Synthesis of Zn, Mn doped Fe3O4 Nanoparticles with Tunable Size
CHENG Tian-Sheng, PAN Jiong, XU Ying-Ying, BAO Qun-Qun, HU Ping, SHI Jian-Lin
Synthesis of Zn, Mn doped Fe3O4Nanoparticles with Tunable Size
CHENG Tian-Sheng1,2,3, PAN Jiong4, XU Ying-Ying1,2,3, BAO Qun-Qun1,2,3, HU Ping1, SHI Jian-Lin1
(1. State Key Laboratory of High Performance Ceramics and Superfine Microstructures, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China; 2. University of Chinese Academy of Sciences, Beijing 100049, China; 3. Shanghai Tech University, Shanghai 201210, China; 4. Tongji University, Shanghai 200092, China)
Zn, Mn doped Fe3O4magnetic nanoparticles have broad application prospects in biomedicine for their excellent magnetic properties. Therein, the most remarkable property of magnetic nanoparticles is size-dependent biomagnetic applications, and size variation also affect their magnetic characteristics. Therefore, based on the specific requirements of size for various biological applications, it is critical to regulate their size. In this study, we synthesized 5–20 nm Zn, Mn doped Fe3O4magnetic nanoparticles by changing reflux time duration, varying metal precursor and adding oil phase reducing agent (1,2-hexadecanediol). It is found that addition of 1,2-hexadecanediol isbeneficial to the formation of smaller nanoparticles, while metal chloride and longer reflux time are helpful to prepare larger particles. Additionally, there exists a positive correlation between particle size and saturation magnetization.
Zn, Mn-Fe3O4nanoparticles; size regulation; magnetic characteristics
Magnetic iron oxide nanoparticles (MNPs) have been researched extensively due to their promising applications for biomedicine such as hyperthermia[1-2], magnetic resonance imaging[3-4]and biolabeling[5]. Magnetic characteristics is highly significant for successful application of MNPs in biomedicine/biotechnology[6]. Recent studies suggest that a metal (Mn, Zn) dopant substitution strategy can achieve high magnetic performance[7-8]. The Zn, Mn doping level, a key factor, can be regulated by simply adjusting the original molar ratio of metal precursors. Moreover, when the ratio of doping Zn and Mn is 2:3, saturation magnetization (s) can reach its maximum[9].
For decades, a variety of preparation methods of MNPs achieved in hydrolytic phase have been developed, including hydrothermal reaction[10], Sol-Gel process[11]and coprecipitation method[12]. Magnetic iron oxide nanoparticles can also be obtained by organic-phase thermal decomposition of metal precursor, for example, decomposition of Fe(acac)2followed by oxidation to Fe3O4in the presence of oleylamine and oleic acid[6,13]. Recent studies showed that organic-phase thermal decomposition methods could overcome the shortcomings of traditional hydrolytic one: it allows more strict control on particles uniformity and structure[14-15].
Size-dependent biomagnetic applications are the most remarkable properties of MNPs[16-17]. Different biological applications have specific requirements on particle size. For example, in the separation of cells, the size of MNPs should be 8 to 10 nm[17-18], but magnetothermal therapy requires larger nanoparticles, resulting from their higher specific absorption rate[19-20]. On the other hand, the size variation also affects the coercivity (c) and saturation magnetization (s) of the magnetic nanoparticles. However, it remains a great challenge to control MNPs size due to the lack of sufficient understanding of formation mechanism of magnetic particles referring to nucleation and growth process[21]. Therefore, it is necessary to study the effects of related parameters on particle size so that we can synthesize magnetic particle with tunable size. In this study, we present a one-step preparation of 5-20 nm Zn, Mn doped Fe3O4(ZnMn-Fe3O4) nanoparticles, which can adapt to broader biomagnetic applications.
1 Experimental
Material Oleic acid (OAc) and Oleylamin (OAm) were purchased from Sigma-Aldrich. Other chemicals were purchased from Shanghai Aladdin Biochemical Technology Co. Ltd.
Preparation of 5 nm ZnMn-Fe3O4nanoparticles Oleylamine (3 mmol), oleic acid (3 mmol), Fe(acac)3(1 mmol), Zn(acac)2(0.2 mmol) , Mn(acac)2(0.3 mmol) and 1,2-hexadecanediol (5 mmol) were placed in a 50 mL three-neck round-bottom flask in the presence of 15 mL benzyl ether. The mixture was heated to 200 ℃ for 30 min and then to 300 ℃ for 1 h under argon atmosphere. After cooling to room temperature, excess ethanol was added to the solution, then a black powder precipitated and was collected by centrifugation. The magnetic nanoparticles were redispersed in cyclohexane.
Preparation of 10 nm ZnMn-Fe3O4nanoparticles Oleylamine (3 mmol), oleic acid (3 mmol), Fe(acac)3(1 mmol), Zn(acac)2(0.2 mmol) and Mn(acac)2(0.3 mmol) were placed in a 50 mL three-neck round-bottom flask in the presence of 15 mL benzyl ether. The mixture was heated to 200 ℃ for 30 min and then to 300 ℃ for 1 h under argon atmosphere. After cooling to room temperature, excess ethanol was added to the solution, then a black powder precipitated and was collected by centrifugation. The magnetic nanoparticles were redispersed in cyclohexane.
Preparation of 15 nm ZnMn-Fe3O4nanoparticles Oleylamine (3 mmol), oleic acid (3 mmol), Fe(acac)3(1 mmol), ZnCl2(0.2 mmol) and MnCl2(0.3 mmol) were placed in a 50 mL three-neck round-bottom flask in the presence of 15 mL benzyl ether. The mixture was heated to 200 ℃ for 30 min and then to 300 ℃ for 1 h under the atmosphere of argon. After cooling to room temperature, excess ethanol was added to the solution. The blackprecipitate was collected by centrifugation and washed with ethanol prior to redispersion in cyclohexane for further use. Similarly, by prolonging reaction time to 1.5 or 2 h, 20 nm ZnMn-Fe3O4nanoparticles can be prepared.
Characterization Transmission electron microscopy (TEM) was performed on JEM-2100F instruments. The XRD studies were conducteda Rigaku D/MAX-2250V diffractometer with a Cu Kα radiation source (40 kV, 120 Ma). Magnetic measurements were performed on a Vibrating Sample Magnetometer (VSM). FT-IR spectra were recorded by an IRPRESTIGE-21 spectrometer (Shimadzu) using KBr pellets. Size distribution were measured on Nano-Zetasizer (Malvern Instruments Ltd.).
2 Results and discussion
Reductive effect As shown in Fig. 1(a), monodispersed ZnMn-Fe3O4nanoparticles about 5 nm were fabricated by decomposition of Fe(acac)2, Mn(acac)2and Zn(acac)2in the presence of oleylamine, oleic acid and 1,2-hexadecanediol. Under the same experimental conditions, if 1,2-hexadecanediol was not added, the size of particles increased from 5 nm to 10 nm (Fig. 1(b)), which were further proved by the alteration of size distribution (Fig. 1(c, d)). Organic-phase thermal decomposition in fact is a process that activates metal atoms to nucleate and grow[21-22], so the size of particles is mainly dependent on the nucleation and growth rate of metal atoms. Herein, the addition of strong reductive 1,2-hexadecanediol contributed to facile reduction of M(acac)2to M0(M=Zn, Mn), leading to faster consumption of metal acetylactone and nucleation of particles, as a result, smaller nanoparticles were formed.

Fig. 1 TEM images of ZnMn-Fe3O4 nanoparticles and histograms of their size distributions obtained by Fe(acac)3, Mn(acac)2 and Zn(acac)2with (a, c) and without (b, d) adding 1,2-hexadecanediol
Metal precursor effect Metal precursorsplay an important role in size regulation of magnetic nanoparticles. The size of nanoparticles synthesized by metal chloride as precursor is about 15 nm with narrow size distribution (Fig. 2(a, b)). The high resolution TEM (Fig. 2(c)) and selected area electron diffraction (SAED) pattern (Fig. 2(d)) suggest that the particles have high-quality crystallinity. In contrast, using acetylacetone as metal precursor, the size of obtained nanoparticles is about 10 nm (Fig. 1(b)). According to chemical bond theory, metal chloride is more stable than metal acetylacetone,thus it requires higher decomposition temperature. Therefore, the addition of metal chloride may lead to slower nucleation rate, more metal precursor could deposit surrounding the nuclei formed in mixture and resulting in larger nanoparticles.
Reflux time effect It is also important to study the effect of reaction time on the size of nanoparticles. In this work, we found that larger particles could be obtained by longer reflux time. As shown in Fig. 3(a), when reflux time was postponed from 1 h to 1.5 h under the same reaction condition, the particles size increased from 15 nm (Fig. 2(a)) to 20 nm (Fig. 3(a)), which were further proved by the alteration of size distribution (Fig. 3(c)). However, further prolongation of the reflux time to 2 h resulted in no distinct increase in the size of nanoparticles (Fig. 3(b, d)), which indicates that the nanocrystals will stop growing when the reaction reaches homeostasis. In the meantime, it seems to produce new nanocrystal with longer reflux time (Fig. 3(b)).
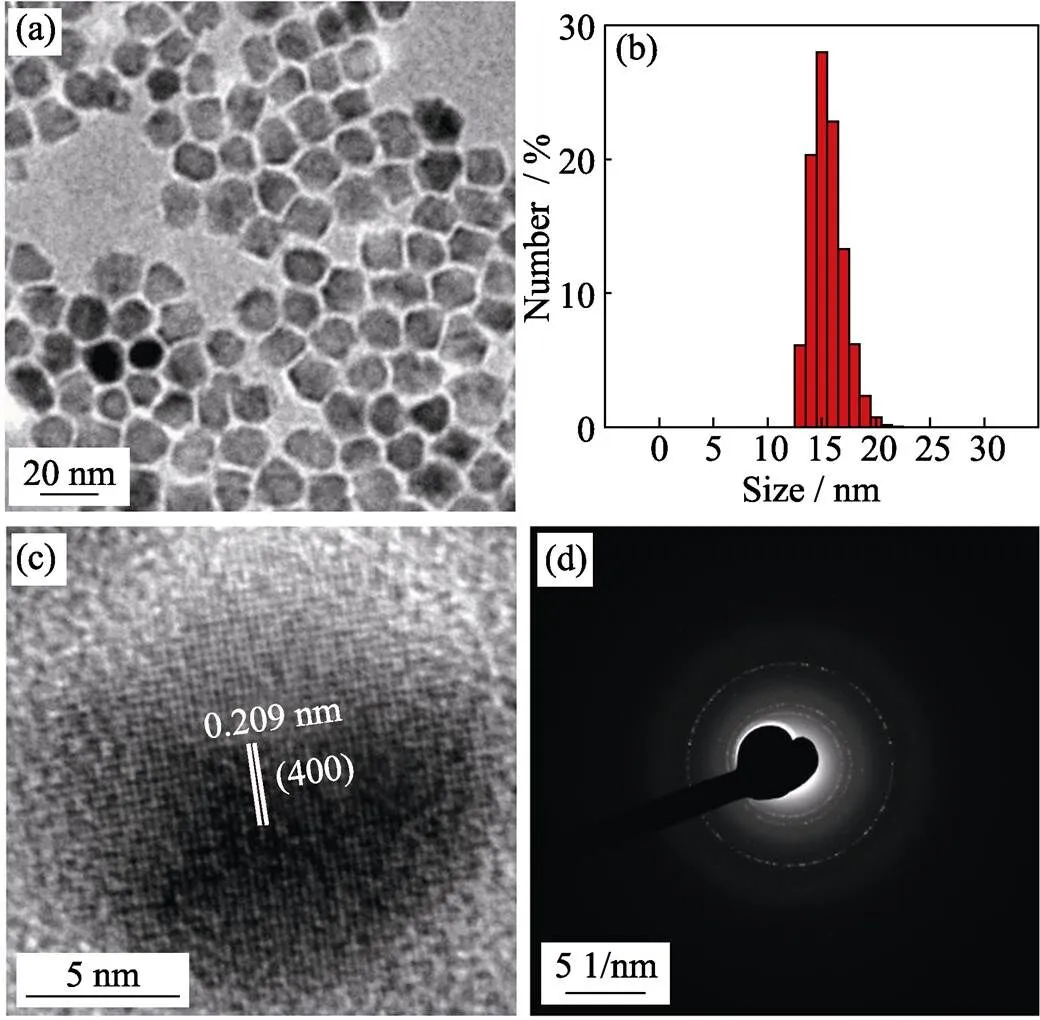
Fig. 2 TEM image (a), histograms of their size distributions (b), high-resolution TEM image (c) and selected area electron diffraction (SAED) pattern (d) of 15 nm-sized ZnMn-Fe3O4 nanoparticles prepared from Fe(acac)3, MnCl2 and ZnCl2
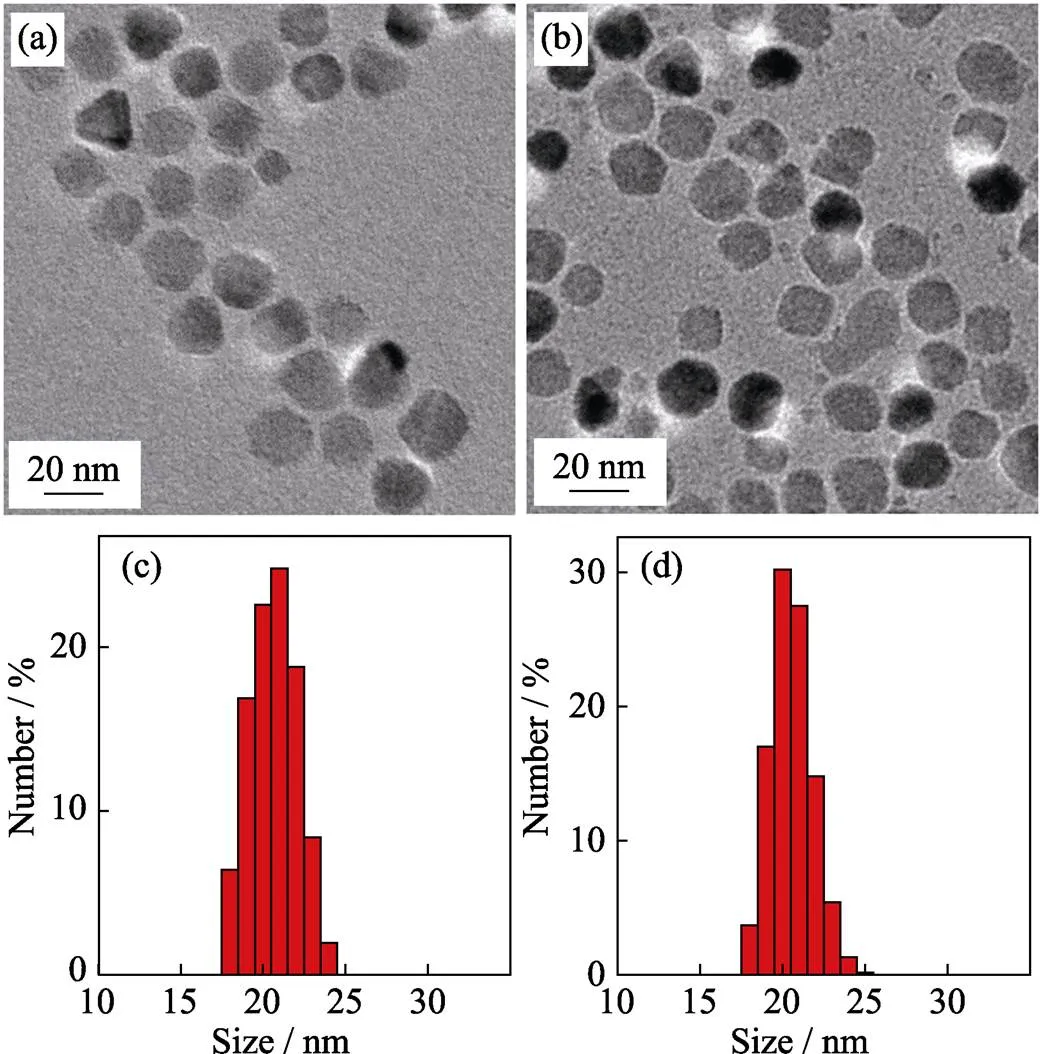
Fig. 3 TEM images of 20 nm-sized ZnMn-Fe3O4 nanoparticles synthesized from Fe(acac)3, MnCl2 and ZnCl2 with different reflux time durations of 1.5 h (a) and 2 h (b), and the corresponding histograms of size distributions of 1.5 h (c) and 2 h (d)
The XRD pattern (Fig. 4(a)) shows a single-phase spinel structure of 5–20 nm ZnMn-Fe3O4nanoparticles and the relative intensity and position of all diffraction peaks/rings accord well with previously reported Zn, Mn doped Fe3O4powder[23], which indicates that crystal structure has little relevance with particle size. Two characteristic stretching were recorded in FT-IR spectrum at 2920 and 2850 cm-1, 1630, and 1410 cm-1corresponding to H-C-H, C-O stretching, respectively (Fig. 4(b)), which reveals that particles are coated by oleylamine and oleic acid. In this case, in addition to acting as reductive, oleylamine and oleic acid also work as surfactant. The organic surfactants can be removed by 2,3-dimercaptosuccinic acid (DMSA) to yield water-soluble nanoparticles[20]. The Zn, Mn doping levels were measured by energy dispersive X-ray spectroscopy (EDS) and the results indicate that 5–20 nm particles have similar element composition (Fig. 4(c)).
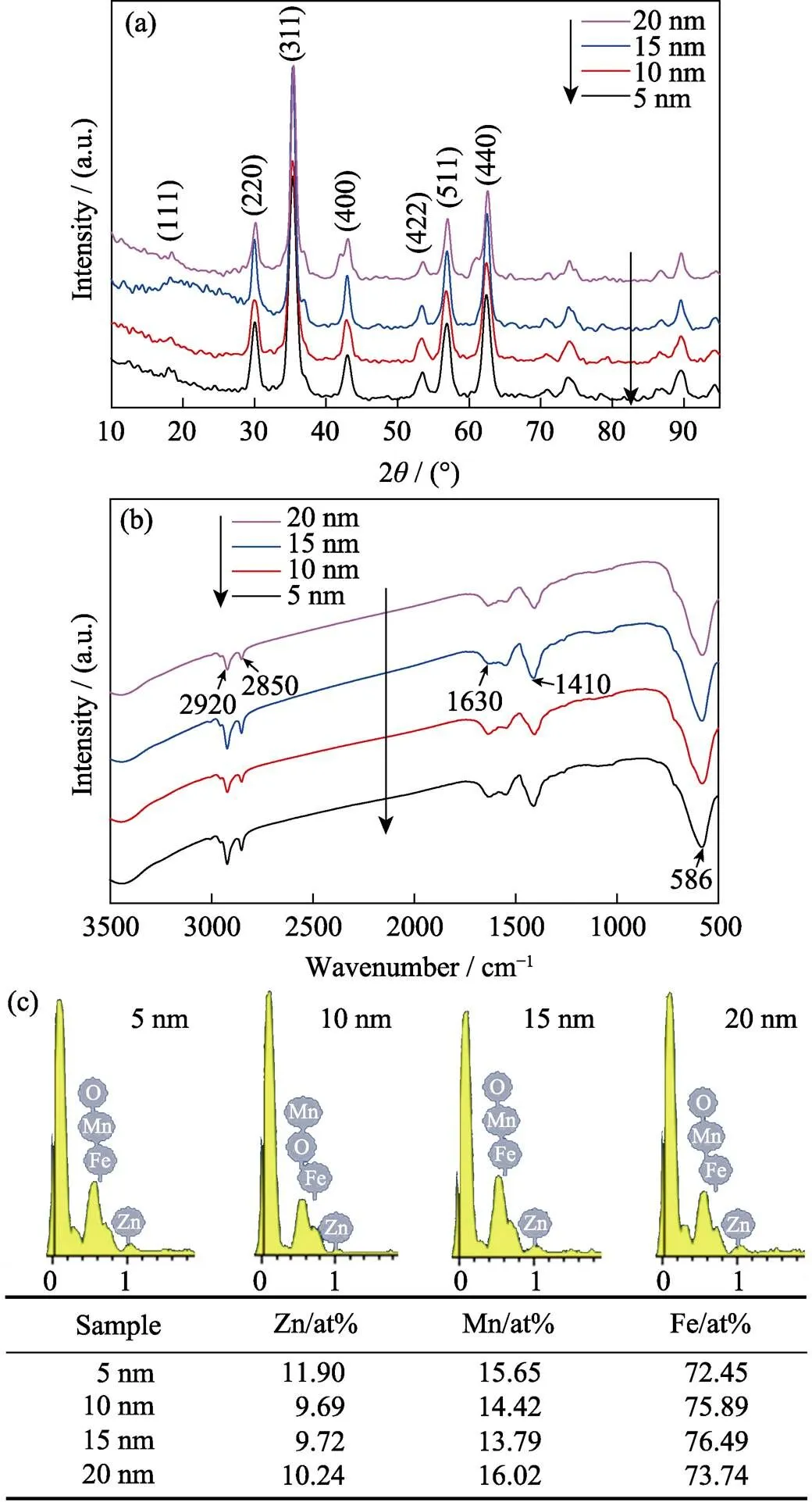
Fig. 4 XRD patterns (a), FT-IR spectra (b) and energy dispersive X-ray spectroscopy (EDS) data (c) of 5 nm, 10 nm, 15 nm, and 20 nm-sized ZnMn-Fe3O4 nanoparticles
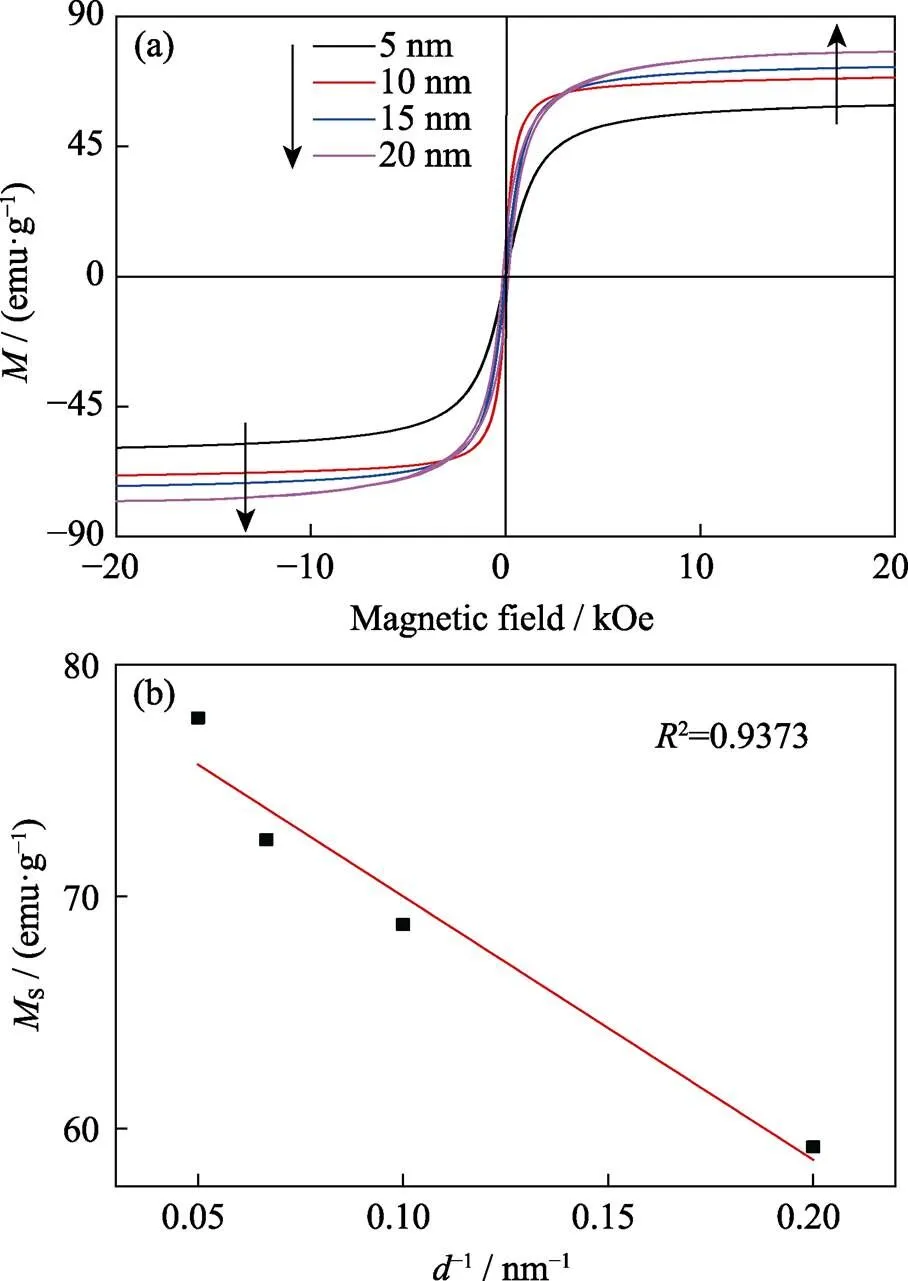
Fig. 5 Magnetic hysteresis curve (a) and d-1-dependent Ms curve (b) of 5–20 nm-sized ZnMn-Fe3O4 nanoparticles
Size effect on magnetic characteristics Magnetic characteristics of MNPs with different size were measured by VSM at 300 K, and the results are shown in Fig. 5(a). The hysteresis curve of MNPs in 5–15 nm exhibits superparamagnetism without remnant magnetization. However, 20 nm particles are ferromagnetic with a coercivity of 150 Oe (1 Oe≈79.6 A/m). Saturation magne-tization (s) value of MNPs gradually increased as the size of MNPs increased from 5 to 20 nm, and reached the maximum of 77.7 emu/g(emu/g=4π×10–7Wb?m/kg). As Fig. 5(b) shown,sand d-1(the reciprocal of the nan-oparticles size) display an approximate linear relation-ship, which is consistent with previous reports[24-25].
3 Conclusion
We have successfully synthesized 5–20 nm ZnMn-Fe3O4by changing metal precursors, varying reflux time and adding 1,2-hexadecanediol. These ferrite nanoparticles with different particle sizes could adapt to the needs of extensive biomagnetic applications. Smaller sized nanoparticles could be obtained by introducing extra reducing power 1,2-hexadecanediol, andwe can prepare larger particles by using more stable metal precursor and extending reflux time. These seem to indicate that powerful reducing agent and metal precursor with low decomposition temperature will yield smaller particles. Meanwhile, the magnetic characteristics of ZnMn-Fe3O4are closely related to particle size. Understanding how the different parameters impact on particle size is extremely critical to develop a more predictive and controllable way to synthesize the MNPs.
[1] Noh S H, Na W, Jang J T,Nanoscale magnetism controlsurface and exchange anisotropy for optimized ferrimagnetic hysteresis, 2012, 12(7): 3716–3721.
[2] Di Corato R, Bealle G, Kolosnjaj-Tabi J,Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes., 2015, 9(3): 2904–2916.
[3] Na H B, Song I C, Hyeon T. Inorganic nanoparticles for MRI contrast agents, 2009, 21(21): 2133–2148.
[4] Gao J H, Gu H W, Xu B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications., 2009, 42(8): 1097–1107.
[5] Wan Y, Cheng G, Liu X,Rapid magnetic isolation of extracellular vesicleslipid-based nanoprobes., 2017, 1: 0058.
[6] Lu A H, Salabas E L, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application, 2007, 46(8): 1222–1244.
[7] Hochepied J F, Pileni M P. Magnetic properties of mixed cobalt- zinc ferrite nanoparticles., 2000, 87(5): 2472–2478.
[8] Arulmurugan R, Jeyadevan B, Vaidyanathan G,Effect of zinc substitution on Co-Zn and Mn-Zn ferrite nanoparticles prepared by co-pecipitation., 2005, 288: 470–477.
[9] Jang J T, Nah H, Lee J H,Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles, 2009, 48(7): 1234–1238.
[10] Deng H, Li X, Peng Q,Monodisperse magnetic single- crystal ferrite microspheres., 2005, 44(18): 2782–2785.
[11] Woo K, Lee H J, Ahn J P,Sol–Gel mediated synthesis of Fe2O3nanorods, 2003, 15(20): 1761–1764.
[12] Wu J H, Ko S P, Liu H L,Sub 5 nm magnetite nanoparticles: synthesis, microstructure, and magnetic properties, 2007, 61(14/15): 3124–3129.
[13] Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents, 2010, 62(11): 1064–1079.
[14] Lee J H, Huh Y M, Jun Y w,Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging, 2007, 13(1): 95–99.
[15] Singh M, Ramanathan R, Mayes E L H,One-pot synthesis of maghemite nanocrystals across aqueous and organic solvents for magnetic hyperthermia., 2018, 12: 250–259.
[16] Fortin J P, Wilhelm C, Servais J,Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia, 2007, 129(9): 2628–2635.
[17] Gu H, Xu K, Xu C,Biofunctional magnetic nanoparticles for protein separation and pathogen detection., 2006(9): 941–949.
[18] Xu H, Aguilar Z P, Yang L,Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood, 2011, 32(36): 9758–9765.
[19] Lee J H, Jang J T, Choi J S,. Exchange-coupled magnetic nanoparticles for efficient heat induction, 2011, 6(7): 418–422.
[20] Lartigue L, Innocenti C, Kalaivani T,Water- dispersible sugar-coated iron oxide nanoparticles. An evaluation of their relaxometric and magnetic hyperthermia properties, 2011, 133(27): 10459–10472.
[21] Wu L, Mendoza-Garcia A, Li Q,Organic phase syntheses of magnetic nanoparticles and their applications., 2016, 116(18): 10473–10512.
[22] Sun S H, Zeng H, Robinson D B,Monodisperse MFe2O4(M = Fe, Co, Mn) nanoparticles., 2004, 126(1): 273–279.
[23] Qu Y, Li J, Ren J,Enhanced magnetic fluid hyperthermia by micellar magnetic nanoclusters composed of Mn(x)Zn(1-x)Fe(2)O(4)nanoparticles for induced tumor cell apoptosis., 2014, 6(19): 16867–16879.
[24] Rong C b, Li D, Nandwana V,Size-dependent chemical and magnetic ordering in L10-FePt nanoparticles., 2006, 18(22): 2984–2988.
[25] Jun Y W, Huh Y M, Choi J S,Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosismagnetic resonance imaging., 2005, 127(16): 5732–5733.
鋅錳摻雜Fe3O4納米顆粒的尺寸可控合成
程田盛1,2,3, 潘炯4, 徐鷹鷹1,2,3, 鮑群群1,2,3, 胡萍1, 施劍林1
(1. 中國科學院 上海硅酸鹽研究所, 高性能陶瓷和超微結構國家重點實驗室, 上海 200050; 2. 中國科學院大學, 北京 100049; 3. 上海科技大學, 上海 201210, 4. 同濟大學, 上海 200092)
鋅錳摻雜的Fe3O4納米顆粒具有優異的磁性能, 在生物醫藥領域有廣泛的應用前景。磁性納米顆粒的尺寸與其磁學性質以及生物磁性應用密切相關。因此, 為了適應不同生物應用對尺寸的需求, 研究其尺寸調控具有重要的意義。在本研究中, 我們采用高溫熱分解法, 通過加入還原劑1,2-十六烷二醇, 改變金屬前軀體和回流時間成功制備了尺寸在5~20 nm的鋅錳摻雜Fe3O4納米顆粒。研究發現:強還原劑1,2-十六烷二醇的加入有利于合成小尺寸的納米顆粒, 而以金屬氯化物作為金屬前軀體和延長回流時間可以進一步合成更大尺寸的納米顆粒; 納米顆粒的飽和磁化強度隨著尺寸的增大而增大。
鋅錳摻雜Fe3O4納米顆粒; 尺寸調控; 磁學性質
TQ174
A
1000-324X(2019)08-0899-05
10.15541/jim20190013
2019-01-06;
Modified date: 2019-01-24
National Natural Science Foundation of China (51702349); Science Foundation for Youth Scholar of State Key Laboratory of High Performance Ceramics and Superfine Microstructures (SKL201704); Shanghai Yangfan Program (16YF1412800).
CHENG Tian-Sheng (1994-), male, candidate of Master degree. E-mail: chengtsh@shanghaitech.edu.cn
Corresponding author:HU Ping, associate professor. E-mail: huping@mail.sic.ac.cn; SHI Jian-Lin, professor. E-mail: jlshi@mail.sic.ac.cn