兩個基于CdⅡ金屬有機骨架化合物在金屬離子和有機分子發光傳感中的應用
張春麗
(宿州學院化學化工學院,宿州 234000)
In recent years, metal-organic frameworks(MOFs)have attracted quite a few attentions because of their fascinating structures and properties.They have applications in a wide range of fields,such as gas storage and separation[1-3],luminescence[4-6]and catalysis[7-10].As we all know,Fe3+is abundant in the industrial water.Though Fe3+is low toxicity for people and animals,high Fe3+concentration will change the sensory properties of water and lead to environmental contamination.Salicylaldehyde is used widely both in organic and drug synthesis as an important intermediate product.However,it is irritating to the respiratory tract or eyes,and induces cough or chest pain after inhalation.Thus,detecting them in the environment effectively has drawn much attention.Recent studies show that the fluorescence method based on the luminescent metal-organic frameworks (MOFs)has special advantages,such as high sensitivity,quick response time,and actual time monitoring[11-15].
In this work,we report two new cadmium metalorganic frameworks (MOFs),{[Cd (L)(fma)]·0.5H2O}n(1),{[Cd(L)0.5(sdb)]·DMF}n(2), (L=E,E-2,5-dihexyloxy-1,4-bis-(2-pyridin-vinyl)benzene,H2fma=fumaric acid,H2sdb=4,4′-sulfonyldibenzoic acid).MOF 1 is a noninterpenetrated 2D+2D→2D parallel stacked 4-connected sql network with the point symbol of{44.62},while MOF 2 exhibits a interpenetrated 2D+2D→3D inclined polycatenated 4-connected sql network with the point symbol of {44.62}.Fluorescence titrations were carried out,and the results confirm that Fe3+ion and salicylaldehyde showed quenching effects on the luminescence of MOFs 1 and 2.Quenching mechanisms also have been studied.
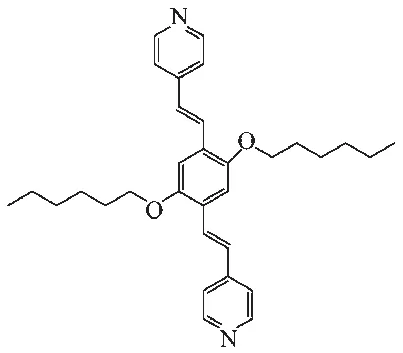
Scheme 1 Structure of ligand L
1 Experimental
1.1 Materials and general methods
The reagents except ligand L were commercial available and were used directly without purification.IR spectra were recorded on a Nicolet (Impact 410)spectrometer with KBr pellets(5 mg of the sample in 300 mg of KBr)in the range of 400~4 000 cm-1.C,H and N elemental analysis was performed with a Perkin Elmer 240C elemental analyzer.The as-synthesized MOF was characterized by thermogravimetric analyses(TGA)on a Perkin Elmer thermogravimetric analyzer,Pyris 1 TGA,from r.t.to 650 K using a heating rate of 10 K·min-1under a N2atmosphere.Powder X-ray diffraction (PXRD)measurements were performed on a Bruker D8 Advance X-ray diffractometer by using Cu Kα radiation (λ=0.154 18 nm)in the 2θrange of 5°~50°,and the X-ray tube was operated at 40 kV and 40 mA.Fluorescence spectra for the MOFs were conducted with an F-4600 FL Spectrophometer at room temperature.
1.2 Synthesis of L
L was synthesized by the similar procedure previously reported[16]. 1,4-di-bromo-2,5-dihexyloxybenzene (2.18 g,5 mmol),4-vinylpyridine (1.6 g,15 mmol),Pd(OAc)2(36 mg,3%mmol),tris(2,4,6-trimethoxyphenyl)phosphane (106 mg,4 mmol),Et3N (5 mL)and CH3CN (35 mL)were mixed in a 100 mL Schlenk flask and refilled with N2three times.The reaction solution was heated at 93℃for three days.The resulting mixture was concentrated in vacuo.The crude product was purified with column chromatography on silica gel eluted with petroleum ether/ethyl acetate (1∶3,V/V)to give the product as a yellow solid(2.0 g,82%).1H NMR (400 MHz,CDCl3):δ8.58 (d,J=6.1 Hz,4H),7.67 (d,J=16.5 Hz,2H),7.38 (d,J=6.1 Hz,4H),7.13 (s,2H),7.08 (d,J=16.5 Hz,2H),4.08(t,J=6.5 Hz,4H),1.94~1.84 (m,4H),1.61~1.50 (m,4H),1.45~1.33 (m,8H),0.91 (t,J=7.1 Hz,6H).Anal.Calcd.for L(%):C,79.30;H,8.32;N,5.78.Found(%):C,79.37;H,8.29;N,5.75.
1.3 Syntheses of MOFs
Synthesisof{[Cd(L)(fma)]·0.5H2O}n(1):A mixture of L (4.8 mg,0.01 mmol),H2fma (2.3 mg,0.02 mmol),Cd(NO3)2·6H2O (15 mg,0.1 mmol),DMF (3.0 mL)and H2O (2.5 mL)was placed in a 20 mL glass vial and heated at 100℃for three days.The resultant orange columnar crystals were washed with fresh DMF,and collected.The yield of the reaction was ca.41%based on L ligand.Elemental analysis calculated for C36H43CdN2O6.5(%):C,60.04;H,6.02;N,3.89.Found(%):C,60.09;H,6.09;N,3.91.
Synthesis of{[Cd(L)0.5(sdb)]·DMF}n(2):A mixture of L (4.8 mg,0.01 mmol),H2sdb (6.1 mg,0.02 mmol),Cd(NO3)2·6H2O (31 mg,0.1 mmol),DMF (5.0 mL)and H2O (0.5 mL)was placed in a 20 mL glass vial and heated at 100℃for three days.The resultant orange block crystals were washed with fresh DMF,and collected.The yield of the reaction was ca.46%based on L ligand.Elemental analysis calculated for C33H35CdN2O8S(%):C,54.14;H,4.82;N,3.83.Found(%):C,54.25;H,4.80;N,3.84.
1.4 X-ray crystallography
Single crystals of MOF 1 and 2 was collected on a Bruker SMART APEX CCD diffractometer using graphitemonochromated Mo Kαradiation(λ=0.071 073 nm)at 296 K.Structure solutions were solved by direct methods and the non-hydrogen atoms were located fromthetrial structures and then refined anisotropically with SHELXTL using full-matrix least-squares procedures based on F2values[17].The hydrogen atom positions were fixed geometrically at calculated distances and allowed to ride on the parent atoms.During the refinement of MOF 1,the 4-vinylpyridine and hexyloxy groups (C18-C24,N2/C18′-C24′,N2′and C32-C36/C32′-C36′atoms)were found to be disordered between two positions,the resulting SOFs(site of occupancies)of these groups were refined to be 0.65/0.35 and 0.5/0.5,respectively.And some needed restraints (DFIX,SADI and SIMU)were applied to ensure a reasonable refinement of this disorder model.Two level A alerts were reported in CheckCIF report,both of which majorly reflect the disorder nature of this structure.The corresponding responses have been embedded into the final CIF file.The crystal and refined data are collected in Table 1.Selective bond distances and angles are given in Table 2.
CCDC:1828500,1;1544271,2.
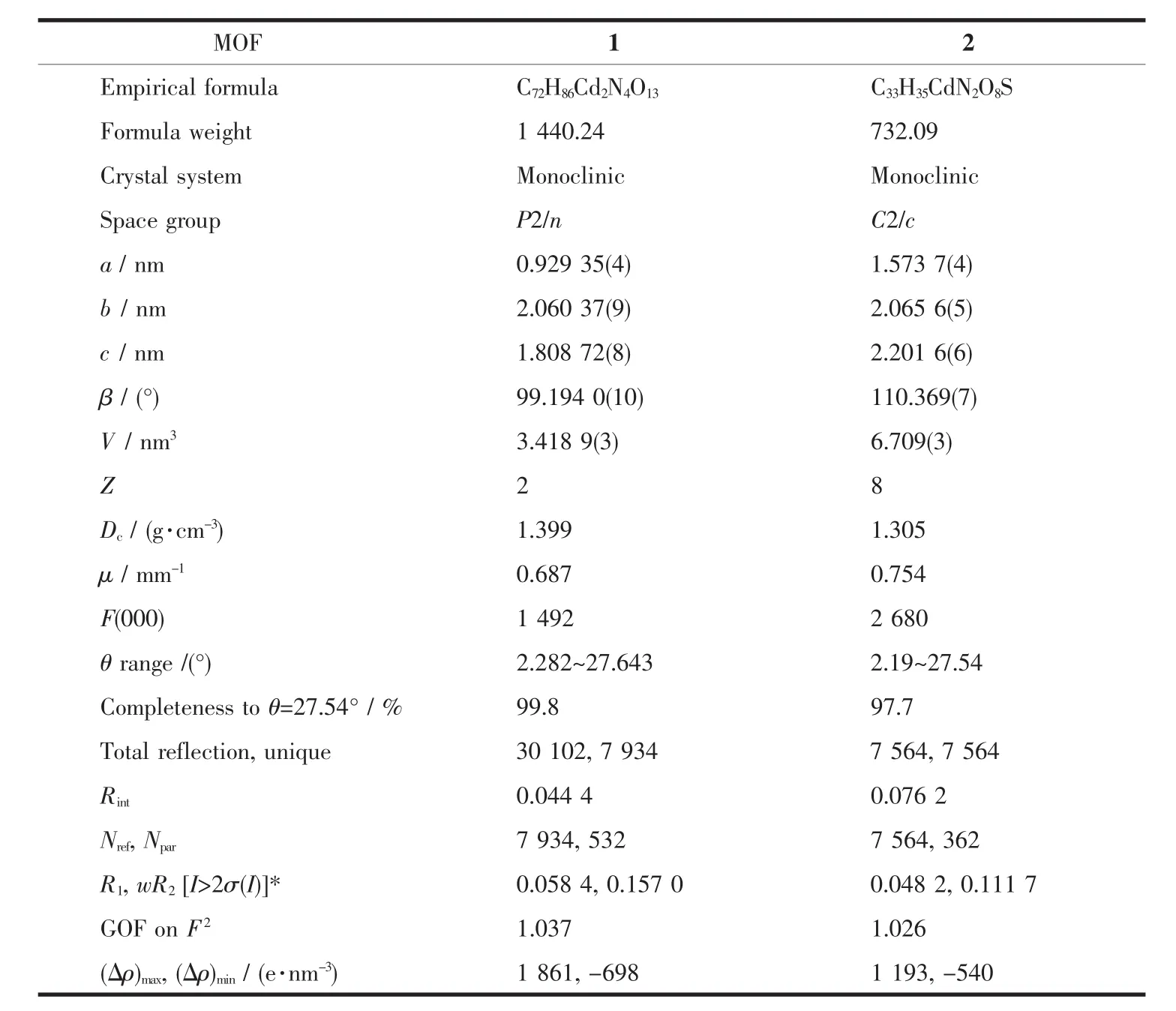
Table 1 Crystal data and structure refinement for MOF 1 and 2
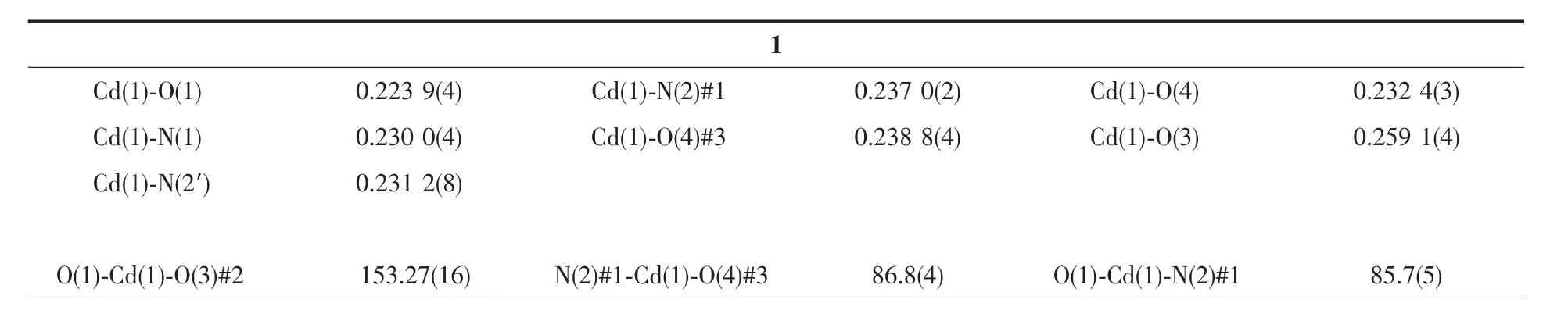
Table 2 Selected bond lengths(nm)and angles(°)for MOF 1 and 2
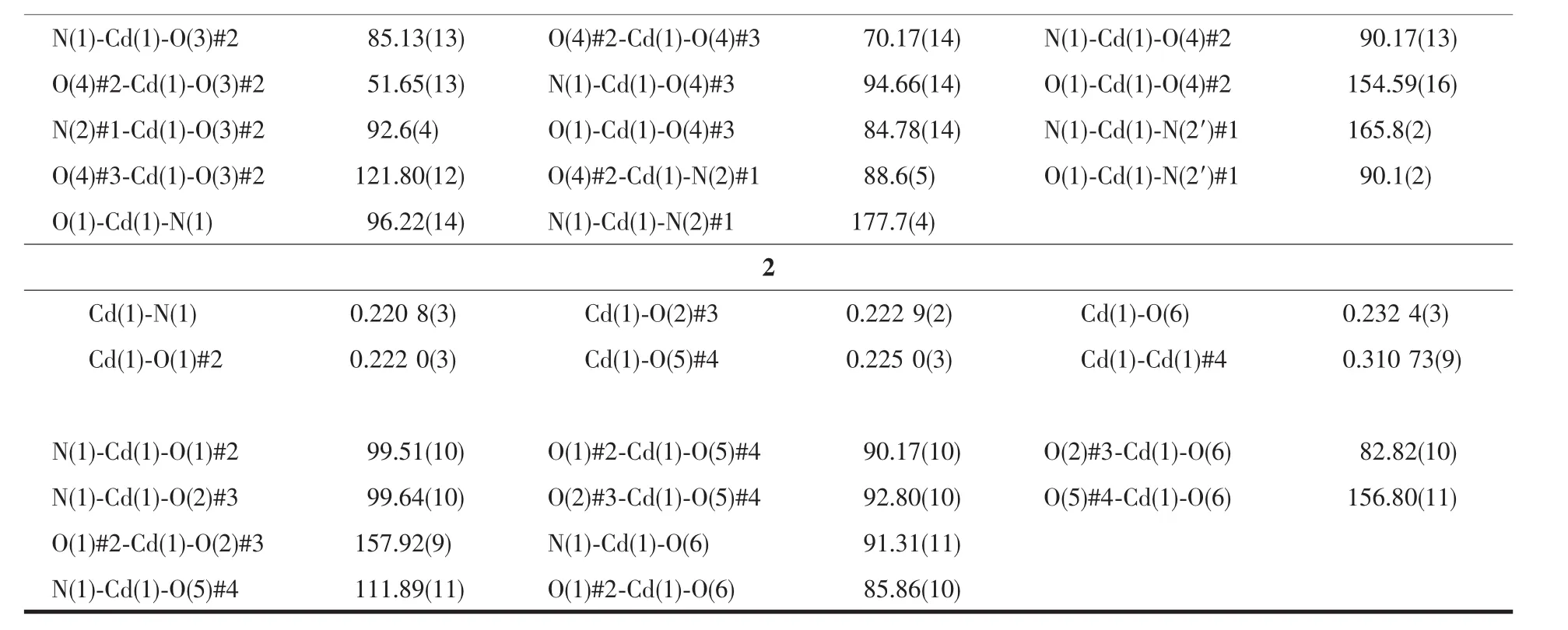
Continued Table 2
2 Results and discussion
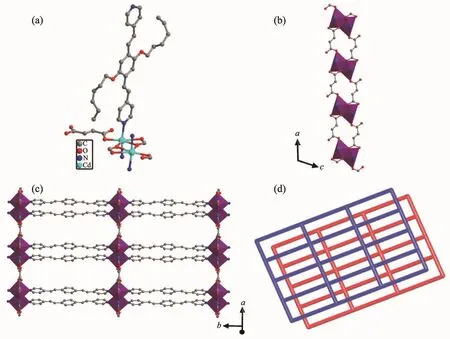
Fig.1 (a)Coordination environment of 1;(b)1D chain constructed by fma2-ligand and cadmium ion;(c)Views of 2D sheets;(d)Schematic representation of an sql poly-catenation of 1
2.1 Crystallographic description
2.1.1 Description of the crystal structure of{[Cd(L)(fma)]·0.5H2O}n(1)
Single-crystal structure analysis reveals that 1 crystallizes in the monoclinic crystal system with P2/n space group.The asymmetric unit of 1 consists of one CdⅡmetal center,one L ligand,one fma2-anion and a half of lattice H2O molecule.Each CdⅡcenter is seven-coordinated by five oxygen atoms from three different fma2-ligands and two nitrogen atoms from two different L ligands to form a pentagonal bipyramid(Fig.1a).The five oxygen atoms from three fma2-ligands locate in the equatorial plane,and two N atoms from two L ligands occupy the axial positions.The carboxylate groups adopt two coordination modes(Mode Ⅰ and Ⅱ of Fig.2)to connect two Cd centers to generate[Cd2(CO2)4]as a secondary building unit(SBU).The fma2-ligands connect SBUs to form 1D chains (Fig.1b).These 1D chains are connected by L ligand in the axial direction to form the 2D sheet(Fig.1c).
To better understand the nature of this intricate framework,we apply a topological approach,which reduces multidimensional structures to simple nodes and connection nets.The SBUs can be regarded as 4-connected nodes and the two ligands act as linkers.Thus,the whole structure can be characterized as a non-interpenetrated 2D+2D→2D parallel stacked 4-connected sql network with the point symbol of{44.62}(Fig.1d).
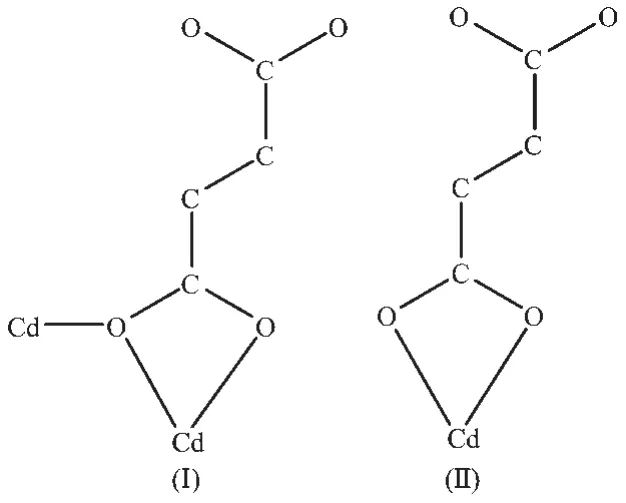
Fig.2 Coordination modes of carboxylic groups in MOF 1
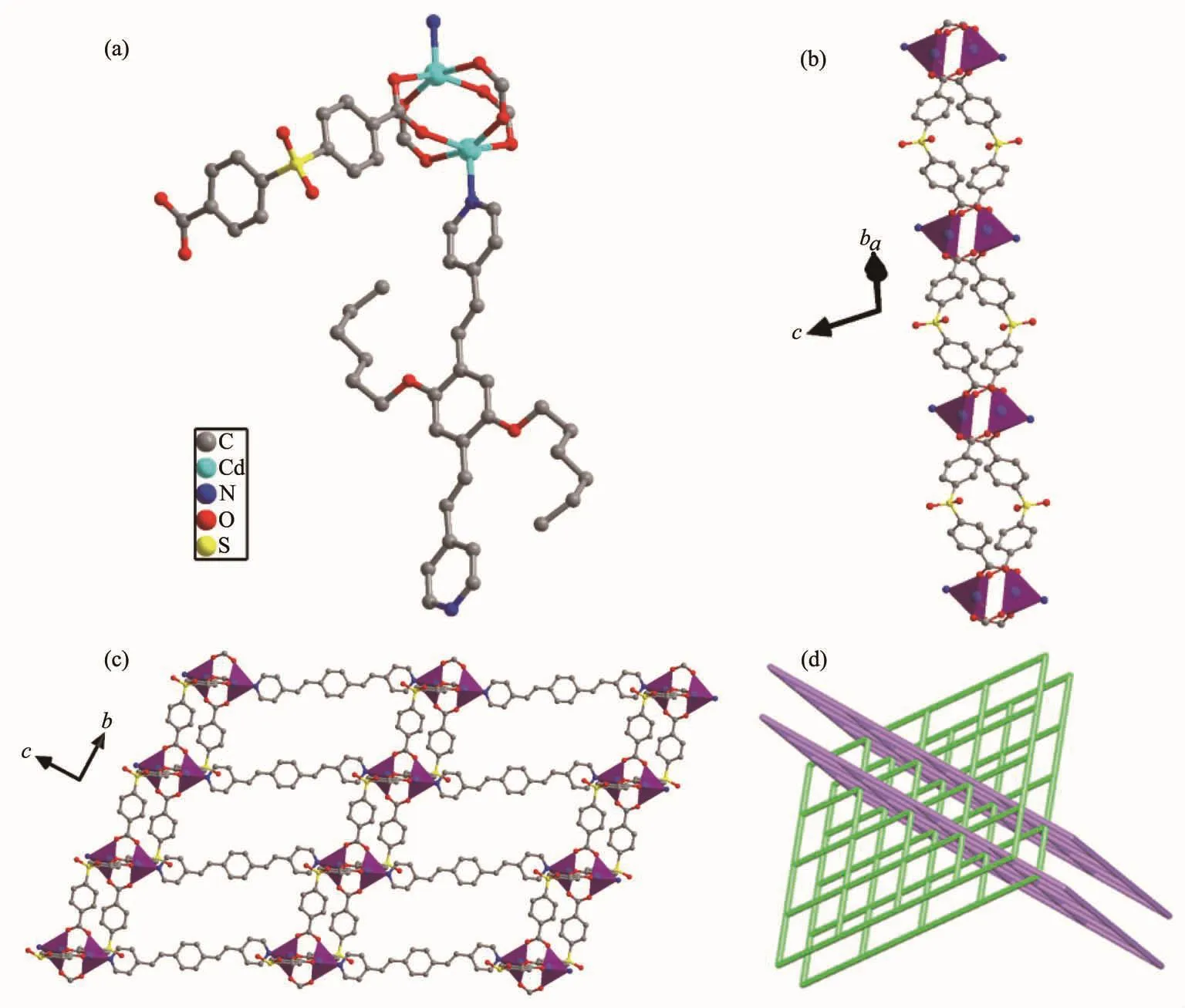
Fig.3 (a)Coordination environment of 2;(b)1D chain constructed by sdb2-ligand and cadmium ion;(c)Views of 2D sheets;(d)Schematic representation of an sql poly-catenation of 2
2.1.2 Description of the crystal structure of{[Cd(L)0.5(sdb)]·DMF}n(2)
Single-crystal structure analysis reveals that 2 crystallizes in the monoclinic crystal system with C2/c space group.The asymmetric unit of 2 consists of one CdⅡmetal center,a half of L ligand,one sdb2-anion and one lattice DMF molecule,which was removed by the SQUEEZE routine in PLATON.Each CdⅡcenter is five-coordinated by four oxygen atoms from four different sdb2-ligands and one nitrogen atom from one L ligand to form a square pyramid (Fig.3a).Four carboxylate groups from four different sdb2-ligands connect pairs of Cd ions to generate a dinuclear CdⅡsecondary building unit (SBU)[Cd2(CO2)4]in which the axial positions are occupied by L ligand.The sdb2-ligandsconnect the[Cd2(CO2)4](SBU)toform1Dchains(Fig.3b).These 1D chains are connected by L ligand in the axial direction to form the 2D sheet (Fig.3c).If the[Cd2(CO2)4]SBUsareregarded as4-connected nodes and the two ligands act as linkers,the whole structure can be characterized as a interpenetrated 2D+2D→3D inclined polycatenated 4-connected sql network with the point symbol of{44.62} (Fig.3d).
2.2 Thermal stability and powder X-ray diffraction(PXRD)
To examine the thermal stabilities of 1 and 2,TG analyses were carried out (Fig.4).The thermogravimetric curve of 1 indicating a weight loss of 1.19%(Calcd.1.25% )was observed from 100 to 180 ℃attributed to the loss of the one lattice water molecule.Further weight loss indicates the decomposition of coordination framework from 340℃.The TG thermogravimetric curve of 2 presented a 10.00%(Calcd.9.98%)weight loss of one solvent DMF molecule from 50 to 210℃,and the framework started to decompose at 380℃.To confirm whether the crystal structures are truly representative of the bulk materials,the powder X-ray diffraction (PXRD)patterns were recorded for 1 and 2,and they are comparable to the corresponding simulated patterns calculated from the single-crystal diffraction data (Fig.5),indicating a pure phase of each bulky sample.
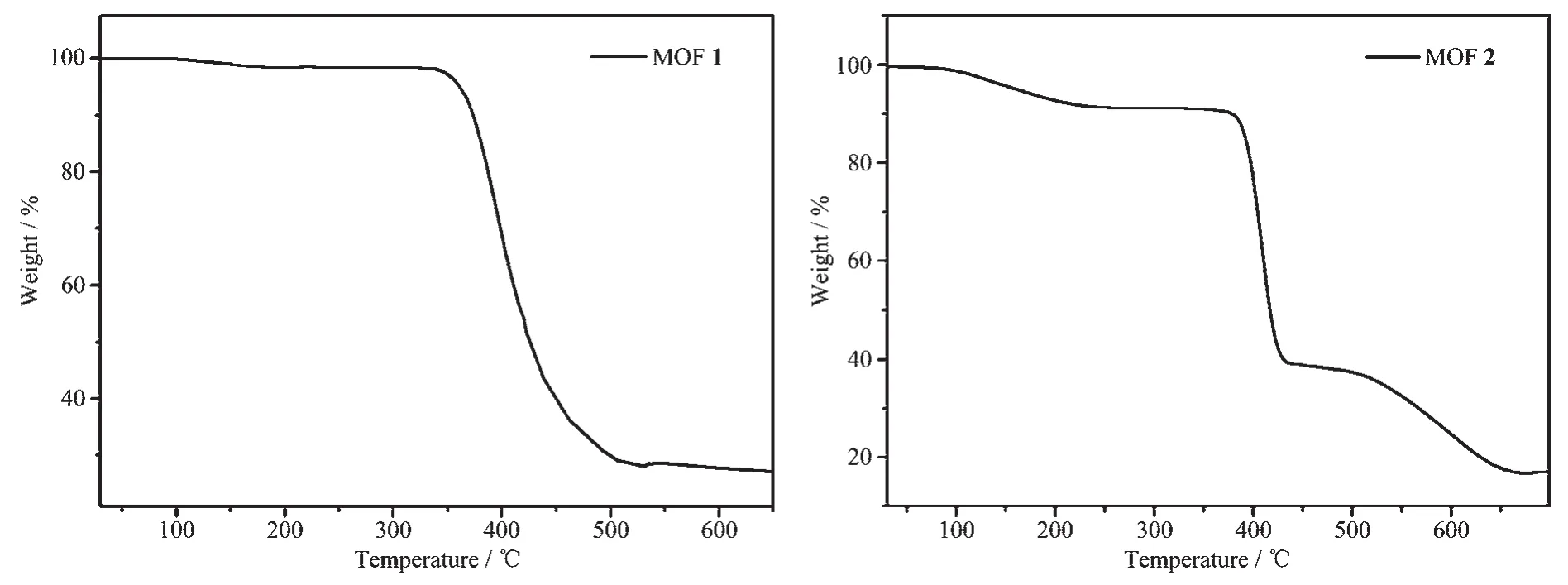
Fig.4 TG curves of MOFs 1 and 2
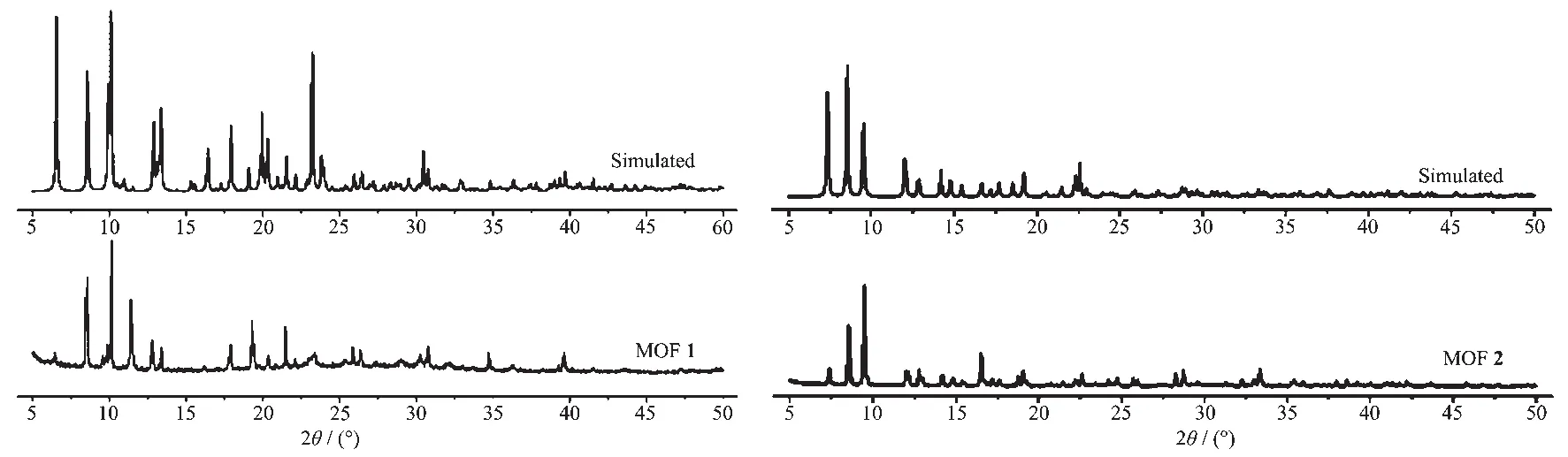
Fig.5 PXRD patterns of MOFs 1 and 2
2.3 Metal cations and organic molecules sensing
The studies of the selectively sensing ability for different metal cations were performed.Different DMF(100 μL)solutions containing 0.05 mol·L-1G (NO3)x(G=Al3+,Co2+,Cr3+,K+,Mg2+,Na+,Zn2+,Cu2+,Fe3+)were added into the suspension of MOFs 1 and 2,respectively.To our surprise,Fe3+showed an excellent quenching effect on the luminescence of MOFs 1 and 2.There was a significant change of luminescence intensity which decreased to 99%for 1 and 96%for 2,respectively (Fig.6).However,the luminescence quenching efficiency was not good for other cations,most for under 50%.To illuminate the possible mechanism of luminescence quenching by Fe3+,we studied the liquid UV-Vis absorption spectra of Fe3+,MOFs 1 and 2 (Fig.7).Obviously,Fe3+ion in DMF had a wide absorption band from 270 to 450 nm,which covered the whole ranges of MOFs 1 and 2.Therefore,the luminescence decrease of MOFs 1 and 2 after addition of Fe3+can be put down to the competition of absorbing light source energy between Fe3+and these two MOFs.Fe3+ion filters the light adsorption of MOFs 1 and 2.Naturally,the luminescence intensitiesof MOFs1and 2 areobviouslydecreased[18-21].
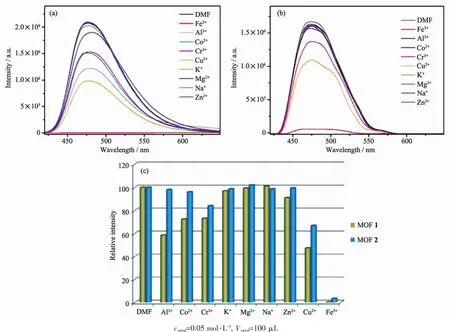
Fig.6 Fluorescence spectra of MOFs 1 (a)and 2 (b)in DMF suspension added different metal ions;Relative emission intensity in the presence of different metal ions (c)
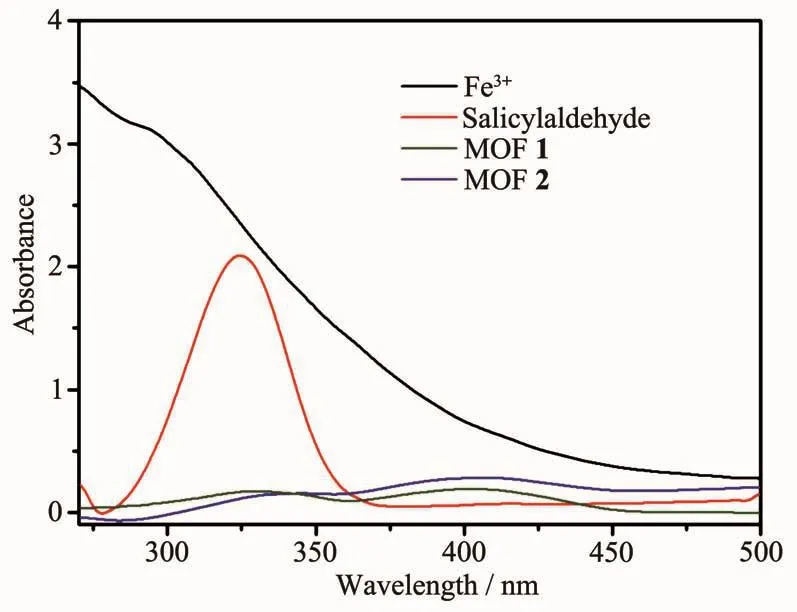
Fig.7 Liquid UV-Vis spectra of salicylaldehyde and MOFs 1 and 2,Fe3+in DMF
Furthermore,we carried out fluorescence sensing experiment to research the effect of organic molecules on MOFs 1 and 2.A few organic molecules were chosen to investigate,such as DMF,1,4-dioxane,N-methyl-2-pyrrolidone (NMP),acetonitrile,ethyl acetate(EA),isopropanol (IPA),ethanol,methanol,acetone,tert-butanol,salicylaldehyde.20μL different organic molecules mentioned above were added into the suspension of MOFs 1 and 2,respectively.Interestingly,only salicylaldehyde shows a definite quenching effect on the luminescence of MOFs 1 and 2(Fig.8).The quenching efficiency can reached 46%for 1 and 41%for 2.According to the UV-Vis absorption spectrum (Fig.7),salicylaldehyde in DMF has an absorption band in 325 nm,it covers partial absorption band of MOFs 1 and 2.Hence,the luminescent intensity of them was decreased partly.
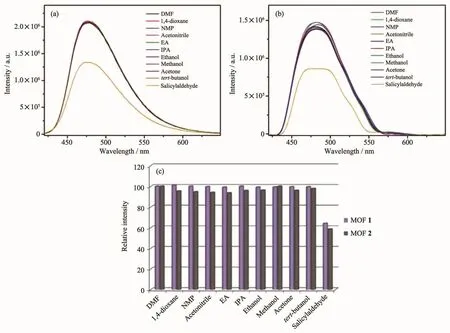
Fig.8 Fluorescence spectra of MOFs 1 (a)and 2 (b)in DMF suspension added 20 μL pure organic molecules;(c)Relative emission intensity in the presence of pure organic molecules
3 Conclusions
In summary,tw o new cadmium metal-organic frameworks have been successfully synthesized.Luminescence sensing measurements indicate that they can be developed as highly selective probes for detection of Fe3+in DMF solutions.We presumed that the luminescence quenching effect depended on the UV-Vis competitive adsorption.We also explored the potential of MOFs 1 and 2 for detecting organic molecules.The results show that salicylaldehyde presents the best quenching effect on these two MOFs.

