順、反-1,2-環(huán)己二胺對有機-無機雜化銻碘異構體的影響
梁志文 陳曉柔 于 慧 魏振宏 張秀秀 蔡 琥
(南昌大學化學學院,南昌 330031)
0 Introduction
During the past decades,organic-inorganic hybrid compounds have received considerable attention due to their opportunity to combine useful properties of both components[1-3],in which the inorganic component supplies the features of adjustable mechanical composition,large polarity,good optoelectronic properties,thermal stability and electron mobility;and the organic component usually acts as a structuredirecting agent and greatly affects the structure of the inorganic part,as well as balances the charge from the inorganic component.
As regards the metal-halide anions,an extensive work has been devoted to the semiconducting metal halide anions(Sn,Pb,Bi,Sb)because the semiconductor metals have an important application in photovoltaic cells because they have suitable band gap width,high light absorption coefficient,the equilibrium electron hole injection distance,and electronic mobility characteristics[4-11].On the other hand,it has been demonstrated that the organic amines as popular templates with tunable size,charge and shape can have a great effect on the final structures and properties of hybrids[12-13].Most popularly used organic amine cations incorporated in hybrids are either alkylammonium[14-15]or single ring aromatic ammonium cations[16].As have been already pointed out,the skeleton of diammonium cations is the key to the formation of novel inorganic structures[17-19].
1,2-Cyclohexanediamine(DAC)is an extraordinary interesting molecule because it displays two different stable cis-and trans-boat configurations as shown in Scheme 1[20-22].Not only its adjacent two primary amines may have a synergistic effect on the finial structure,but also the cis-and trans-1,2-cyclohexanediamines can react with the semiconducting metal halide to give different structures of organic-inorganic hybrid compounds,which will help to lay a solid foundation for the following structureproperty researches[23-27].

Scheme 1 cis-and trans-configurations of 1,2-cyclohexanediamine
So,we prepared two organic-inorganic hybrid isomers (cis-1,2-DACH2)[SbI5]·H2O (1)and{(trans-1,2-DACH2)[SbI5]·H2O}n(2)by reactions of cis-and trans-1,2-DAC with semiconducting metal halide iodoantimonate(Ⅲ),respectively.Herein,we report the syntheses,characterizations,fluorescent properties and DFT calculation of compounds 1 and 2.
1 Experimental
1.1 Instruments and materials
The starting materials antimony triiodide(SbI3),trans-/cis-1,2-DAC and the concentrated hydriodic acid(HI)are commercially available and were used as received.Powder X-ray diffraction data of the samples were recorded on an X-ray powder diffractometer(Beijing Persee Instrument Co.,Ltd.XD-3)with Cu Kα radiation(λ=0.154 06 nm)operating at 40 kV and 15 mA in a range of 5.00°~55.00°(2θ).The elemental analysis of C,H and N were determined using a Vario ELⅢelemental analyzer.The FT-IR spectra were recorded in a range of 4 000~400 cm-1with a Nicolet 5700 Spectrometer using KBr pellets.The UV-Vis spectra were measured at room temperature using a Perkin-Elmer Lambda 900 spectrophotometer.The photoluminescence spectra were conducted on a Hitachi F-7000 fluorescence spectrometer.The DFT calculation of 1 and 2 were performed at the B3LYP level of theory as implemented in the Gaussian 03 program package.
1.2 Synthesis
1.2.1 Compound 1
To a solution of SbI3(0.501 6 g,1.0 mmol)in 10 mL HIaqueoussolution(47%),cis-1,2-DAC(0.222 1 g,2.0 mmol)in 5 mL HI solution(47%)was added and the mixture was heated to 90℃and kept stirring for half an hour.After slowly cooling down to room temperature,the mixture gave orange block crystals of 1,which were filtered and dried under vacuum.Yield:0.614 0 g,68%.Anal.Calcd.for C6H18N2OSbI5(%):C,8.09;H,2.04;N,3.15.Found(%):C,8.21;H,2.02;N,3.46.IR(KBr,cm-1):3 437(s),3 018(s),2 933(s),2 866(s),1 622(m),1 581(s),1 561(s),1 500(s),1 459(s),1 388(w),1 348(w),1 317(w),1 266(w),1 236(w),1 185(w),1 155(w),1 107(w),1 087(w),1 036(w),1 016(w),955(w),890(w),853(w),806(w),752(w),674(w),613(w),566(w),494(w),423(w).
1.2.2 Compound 2
The similar procedures for synthesis of compound 1 based on trans-1,2-DAC(0.223 1 g,2.0 mmol)and SbI3(0.502 3 g,1.0 mmol)in the concentrated HI aqueous solution gave orange block crystals of 2.Yield:0.658 7 g,74%.Anal.Calcd for C6H18N2OSbI5(%):C,8.09;H,2.04;N,3.15.Found(%):C,8.23;H,1.89;N,2.34.IR (KBr,cm-1):3 561(m),3 494(m),3 066(m),2 989(s),2 942(s),2 866(s),1 625(w),1 586(s),1 544(m),1 502(m),1 477(s),1 441(m),1 388(w),1 366(w),1 354(w),1 317(w),1 277(w),1 261(w),1 237(w),1 205(w),1 175(w),1 130(w),1 079(w),1 061(m),1 023(w),1 006(w),998(m),930(w),898(w),874(w),837(w),767(w),507(w),434(w).
1.3 X-ray crystallography
Diffraction data of two block single crystals with dimensions of 0.20 mm×0.18 mm×0.15 mm for 1 and 0.25 mm×0.20 mm×0.15 mm for 2 were collected on a Bruker SMART CCD area detector diffractometer with graphite monochromated Mo Kα radiation(λ=0.071 073 nm).The crystal structures were solved by direct methods using SHELXSL-97.Non-hydrogen atoms were first refined isotropically followed by anisotropic refinement by full matrix least-squares calculations based on F2(SHELXL-97)[28].Hydrogen atoms on carbon and nitrogen atoms were placed in idealized positions and treated as riding atoms.While hydrogen atoms on water were first located in the difference Fourier maps then positioned geometrically and allowed to ride on their respective parent atoms.Crystal data and structure refinement results of compounds 1 and 2 were summarized in Table 1.
CCDC:1818719,1;1818718,2.

Table 1 Crystallographic data for compounds 1 and 2
2 Results and discussion
2.1 Synthesis and characterization
The phase purities of 1 and 2 were verified using the powder X-ray diffraction (PXRD)patterns which matched very well with the simulated ones in terms of the single-crystal X-ray data as shown in Fig.1.TG analysis showed that compounds 1 and 2 have similar curves,both firstly lost their crystalline water at a range of 25~120 ℃,then completely decomposed their whole skeletonsat a rangeof 270~350℃ (Fig.2).
2.2 Structure comparison
Compounds 1 and 2 are isomers,both crystallize in the monoclinic system with P21/c space group.In their asymmetric units,both have a [SbI5]2-anion,a protonated 1,2-cyclohexanediamine cations (1,2-DACH22+)and a solvated water.It can be seen from Fig.3 that the cis-and trans-1,2-DACH22+keep their original configurations after reactions with SbI3.

Fig.1 Powder X-ray diffraction patterns of compounds 1 and 2

Fig.2 TGcurves of compounds 1 and 2
In compound 1,two [SbI6]octahedra are first constructed into a dimer [Sb2I10]by sharing with the II edge.In the dimer,there is a symmetric center locating at the middle of Sb(1)…Sb(1A)bond.The crystallographically independent antimony atom is coordinated by six I atoms in a significantly distorted octahedral coordination environment with bond lengths ranging from 0.282 40(6)to 0.329 49(7)nm and the maximum bond angle I(4)-Sb(1)-I(3)of 175.500(18)°(Table 2),consistent with those found in other halogenoantimonate(Ⅲ)[29-30].On the other side,the cis-1,2-DACH22+works as a bridge connecting the [Sb2I10]dimers by hydrogen bonds N-H…I along b direction to form a chain,and the solvated water is linked to the chain by the N-H…O hydrogen bond(Fig.4).
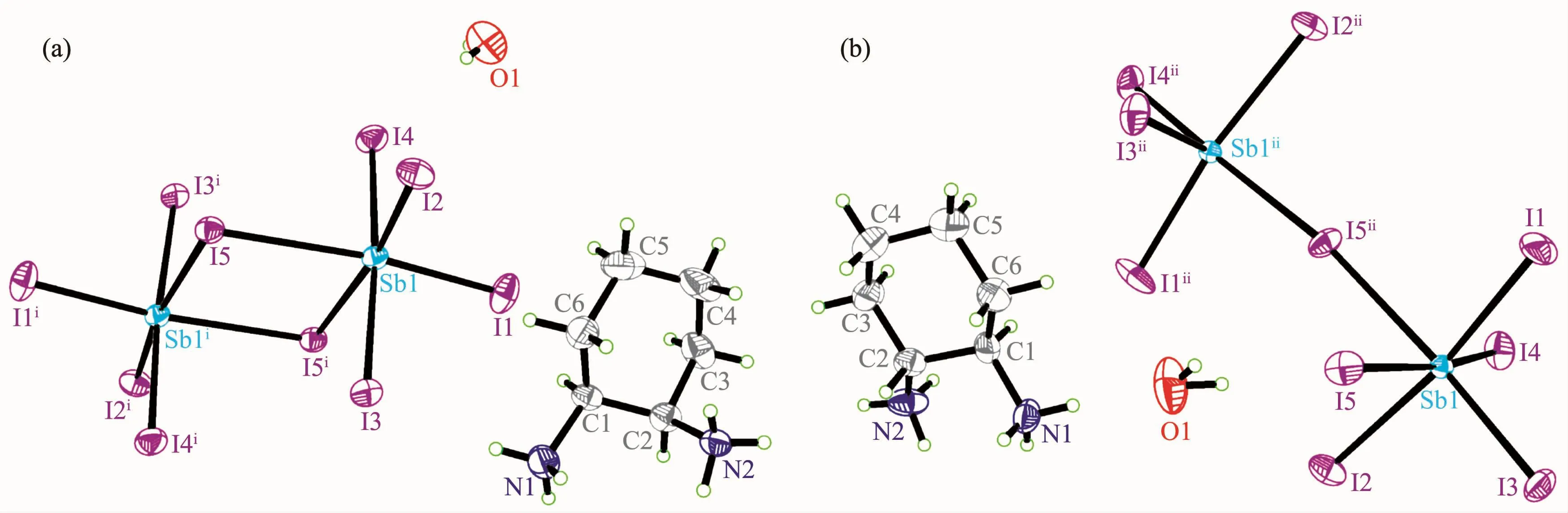
Displacement ellipsoids probability level:50%;Symmetry codes:i 2-x,2-y,-z for 1;ii 3.5-x,-0.5+y,-0.5-z for 2

Table 2 Selected bond lengths(nm)and angles(°)for compounds 1 and 2

Continued Table 2
While,in compound 2,the [SbI6]2-ions are bridged by I5 atom to form a one-dimensional chain along b direction,and the trans-DACH22+ions are connected to the anionic chain by hydrogen bonds in addition to the ionic bond between them.As shown in Table 2,the Sb-I distances,ranging from 0.280 92(11)to 0.322 64(12)nm for the terminal iodine atoms and from 0.283 42(11)to 0.314 19(16)nm for the bridging iodine ones,closely agree with those observed in other zigzag chain structures[31-32].The trans-DACH22+and solvated water act as two connecting-points tying together the one-dimensional strands into twodimensional layered step-like structure by hydrogen bonds(Fig.5).

Fig.4 Anionic dimers [Sb2I10]2-connecting the cis-DACH22+cations by hydrogen bonds

Fig.5 trans-DACH22+cations connected to the 1D chain by hydrogen bonds
2.3 Absorption and fluorescent spectra
The room temperature absorptions of compounds 1 and 2 showed very similar curves as shown in Fig.6.In the absorption spectrum of compound 1,there were three obviously peaks at 235,362 and 429 nm,which can be attributed to the charge transfer transitions in the ligand,between the organic and inorganic layers,and within the inorganic layers.This is because the organic-inorganic hybrid is a type of semiconductor quantum well structure,typically with small band gap inorganic sheets(carrier)alternating with larger band gap organic layer(well)[33].Compared with compound 1,the corresponding peaks in compound 2 were located at 245,348 and 499 nm which have red shifts,consistent with the rule that the energy gap decreases as the dimensionality increases.The solid fluorescent spectra of compounds 1 and 2 are showed in Fig.7.At the excitation wavelength of 365 nm,compounds 1 and 2 exhibited the emission peaks at 566 and 568 nm,respectively,both could be ascribed to the inorganic semiconducting moieties [SbI6][34-36].

Fig.6 UV-Vis spectra of compounds 1 and 2 in solid states

Fig.7 Emission spectra of compounds 1 and 2 in solid states
2.4 DFT calculations
The energy difference between the two configurations of free 1,2-DAC is about 22 kJ·mol-1from DFT calculations,in which the trans-configuration is more stable than the cis-one.After protonation,the energy difference is little increased to about 28 kJ·mol-1,and configuration conversion was expected between transand cis-configurations.However,compounds 1 and 2 lost their crystalline water at 25~120 ℃ (Fig.2),so it is difficult to discuss the configuration inversion.
3 Conclusions
In this paper,by properly choosing 1,2-cyclohexanediamine(1,2-DAC)with cis-and trans-configurations to react with semiconductor halide SbI3,two different organic-inorganic halide (cis-1,2-DACH2)[SbI5]·H2O(1)and{(trans-1,2-DACH2)[SbI5]·H2O}n(2)were obtained.The single crystal diffraction and DFT calculations revealed that although compounds 1 and 2 are isomers,they are totally different in their properties.The relationship between structure-property is of great significance for further research on the organic-inorganic hybrid materials in practical.
Acknowledgements:We thank the National Natural Science Foundation of China (Grants No.21571094,21661021,21865015)and the Jiangxi Provincial Department of Science and Technology(Grant No.20161BAB203073) for financial support.

