of 10 ℃·min-1under N2atmosphere on Perkin-Elmer TGA-7 thermogravimetric analyzer.Powder X-ray diffraction(PXRD)determinations were performed on an X-ray diffractometer(D/max 2500 PC,Rigaku)at 50 kV,30mA by using Cu Kα radiation(λ=0.154 06 nm)with the 2θ range of 5°~50°.The variable-temperature magnetic susceptibility measurements were performed on the Quantum Design SQUID MPMS XL-7 instruments.Photocatalytic experiments were evaluated by the degradation ofmethylene blue (MB)under UV light irradiation using a 300 W metal-halide lamp as the light source.0.5 mL of 30%(w/w)hydrogen peroxide was injected into 20.0 mL 10 mg·L-1methylene blue(MB)aqueous solution with 10 mg powdered catalyst(30min dark adsorption pretreated).During the degradation,the reaction solution was sampled at specific time-points and centrifuged to remove the photocatalysts in order to monitor the absorption curves of MB(500~700 nm)by using a Hitachi U-3500 UV-Vis spectrometer.
1.2 Synthesis
1.2.1 Synthesis of{[Mn2(L)2(H2O)5]·2H2O}n(1)
A mixture of H2L (0.005mmol,2.5mg),MnCl2·4H2O (0.010 mmol,2.0 mg),a drop of 0.5 mol·L-1NaOH aqueous solution,and 1 mL H2O was sealed in a pressure-resistant glass tube and heated at 130℃for 3 days,and then cooled to room temperature at a descent rate of 5℃·h-1.The colorless block crystals of 1 were obtained with the yield of about 37%based on H2L.Anal.Calcd.for C56H38Mn2N4O23(%):C,54.03;H,3.08;N,4.50.Found(%):C,54.43;H,3.12;N,4.56.IR(KBr,cm-1):3 337(m),2 367(m),1 715(s),1 674(vs),1 559(vs),1 403(s),1 348(s),1 254(s),1 199(m),1 125(w),983(m),901(w),772(m),738(s),684(m),623(w),575(w).
1.2.2 Synthesis of[Cd(L)(H2O)2]n(2)
A mixture of H2L (0.005 mmol,2.5 mg),and CdCl2·2.5H2O(0.010 mmol,2.3 mg),and 1 mL H2O wassealed in a pressure-resistantglass tube and heated at 130℃for 3 days,and then cooled to room temperature ata descent rate of 5℃·h-1.The orange block crystals of 2 were obtained with the yield of about 45%based on H2L.Anal.Calcd.for C28H16CdN2O10(%):C,51.51;H,2.47;N,4.29.Found(%):C,51.53;H,2.51;N,4.28.IR (KBr,cm-1):3 317 (m),2 360(m),1 716(vs),1 669(vs),1 539(s),1 442(m),1 405(s),1 350(vs),1 253(s),1 198(s),1 117(m),980(m),887(m),840(w),768(m),736(m),686(m),628(m),553(w).
1.3 X-ray crystallography
Structural integrity single crystals of 1 and 2 were carefully selected under an optical microscope and fixed to thin glass fibers.After that,single-crystal X-ray diffraction analyseswere performed on a Siemens SMART diffractometer using Mo Kα radiation(λ=0.071 073 nm)at 200(2)K for 1,and 295(2)K for 2,respectively.The structures of two obtained CPs were solved by direct methods,with the non-hydrogen atoms refined anisotropially by using the SHELXTL packagewith F2values based full-matrix least-squares procedure[14].All the hydrogen atoms except those for water molecules were generated geometrically with fixed isotropic thermal parameters,and included in the structure factor calculations[15].And the hydrogen atoms attached to oxygen were refined with O-H 0.085 nm and Uiso(H)=1.2Ueq(O).The crystallographic data and the details of the crystal structures are listed in Table 1.Selected bond lengths and angles for complexes 1 and 2 are listed in Table S1.
CCDC:1848147,1;1848148,2.
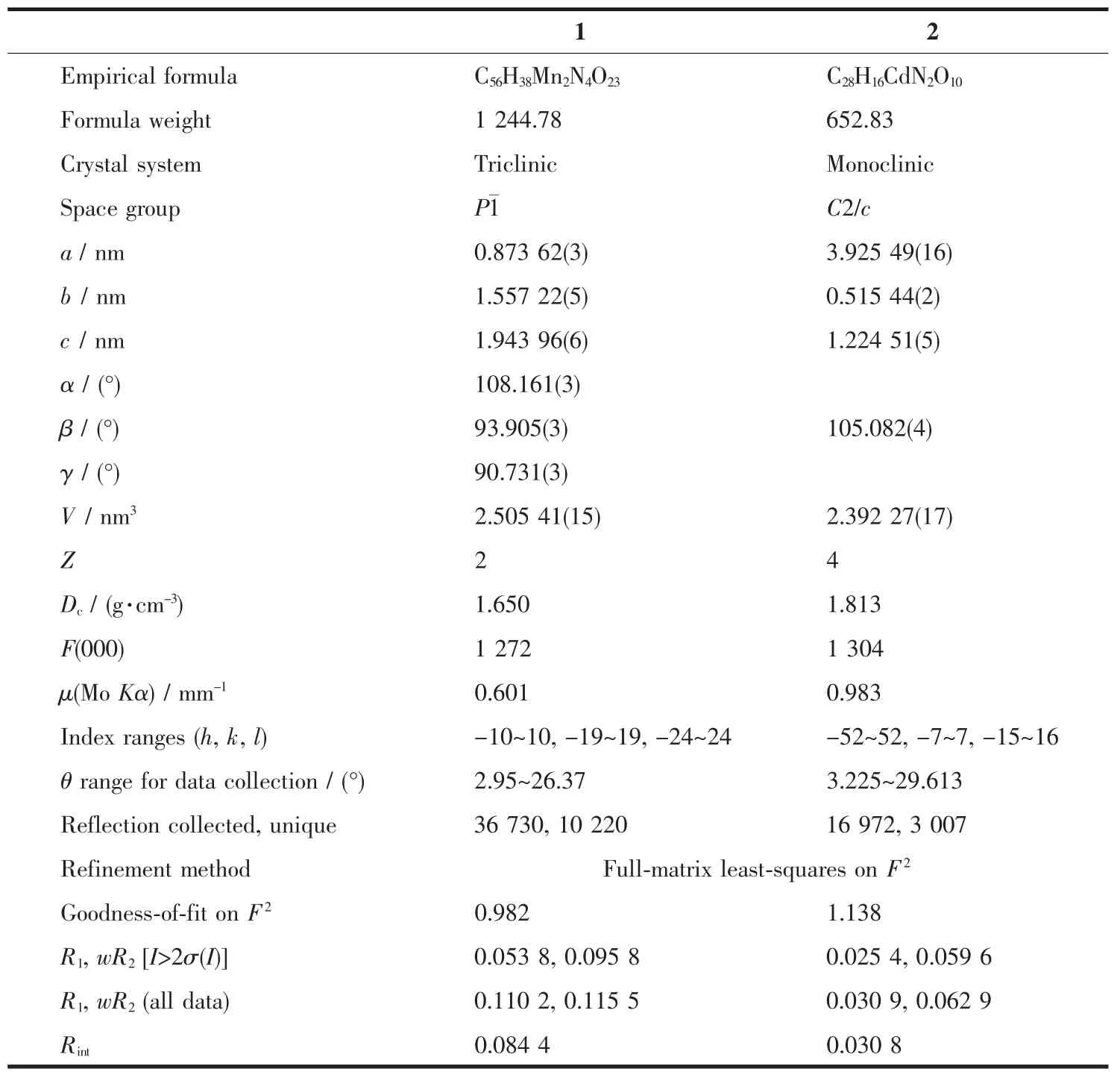
Table 1 Crystal structure parameters of complexes 1 and 2
2 Results and discussion
2.1 Crystal structure of{[M n2(L)2(H 2O)5]·2H2O}n (1)
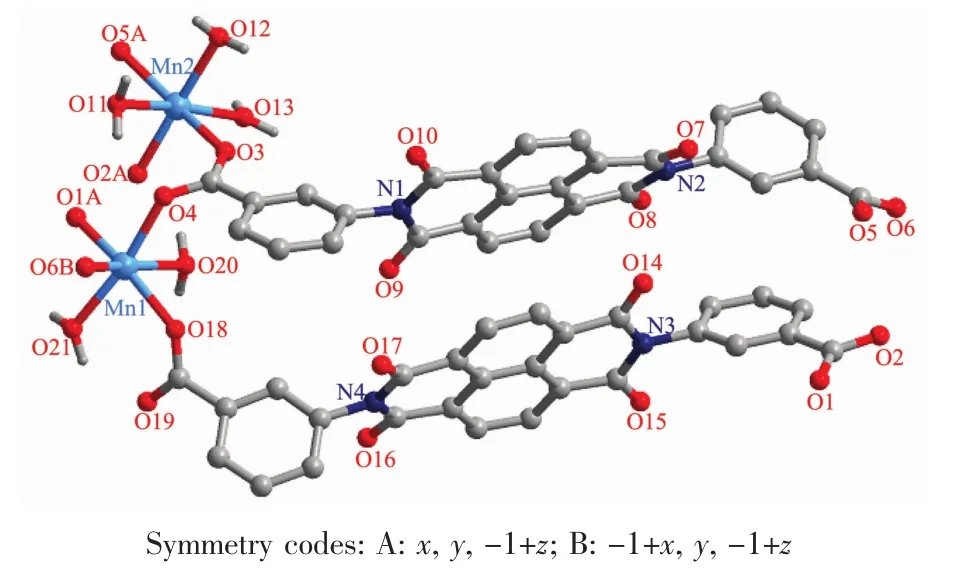
Fig.1 Asymmetric unitof 1
X-ray crystallography reveals that complex 1 crystallizes in the triclinic system,space group P1.As shown in Fig.1,the asymmetric unit consists of two Mnギions,two L2-ligands,five coordinated water molecules,and two lattice watermolecules.Mn(1)is located in a distorted {MnO6}octahedral geometry,completed by four carboxyl O atoms(O4,O18,O1A and O6B)from four distinct L2-ligands,and two water molecules(O20 and O21).Mn(2)lies in the center of a similar{MnO6}octahedral geometry,surrounded by three carboxylO atoms(O3,O2A and O5A)from three L2-ligands,and three coordinated water molecules(O11,O12 and O13).Besides,the Mn-O bond lengths are in the range of 0.212 6(2)~0.228 3(7)nm.
In the assembly of complex 1,the H2L ligands are completely deprotonated and adopts two different kinds of coordination modes:(κ1-κ0)-(κ1-κ1)-μ3(ModeⅠ,Scheme 2)with the dihedral angles ofα1,β1and γ1being 57.29°,86.26°and 37.22° (α1,β1,γ1corresponding to the dihedral angles between the aromatic functional groups of A/B,B/C and A/C),and(κ1-κ1)-(κ1-κ1)-μ4(Mode Ⅱ ,Scheme 2)with the dihedral angles of being 75.57°,56.60°and 18.98°.It is noteworthy that the μ2-η1:η1carboxyl groups of two kinds L2-ligands bridged Mnギions to form an infinite 1D{Mn3(COO)2}chain with the nearest Mn…Mn distances being 0.458 8 nm and 0.503 3 nm,respectively (Fig.2,and Fig.S1).The L2-ligands act as pillars to bridge the 1D{Mn3(COO)2}chains,finally leaving a 2D sheet(Fig.3).Adjacent sheets interact with each other or guestwatermolecules through O-H…O hydrogen bonds (Table S2),finally giving a 3D supramolecular structure (Fig.S2).Among them,the lattice watermolecules cooperate with the coordinated water molecules through O-H…O hydrogen bonding interactions,forming an interesting water cluster(Fig.S3).
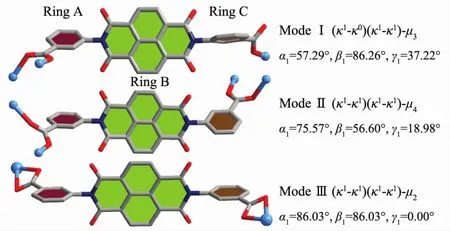
Scheme 2 Coordinationmodes of H2L in complexes 1 and 2
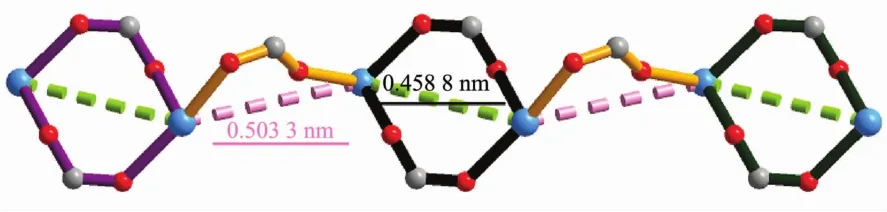
Fig.2 One dimensional{Mn3(COO)2}chain in 1
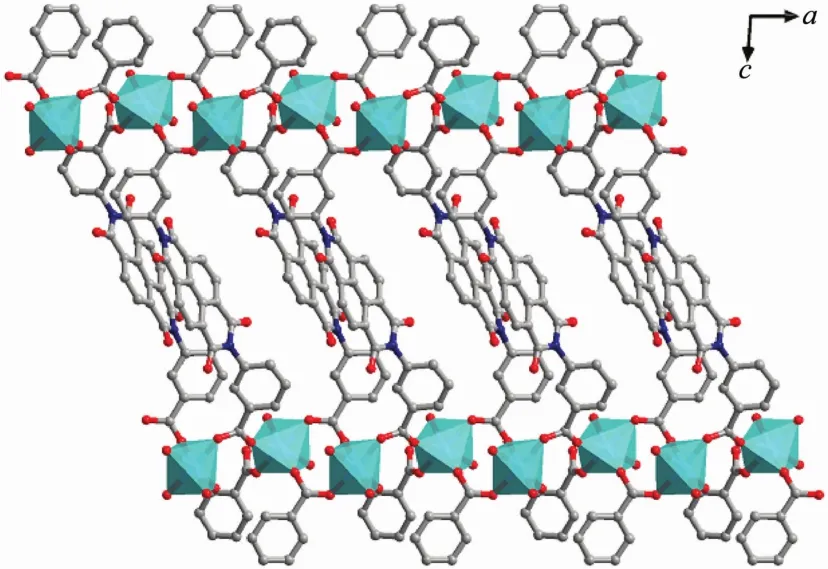
Fig.3 Two dimensional sheetof complex 1 views along b axis
2.2 Crystal structure of[Cd(L)(H 2O)2]n(2)
Complex 2 crystallizes in the monoclinic system C2/c and the structure contains a half of Cdギions,a halfof L2-ligands,and one coordinated watermolecule(Fig.4).The central Cdギion is located in distorted{CdO6}triangular prism coordination geometry,surrounded by four carboxyl oxygen atoms from two L2-ligands(O1,O2,O1A and O2A),and two coordinated water molecules(O1W and O1WA).And the Cd-O bond distances range from 0.220 3(9)to0.251 5(5)nm.
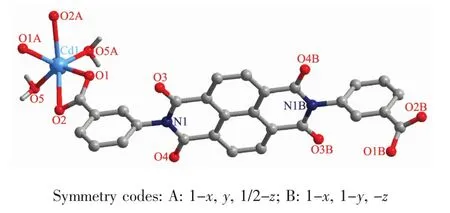
Fig.4 Asymmetric unitof 2
Different from that in complex 1,the H2L ligand adopts(κ1-κ1)-(κ1-κ1)-μ2(Mode Ⅲ,Scheme 2,with α1,β1and γ1being 86.03°,86.03°and 0.00°)coordination mode to bridge two Cdギions by using the chelating η2carboxyl groups,leaving a 1D chain with the neighbouring Cd…Cd distance of 2.029 5 nm(Fig.5).Expanded by the O-H…O hydrogen bonds along b axis,a 2D sheet is constructed (Fig.S4).Those 2D sheets interactwith adjacent ones through C/O-H…O hydrogen bonds (Table S2),finally giving a 3D supramolecular structure(Fig.S5).
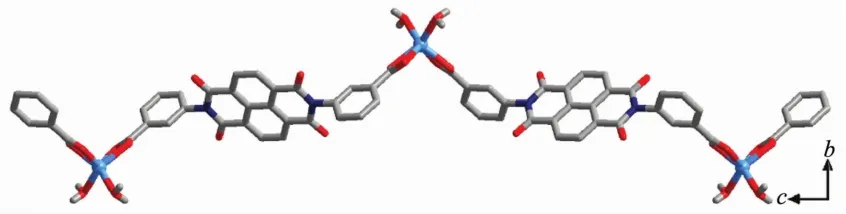
Fig.5 One dimensional polymeric chain structure of 2 view along a axis
2.3 Structural com parison
Up to now,only one CP based on 3,3′-(1,3,6,8-tetraoxobenzol[lmn][3,8]-phenanthroline-2,7(1H,3H,6H,8H)diyl)-di-benzoic acid)(H2L),namely,NKUMOM-3[14],has been reported.We designed two novel CPs here and compared those structures.As can be seen in the Scheme 2,the L2-adopted two different coor-dination modes:(κ1-κ0)-(κ1-κ1)-μ3(Mode Ⅰ)and(κ1-κ1)-(κ1-κ1)-μ4(Mode Ⅱ)in 1,(κ1-κ1)-(κ1-κ1)-μ2(Mode Ⅲ)in 2,and(κ1-κ0)-(κ1-κ0)-μ2(Mode Ⅳ)in NKUMOM-3.When comparing the structures of those CPs,we can notice that the bridging μ2-η1∶η1carboxyl groups,chelatingη2carboxyl groups,as well as the monodentateη1carboxyl groups,are crucial role in forming different architectures.And the coordinated solvent molecules also greatly affect the final structures,for their steric hindrance and bridging effects after coordinating withmetal ions.Besides,although all the metal ions in above mentioned CPs are pentacoordinated with atoms from L2-ligands and solvent molecules,the coordination preference of those central metal ions also adds one hand in adjusting the structure diversity.
2.4 Powder X-ray diffraction and thermogravimetric analysis
In order to check the phase purity of the complexes,the PXRD patterns were checked at room temperature.As shown in Fig.S6,the peak positions of the simulated and experimental PXRD patterns were in agreementwith each other,demonstrating the good phase purity of the complexes.The dissimilarities in intensitymay be due to the preferred orientation of the crystalline powder samples.
To examine the thermal stability of two CPs,the thermogravimetric analyses were carried out from ambient temperature up to 800℃,and the results are given in Fig.6.For complex 1,the firstweight loss of 2.91%before 85℃,corresponds to the loss of lattice water molecules (Calcd.2.89%),then the release of coordinated water molecules (Found:7.17%;Calcd.7.23%)occurred in the temperature range of 85~195℃.The architecture can exist stably until the temperature up to 450℃.For complex 2,the first weight loss of 5.37%before 180℃,corresponds to the release of coordinated watermolecules(Calcd.5.51%).Beyond 435℃,there was a rapid weight loss,suggesting the decomposition of complex 2.Both two TG curves show the high thermal stabilities of two complexes,which is important for the CPs as functionalmaterial in practical application.

Fig.6 TG curves of complexes 1 and 2
2.5 M agnetic property
Variable-temperature susceptibility of complex 1 was measured in the temperature range of 2~300 K with an applied magnetic field of 1 000 Oe.For complex 1,theχMT value at room temperature are 7.72 cm3·K·mol-1(Fig.7),smaller than that for two isolated Mnギ cations(8.80 cm3·K·mol-1),which can be attributed to the contribution to the susceptibility from orbital angular momentum at higher temperatures[16].With the temperature decreasing,theχMT value decreased continuously to 6.81 cm3·K·mol-1at 2 K.And the temperature dependence ofχMfollows the Curie-Weiss law χM=C/(T-θ)with C=7.78 cm3·K·mol-1,θ=-7.92 K(Fig.S7).The negative value ofθindicates the presence of the antiferromagnetic interactions between the Mnギcations in complex 1[17-18].
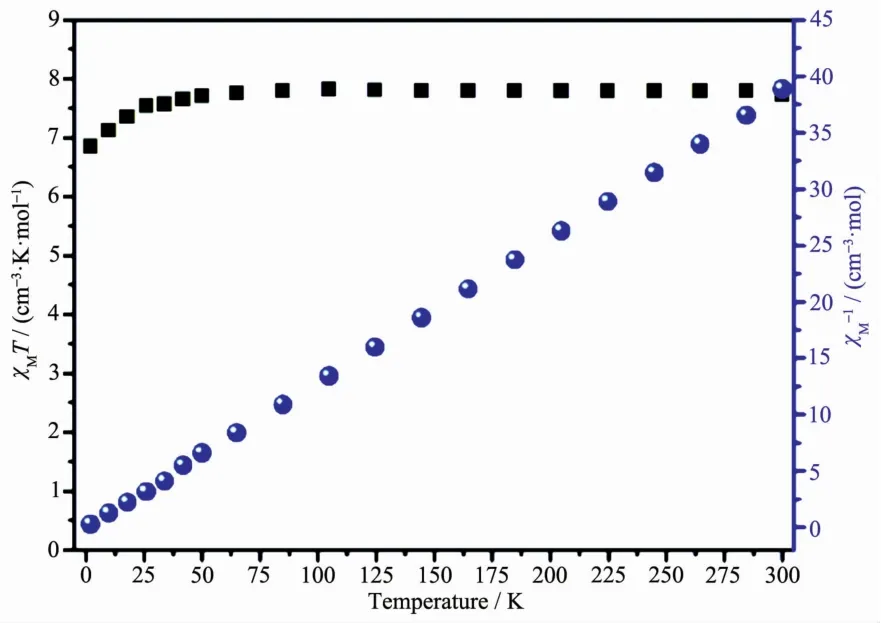
Fig.7 χM T and χM-1 versus T plots for complex 1
2.6 Photocatalytic activity
The photocatalytic activity of complex 2 as photocatalyst in degradatingmethylene blue(MB)was investigated.The decomposition of dye MB was monitored by the characteristic absorption band at 664 nm.As illustrated in Fig.8,changes in the concentration of MB solution are plotted vs irradiation time,with the degradation efficiency of MB being 74.1%after 120 minute.Under the same conditions,the total catalytic degradation efficiency of the control experiment under illumination after 120 minute was 15.7%.The kinetic data for the degradation of MB can be well fitted by the apparent first-order reaction model,ln(C/C0)=-kt(Fig.S8),where k is the rate constant,C0and C are the concentration of MB at irradiation time t=0 and t,respectively.After calculation,the rate constant value of k was found to be 0.010 7min-1.
The possiblemechanism for the MB degradation is proposed as described in the previous literature[19-20].Under the irradiation of UV-Vis light,the organic ligands are induced to produce O-Cd charge transfer promoting electrons from the highest occupied molecular orbital(HOMO)to the lowest unoccupied molecular orbital(LUMO)[21].Therefore,the HOMO strongly needs one electron to return to its stable state.Thus,one electron is captured from water molecules,which is oxygenated to generate the·OH radicals.And then the·OH active species could decompose the MB effectively to complete the photocatalytic process[22].
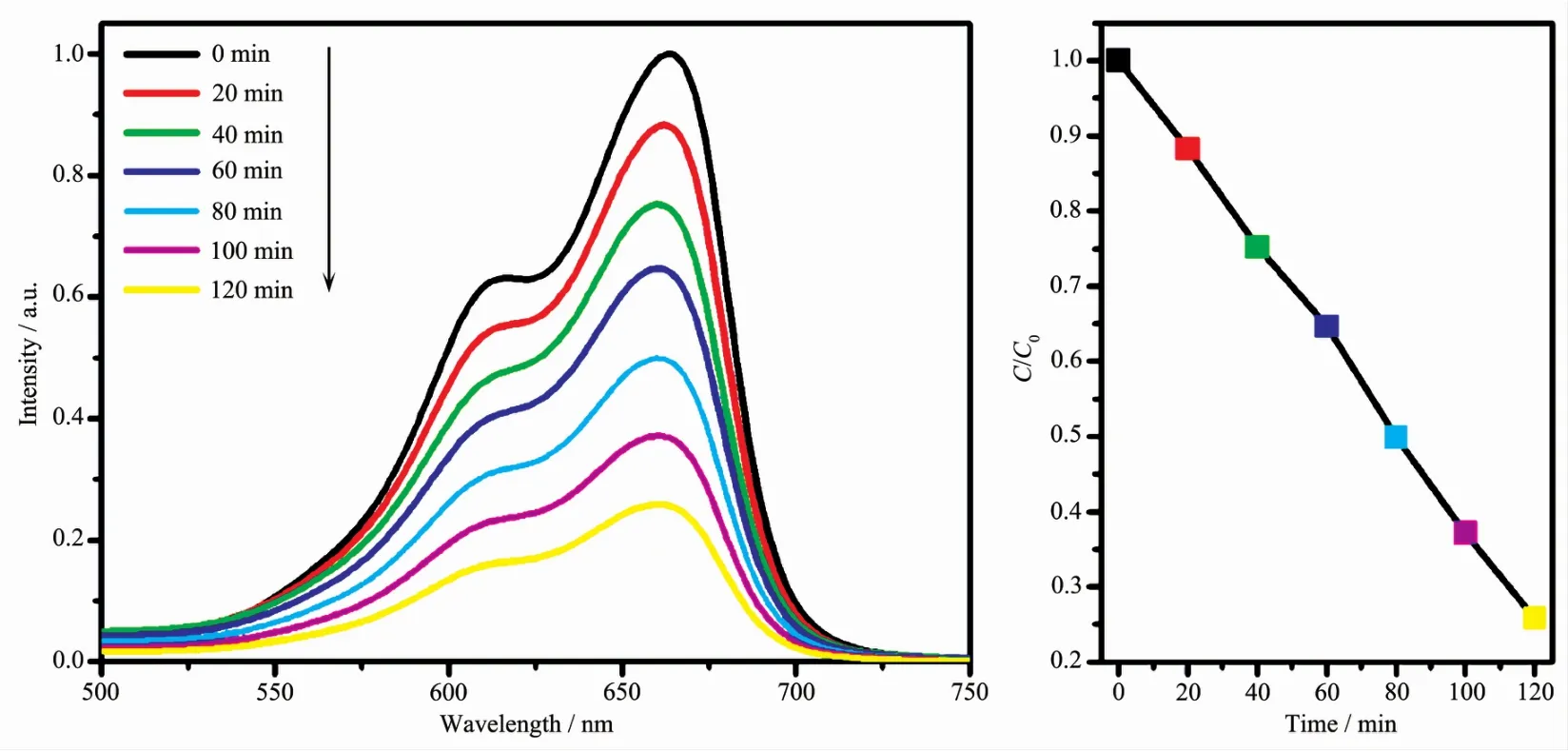
Fig.8 UV-Vis absorption spectra of the MB solutions degraded by complex 2 as photocatalyst under UV irradiation at different time intervals
3 Conclusions
In summary,two novel CPs have been constructed from the π-conjugated ligand of 3,3′-(1,3,6,8-tetraoxobenzol[lmn][3,8]-phenanthroline-2,7(1H,3H,6H,8H)diyl)-di-benzoic acid),with the structures being 2D sheet containing 1D{Mn3(COO)2}chains for 1,and 1D polymeric chain for 2,respectively.With the help of hydrogen bonds,two CPs form 3D supramolecular structure finally.Thermal stability analysis revealed both two CPs show high thermal stabilities up to 450℃.Besides,the variable-temperature susceptibility of 1 indicated there are antiferromagnetic interactions between the Mnギcations.And the photocatalytic tests demonstrated that the obtained complex 2 is good photocatalyst in the degradation of MB,with the efficiency up to 74.1%after 2 hours.

