Commentary on the“Measurement and correlation of solubility of meropenem trihydrate in binary(water+acetone/tetrahydrofuran)solvent mixtures”☆
Renjie Xu ,Min Zheng ,Jiao Chen ,Hongkun Zhao ,*
1 Guangling College,Yangzhou University,Yangzhou 225009,China
2 College of Chemistry&Chemical Engineering,Yangzhou University,Yangzhou 225002,China
ABSTRACT Article history:Received 2 July 2018 Accepted 20 August 2018 Available online 28 September 2018 Problem was discussed on the reported equation parameters by Zhou and co-workers[Chinese Journal of Chemical Engineering 25(10)(2017)1461-1466]for expressing the meropenem trihydrate solubility in binary(water+acetone and water+tetrahydrofuran)mixtures with the modified Apelblat equation.The reported model parameters do not back-calculate correctly the evaluated solubility as shown in their published work.The reported parameters of the modified Apelblat equation tabulated in Tables 3 and 4 by Zhou and coworkers are in mistake.
Zhou and coworkers determined the meropenem trihydrate solubility in binary(water+acetone and water+tetrahydrofuran)mixtures in their paper published in Chinese Journal of Chemical Engineering[1].Solubility was obtainable over the temperatures ranging from 278.15 K to 303.15 K by using a static technique.As an important part of their paper,Zhou and coworkers expressed the mole fraction solubility of meropenem trihydrate solubility in the two binary mixtures via the modified Apelblat equation

and the CNIBS/Redlich-Kister model:

here x1is the mole fraction of acetone or tetrahydrofuran in initial(water+acetone and water+tetrahydrofuran)mixtures free of meropenem trihydrate.xsrefers to the solubility of meropenem trihydrate in mole fraction in saturated solutions.The parameters in the modified Apelblat equation are referred to A,B and C;and in the CNIBS/Redlich-Kister model,B0,B1,B2,B3and B4.
The reported solubility data by Zhou et al.is of greatimportance and can offer crucialsupportfor the meropenem trihydrate purification.The aim of this comment is merely to show some errors in the authors'reported equation parameters rather than doubt the accuracy of their determined solubility data.For this equation,the parameters'values reported in Table 3 by Zhou and co-workers[1]give the mole fraction solubility which are greatly different from the determined ones.So as to demonstrate the problem,we back-calculate the meropenem trihydrate solubility in acetone(1)+water(2)mixtures with x1=0 at 278.15 K through substituting the reported model parameters of the modified Apelblat equation(A=-580.00,B=23,051.00 and C=87.00)from Table 3[1]into Eq.(1):

The back-calculated solubility values are xS=5.567×10-4and xS=10.87×10-4at 278.15 K and 303.15 K,respectively.The calculated values reported by Zhou et al.are xS=4.103×10-4at 278.15 K and xS=7.812×10-4at 303.15 K which are presented in Table 2 of their published work[1].The back-calculated solubilities by us for meropenem trihydrate in acetone(1)+water(2)mixtures with x1=0 with the published modified Apelblat equation coefficients are about 1.4 times the reported values by Zhou et al.
We subsequently back-calculate the meropenem trihydrate solubility in acetone(1)+water(2)mixtures with x1=0.0058 through substituting the reported equation coefficients(A=-778.00;B=30525.00 and C=117.00)from Table 3 into Eq.(1).

There are some differences in the results between ourcalculated and those reported in their work[1].The back-calculated solubilities by us using the authors'equation parameters,xS=5.758×10-5at 278.15 K and xS=1.596×10-4at 303.15 K,are also far from the data of xS=2.132×10-4at 278.15 K and xS=6.030×10-4at 303.15 K that tabulated in Table 2 of their work[1]for the computed solubility values of meropenem trihydrate in acetone(1)+water(2)mixtures with x1=0.0058.The relative deviations are 72.99%and 73.53%at 278.15 K and 303.15 K,respectively.
The reported parameters'values of Apelblat equation for tetrahydrofuran(2)+water(3)mixed solutions have also shortcoming.If we substitute the parameters'values of A,B and C for the tetrahydrofuran(2)+water(3)solutions with x1=0.307(A=-369.00;B=12527.00;C=56.00)from Table 4 oftheirwork[1]into Eq.(1),we obtain

The back-calculated solubility value is xS=1.528×10-4for meropenem trihydrate in tetrahydrofuran(1)+water(2)at 278.15 K.The back-calculated solubility by us by employing the values of authors'equation parameters is roughly 597%greater than the authors'back-calculated one of xS=0.256×10-4tabulated in the Table 2[1].
With the intention of illustrating the inconsistency clearly,we carry out back-calculations for the meropenem trihydrate solubility in acetone(1)+water(2)and tetrahydrofuran(1)+water(2)mixtures within whole composition range at investigated temperatures according to authors'reported model coefficients from Tables 3 and 4 into Eq.(1).The back-calculated values by us and the reported ones by Zhou and co-workers[1]are presented in Tables 1 and 2 of thepresent communication,along with the values of RAD(relative average deviation)expressed as Eq.(6).
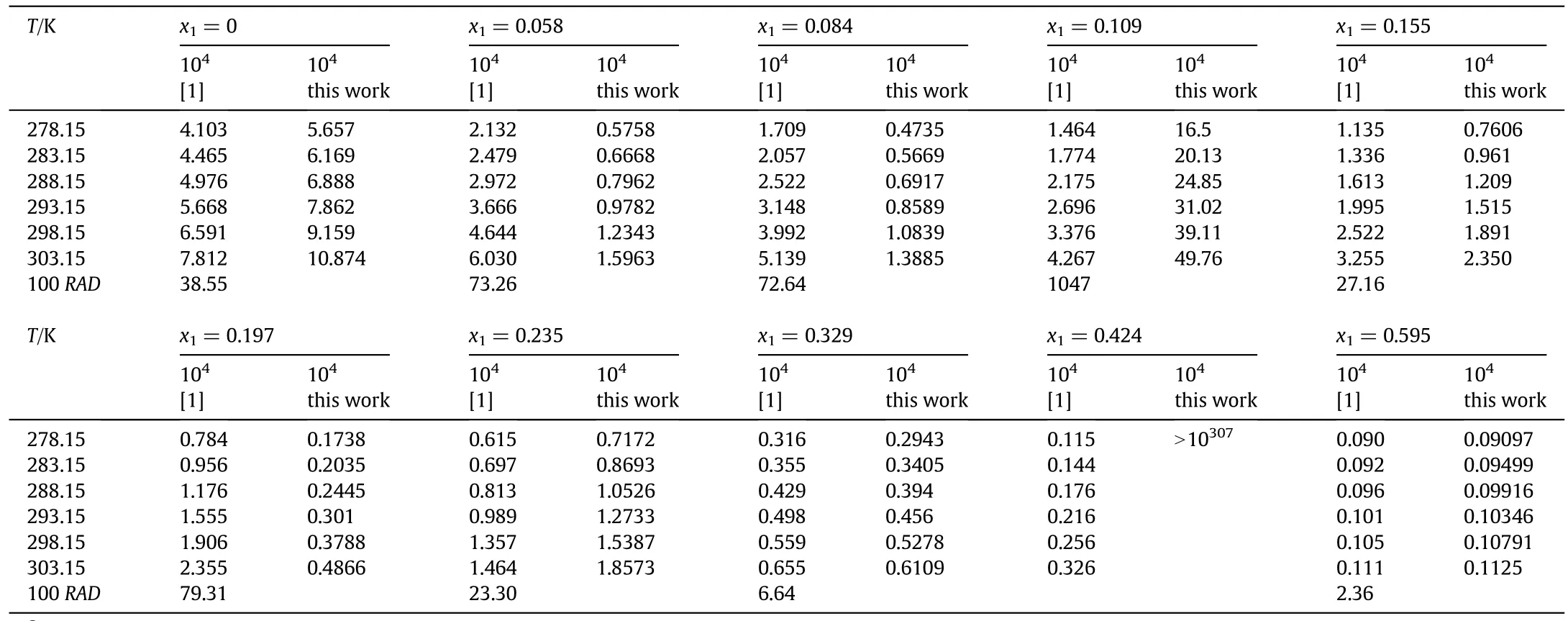
Table 1 The back-calculated mole fraction solubilities x S of meropenem trihydrate in acetone(1)+water(2)mixtures at different temperatures①

Table 2 The back-calculated mole fraction solubilities x S of meropenem trihydrate in tetrahydrofuran(1)+water(2)mixtures at different temperatures①

As can be observed from Tables 1 and 2,the back-calculated solubility values are all disagreement with the reported data by Zhou and co-workers[1]with the exception for the acetone(2)+water(3)solutions with x1=0.595 and fortetrahydrofuran(1)+water(2)solutions with x1=0.152 and 0.605.Some deviations are observed between our back-calculated solubility data and those reported by Zhou et al.
We speculate that Zhou etal.used the following equation(Eq.(7))to correlate their determined solubility values,which is given as Eq.(4)in their published work.

By using A=-580.00,B=23,051.00 and C=87.00 for acetone(1)+water(2)solutions with x1=0,the back-calculated solubilities are xs=1 at all temperatures,which are still noticeably different from their back-calculated ones listed in Table 2 of their work[1].
The computation results performed by us exhibit clearly that the parameters'values of the modified Apelblat equation that Zhou et al.reported in their work[1]cannot express correctly the meropenem trihydrate solubility in acetone(1)+water(2)and tetrahydrofuran(1)+water(2)solutions with most compositions of acetone(2)and tetrahydrofuran(2).Therefore the readers should pay attention to this in using their reported model coefficients.
 Chinese Journal of Chemical Engineering2018年10期
Chinese Journal of Chemical Engineering2018年10期
- Chinese Journal of Chemical Engineering的其它文章
- Recent progress in the green synthesis of rare-earth doped upconversion nanophosphors for optical bioimaging from cells to animals☆
- One-step synthesis of hydrophobic magnesium hydroxide nanoparticles and their application in flame-retardant polypropylene composites☆
- Controllable preparation of ZnO porous flower through a membrane dispersion reactor and their photocatalytic properties☆
- Distribution and degradation kinetics of cyhalodiamide in Chinese rice field environment☆
- Co-pyrolysis characteristics of typical components of waste plastics in a falling film pyrolysis reactor☆
- Reliable,environmentally friendly method for the recycling of spent Ag/α-Al2O3 catalysts using(NH4)2Ce(NO3)6☆
