The universal characteristic water content of aqueous solutions?
Xiao Huang(黃曉),Ze-Xian Cao(曹則賢),3,and Qiang Wang(王強)
1Institute of Physics,Chinese Academy of Sciences,Beijing 100190,China
2University of Chinese Academy of Sciences,Beijing 100190,China
3Songshan Lake Materials Laboratory,Dongguan 523808,China
Keywords:aqueous solutions,stretching vibration of water,concentration dependence,hydration number
1.Introduction
Identifying the types of water and quantifying their fractionsin aqueous solutions can help us to understand their physiochemicalproperties and behaviors.[1-9]To achieve this goal,the first challenge is to quantify hydration water.Accordingly,various technological methods with different spatial and temporal resolutions have been adopted to quantify the hydration number of solute,nh.Regretfully,most of the reported values differ from each other.Recently,following our previous work in the past few years,the universal concentration dependence of the icing/vitri fication behavior for aqueous solutions has been established,[10-13]from which a convenient and reliable method of determining nhis developed.
The value of nh,quanti fied based on concentration dependent crystallization and vitri fication of water,re flects the degree of solutes affecting their adjacent water from the viewpoint of promoting solvent water vitri fication in a dilute solution.The sede fined hydration water can easily vitrify at a moderate and even slow rate,e.g.,as low as 10-2K/s.[14]The critical cooling rate required for initiating vitri fication of bulk water is about 106K/s.[15]For the dilute solution,it can be simply regarded as a mixture of hydrated solute and bulk-like free water.However,witha decrease of water content,more hydration shells become closely compacted and even overlapped and, finally,free water disappears.[5]The question here is whether or not there is an obvious critical concentration point,through which a mixture of hydrated solutes and free water for dilute solutions changes into that of hydrated solutes and con fined water for medium-concentrated ones.
As recently reported,for a series of solutions of electrolytes and organic molecules,their glass-forming ability shows an abrupt change at a critical concentration point,defi ned as M·ncH2O for brevity,where M is solute.[10,13]For M·nH2O with n>nc,some water molecules can easily crystallize into ice upon cooling,even at a high rate.After that,the residual freeze-concentrated solution,which shows high glass-forming ability,can easily vitrify even at a slow cooling rate.In contrast,when water content decreases below that of M·ncH2O,the solutions can easily vitrify totally.Most interestingly,as already con firmed,the value of nc/nhis nearly solute-type-independent for electrolytes and molecules especially with higher molar weights,and nc/nh≈1.7.[12]
Besides glass forming ability,many other properties of water and solute of organic molecule solutions,which are measured at room temperature,also manifest a de flection feature or abrupt change of their concentration dependence with the variation of water content.[16-19]On the side of solvent water,these properties include spin-spin and spin-lattice relaxations,[20]adiabatic compressibility coefficient,[21]diffusion coefficient,[22]viscosity,[19]partial molar volume or vapor pressure,[23]Raman shift,etc. On the side of organic molecules solute,the property mainly refers to the CH-stretching vibration.[24]Due to the lack of a clear physical picture behind this behavior,the molar ratio of water to solute at such a deflection point for concentration dependence of phys-ical properties has often been taken as the hydration number.Actually,in most cases,the concentration of this deflection point is consistent with that of M·ncH2O.[24]
Here,we focus our interest on concentration dependence of O-H stretching vibration of water in solutions of electrolytes at room temperature.We find that,like those scenarios observed in solutions of organic molecules,the O-H stretching vibration of water in solutions of electrolytes also displays obviously a deflection at a characteristic concentration,which,most importantly,is also very consistent with that of M·ncH2O,and nc≈ 1.7nh.Therefore,a universal characteristic water content of aqueous solutions highly correlated with the hydration numbers of solutes for properties measured at low and room temperatures is experimentally con firmed.
2.Experiment
2.1.Samples
High-purity water was prepared by using a Millipore Milli-Q system. The salts CaCl2(CaCl2·2H2O,purity 99.99%),CaBr2(99.98%),Ca(NO3)2(99.99%),Ca(ClO4)2(Ca(ClO4)2·4 H2O 99%),MgCl2(anhydrous 99.99%),MgBr2(99.99%),Mg(NO3)2,Mg(ClO4)2(Mg(ClO4)2·6 H2O 99%),AlCl3(AlCl3·6 H2O 99%),Al(NO3)2(AlCl3·6 H2O 99%),and Al(ClO4)3(Al(ClO4)3·9 H2O 98%)and D2O(99.9%)were purchased from Sigma-Aldrich.
2.2.Raman spectrometric measurements
Raman spectra were measured at 295 K by using a Jobin-Yvon HR800 Raman system.For excitation,the spectral line at 532 nm of a laser with about 100 mW was used.The laser power of 1 mW was focused on the sample surface through the fused SiO2film with a×50 long focus objective.
3.Results and discussion
As an example, figure 1 shows the Raman spectra in a range from 2800 cm-1to 3800 cm-1for solutions of MgCl2of various concentrations measured at 295 K.We choose pure H2O and mixture of H2O with D2O in a molar ratio of H2O:D2O=1:4,respectively,as a solvent.
In Figs.1(a)and 1(b),the solution of D2O for the electrolytes usually has an unaltered hydration number.In Fig.1(b),the coupling of the OH-stretching vibration,both intramolecular and intermolecular,can be effectively suppressed,and thus improves the accuracy of the peak position at around 3400 cm-1from the Raman spectra.Figure 1(c)shows the plots of the locations of the peak maximum of Raman spectra plotted in Figs.1(a)and 1(b),and those reported in Ref.[25]versus n for MgCl2·nH2O or MgCl2dissolved in D2O with dilute H2O with a molar ratio of 4:1.The variation of peak position for the component around 3400 cm-1obtained by Gaussian fitting of the spectra in Fig.1(a),as conventionally done in the literature,is also presented for comparison.Clearly,in all the cases,the peak position of the OH-stretching vibration shows a deflection point,a change of tendency,in their water-content dependence.Noticeably,the de flection occurs at n≈20,corresponding to nc≈1.7nh(nh=12[13]).
The deflection behavior of the water content dependence of peak position of Raman spectra shown in Fig.1(c)can be verified in aqueous solutions of various other electrolytes(Fig.2),where the peak positions of Raman spectra for the OH-stretching vibration,measured at 295 K,are plotted as a function of n/nh.As determined previously,nhranges from 6 for LiCl to 19 for AlCl3.[13]Over such a wide range of nh,the water content dependence of the Raman shift measured on these aqueous solutions inclusively displays a de flection point at n/nh.≈1.7.All of these electrolyte solutions also show similar water content dependence of icing/vitri fication of water to the behavior shown in Fig.1(d).For more details,see Supplementary Information of Ref.[13].
The deflection occurring at M·ncH2O,where nc≈ 1.7nh,may be related to the variation of microstructure of water and solute in aqueous solution as the concentration changes.As mentioned earlier,the solvent water may be found to be in different status,namely free water,con fined water(spatially confined among hydrated solutes),and hydration water,respectively,depending on the water content.To explain the abrupt change in glass forming ability of aqueous solutions at M·ncH2O as shown in Fig.1(d),we have recently suggested that aqueous solutions can be divided into three distinct concentration zones(Fig.1(d)).Solutions within water rich zone III,solvent water is comprised of hydration water and bulk-like free water.Free water can easily crystallize into ice,and,differently,hydration water can easily vitrify.The value of nhis then quantified just based on this characteristic feature of hydration water.When water content decreases below M·ncH2O,the aqueous solutions can easily vitrify,even at a slow cooling rate.It has been suggested that this abrupt change in glass forming ability of solution can be mainly attributed to the disappearance of free water.In other words,solutions change from a mixture of free water and hydrated solutes within zone III to a mixture of hydrated solutes and confined water in zone II.This proposition can be proved by the cold-crystallization behavior of water appearing upon thawing vitrified or deeply supercooled aqueous solutions at concentrations within zone II.Otherwise,if all water molecules in zone II are regarded as hydration water,then solutions within this zone show only devitrification behavior in heating process,such as that of the solutions within zone I, wherein solutes cannott be hydrated to the greatest extent.
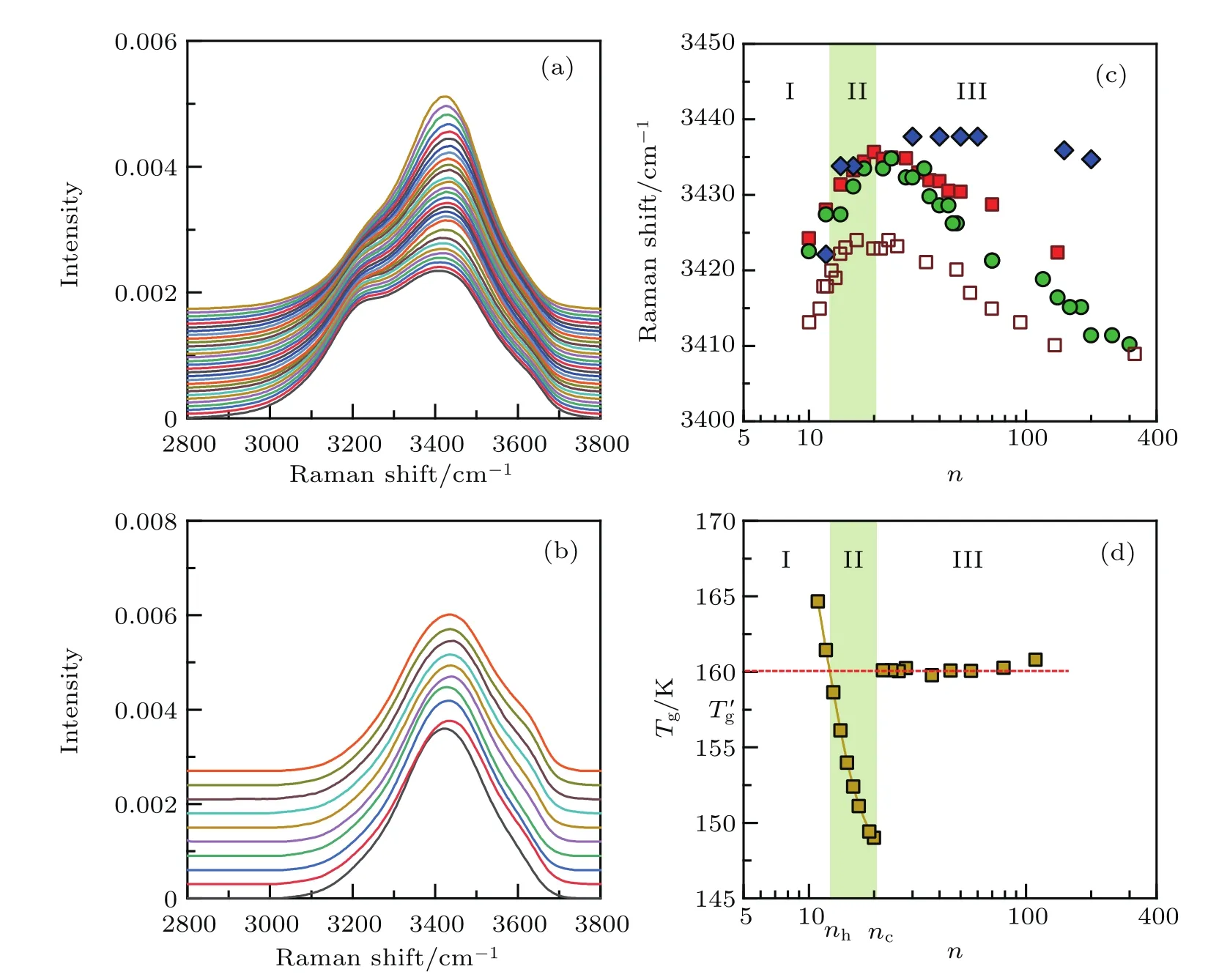
Fig.1.(a)Raman spectra for OH-stretching vibration of H2O for MgCl2solutions,MgCl2·nH2O,with n=300,200-80 in steps of 20,and 50-10 in steps of 2(from bottom curve to top curve).(b)OH-stretching vibration of HDO in solution of MgCl2dissolved in D2O mixed with dilute H2O with a molar ratio of 4:1.Molar ratios of water to solute n are 200(bottom curve),150,60,50,40,30,26,16,14,12(top curve).(c)Peak position for OH-stretching vibration as a function of n plotted on the basis of data from panels(a)(solid circle)and(b)(solid diamond).The data of MgCl2·nH2O reported by Burikov et al.[25]are also shown for comparison(empty square).Moreover,the peak position of the component around 3400 cm-1obtained by Gaussian fitting of the spectra in panel(a)is also presented(solid square).(d)Water content dependence of glass transition temperatures,Tg,of aqueous solution of MgCl2.In dilute solutions with n>nc,glass transition occurs after spontaneous icing in cooling process,thus it involves only the glass transition of freeze-concentrated solution,which manifests an almost constant Tg,de fined as T′g.The extension of T′g-n can intersect the fitting curve of Tg-n for concentrated solutions at a feature point,i.e.,MgCl2·nhH2O,where nhis the hydration number of solute.For more details,see Supplementary Information in Ref.[13].
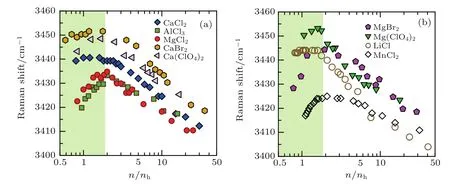
Fig.2.Peak position of Raman spectra for the OH stretching vibration of water at about 3400 cm-1as a function of the ratio of n/nhfor aqueous solutions of various electrolytes M·nH2O.In panel(a)M refers to CaCl2(diamond),CaBr2(hexagon),Ca(ClO4)2(triangle),MgCl2(circle),and AlCl3(square),and in panel(b)M refers to MgBr2(pentagon),Mg(ClO4)2(triangle),LiCl[25](circle),and MnCl2[25](square).
The previously-mentioned division of concentration zone is further con firmed by an obvious change in concentration dependence of OH-stretching vibration of solvent water at M·ncH2O as highlighted in this work.Actually,this nonmonotonic dependence of OH-stretching vibration of water on concentration has already been tentatively discussed based on a proposed change in the con figuration of ion-pairs with water content.[25]With decreasing the water content,the ion-pairs in solution gradually come into contact from the initially isolatingstatus through the solvent-sharing intermediate stage.[26,27]For some solutes,such as LiCl presented in Fig.2(b)(empty circles),the ions always tend to stay separated by hydration water due to a strong cation-water interaction and a relatively weak anion-water interaction.[28]In this case,below a critical water content point,the hydration water can be believed to be structurally insensitive to a further increase of solute content.As a result,the peak position of the O-H stretching vibration remains nearly unchanged at low water content.However,in those aqueous solutions with stronger cation-anion interaction,even if solvent-separated or solvent-sharing ion-pairs are present in water-rich environment,the ion-pairs may come into contact with further reducing the water content.If the fraction of contacting ion-pairs increases with reducing water in the water-poor solutions,the water molecules will become more strongly hydrogen-bound to each other.Consequently,the Raman spectra of the solvent water will be red-shifted as the water content is reduced.This explanation in terms of ionpair con figuration above seems to be implacable to water in aqueous solution of CsCl,because the Cs+1and Cl-1ions will come into contact even in a water-rich solution,[28]yet the solution still manifests this deflection feature of the water content dependence of the OH-stretching vibration.[25]Therefore,the detailed mechanism obtained from the microstructures of the solution needs further investigation.
4.Conclusions
In this work,we have measured the Raman spectra of the O-H stretching vibration of water for aqueous solutions of a series of electrolytes in a wide range of water content.The results clearly exhibit a deflection point,which appears inclusively at the water content corresponding to nc≈1.7nh,where nhrepresents the hydration number of the given solute.The existence of the critical hydration number nc,together with nhwhich divides the aqueous solution into three distinct zones,is studied in the investigation of low temperature behaviors such as icing/vitrification.The critical hydration number ncindicates a turning tendency for the variation of microstructures in the aqueous solution,and it is reasonable that it will exhibit some properties of the solutions at room temperature.This characteristic feature is universal for solutions of organic molecules and even of electrolytes,and it might help us to understand the bizarre variation tendency of properties of aqueous solutions,particularly those with water content in the neighborhood specified by the critical hydration number.
- Chinese Physics B的其它文章
- Theoretical study of overstretching DNA-RNA hybrid duplex?
- influence of carbon coating on the electrochemical performance of SiO@C/graphite composite anode materials?
- Spin glassy behavior and large exchange bias effect in cubic perovskite Ba0.8Sr0.2FeO3-δ?
- Aging mechanism of GaN-based yellow LEDs with V-pits?
- Magnetotransport properties of graphene layers decorated with colloid quantum dots?
- Temperature-dependent subband mobility characteristics in n-doped silicon junctionless nanowire transistor?

