兩種含氮類大豆苷元衍生物對人體紅細胞的抗溶血作用
劉會青 于渼璇 宋靜磊 延 璽*, 徐 寧 郝海軍
(1北京師范大學化學學院,北京 100875)(2北京大學第三醫院風濕免疫科,北京 100083)(3北京化工大學化學學院有機化學系,北京 100029)
0 Introduction
Free radicals are highly reactive species which are predominantly produced during cellular respiration and normal process of metabolism.Many of them serve useful physiological functions[1-2],but they can also damage the biomolecules when present in excess.Reactive oxygen species (ROS),such as superoxide anion free radical(·O2-),hydrogen peroxide(H2O2)and hydroxyl free radical (HO·),can cause cellular injuries and initiate peroxidation of polyunsaturated fatty acids in biological membranes of the human body[3-5].They are known to be the major cause of various chronic and degenerative diseases,including aging,coronary heart disease,inflammation,stroke,diabetes mellitus,atherosclerosis and cancer[6-10].Imbalance between the production of cellular free radicals and the ability of cells to defend against them is referred to as oxidative stress(OS),which has been implicated asapotential contributor to the pathogenesis of acute central nervous system (CNS)injury.After brain injury by ischemic and hemorrhagic stroke or trauma,the production of ROSmay increase,sometimes drastically,leading to tissue damage via several different cellular molecular pathways[11-17].The tissue injury caused by ROS may include DNA damage,protein damage and oxidation of important enzymes in the human body.These events could finally lead to the occurrence of various free-radical related diseases.Moreover,acute brain injury can increase the levels of excitotoxic amino acids,such as glutamate.Cells activated by excess excitotoxic amino acids become overexcited and this over excitation can trigger the ROS formation,which promotes the oxidation of low density lipoprotein (LDL),increasing the risk of atherosclerosis which deteriorates the artery wall[18-19].Autoimmune diseases are chronic diseases,such as rheumatoid arthritis (RA),which is a rather common disease with an about 1%incidence in the Western world[20].It is clear that ROSplay a crucial role in RA since ROS are important mediators of damage to cell structures,damaging both the deoxyribose backbone and the purineand pyrimidinebases[21-22].Epidemiologic studies have indicated that RA preferentially occurs in previously healthy subjects who have low levels of antioxidants[23].Gelderman et al.summarized the role of reactive oxygen species in disease RA development and therapeutic strategies[24].For keeping the balance between the generation and the elimination of free radicals,the human body has evolved a wide array of enzymatic and no enzymatic endogenous antioxidant defence systems[25-26].However,the cellular antioxidant defence systems are imperfect, and regulation antioxidant capability is limited.The free radical in excess can seriously damage human health and the injuries will become more widespread.This is why supplementation of exogenous antioxidants is necessary to maintain human health.The exogenous antioxidants can help scavenge surplus free radicals in human body and prevent propagation of tissue damage and oxidation of LDL in blood,and can adjust immune excessive response in RA.The atherosclerosis and CNS injury can thus be improved by intake of exogenous antioxidants for human being[27-30].Much attention has recently been focused on the potential antioxidant effects of components in the diet[31-33].For example,epidemiological evidence supports a protective action of dietary antioxidants,such as αtocopherol,ascorbic acid and tea polyphenols against atherosclerosis and associated vascular dysfunction[34-37].More recent studies have highlighted the potential cardio protective and ant atherosclerosis effects of polyphenolic compounds,such as genistein and daidzein,available from a wide variety of sources including red wine and soy products,for example,soybean milk and fermented bean card[38-39].The biological activity of daidzein derivatives has been a research focus of scientists[40-43].We are interested in the antioxidant properties of daidzein derivatives containing nitrogen.In the present study,we have investigated the antioxidant activities of two new daidzein derivatives containing nitrogen (target compounds)as inhibitors of ROS.Structures of the compounds used in the study are shown in Fig.1.Their free radical scavenging activities were assessed by examining their ability to capture the hydroxyl free radical,the superoxide anion free radical,and to protect human erythrocyte membrane against oxidative damage induced from AAPH (2,2′-azobis(2-amidinopropane)hydrochloride).

Fig.1 Structures of target compounds L1 and L2
1 Experimental
1.1 Reagents and instruments
Terephthalate(TA),tris base,dimethylsulphoxide(DMSO),AAPH were of the best purity from commercial sources.All other chemicals were of analytical grade commercially available and used without further purification unless otherwise noted.All solutions were freshly prepared before use.Elemental analyses were conducted using a Vario EL elemental analyzer.UVVis absorption spectra were recorded by a spectrophotometer UV-2450 and fluorescence spectra were recorded on a Cary Eclipse fluorescence spectrophotometer with a quartz cuvette (path length=1 cm).1H NMR spectra were obtained using a Bruker Avance DRX 500 MHz.spectrometer.Blood samples were obtained from Red Cross Blood Center of Beijing,and the blood samples were collected from healthy volunteers by venipuncture.
1.2 Synthesis of 4′,7-bis((ethoxycarbonyl)methoxy)isoflavone(2)
Compound 1 (2.50 g,10 mmol)and anhydrous K2CO3(5.50 g,40 mmol)were dissolved in anhydrous acetonitrile (80 mL)and the mixture was stirred at room temperature for 30 min.Ethyl bromoacetate(3.3 mL,20 mmol)was added dropwise to the solution.The reaction mixture was further stirred at room temperature for 10 min and then heated to reflux.The progress of the reaction was monitored by TLC(Vethylacetate∶Vpetroleumether=1∶2).After 12 h,the mixture was cooled to room temperature and the solvents were removed under reduced pressure.The residue was washed to neutral with distilled water,yielding a white solid which was then added to 3 mol·L-1NaOH aqueous solution(30 mL)to be fully washed.After the filtration,the resultant light yellow solid was further washed to neutral with distilled water.The crude product was then recrystallized with anhydrous ethanol to give compound 2 as a white crystalline solid(3.55 g,82.80%).m.p.124~125 ℃.IR(KBr,cm-1):2 925,1 742,1 631,1 603,1 251,896.1H NMR(DMSO,500 MHz):δ8.45(s,1H),8.04(d,1H,J=8.8),7.51(d,2H,J=8.6),7.11(dd,1H,J1=8.6,J2=2.4),6.98(d,2H,J=8.6),4.9(s,4H),4.29(q,J=6.8),1.25(t,J=6.8).Anal.Calcd.for C23H22O8(%):C 64.78,H 5.20;Found(%):C 64.70,H 5.18.MS:m/z=426.1 for[M+].
1.3 Synthesis of 4′,7-bis(((sodium glycinate)carbonyl)methoxy)isoflavone(L1)

Scheme 1 Synthesis of the target compounds L1 and L2
5.0 mL aqueous solution of glycine (1.70 g,23 mmol)containing NaOH(0.92 g,23 mmol)was added to a solution of compound 2 (1.00 g,2.3 mmol)in ethanol(60 mL).The reaction mixture was heated to reflux for 1 d.The solvents were removed under reduced pressure.The resulting light yellow solid was then washed with a small amount of anhydrous ethanol,filtered,dried and recrystallized with 5%NaCl aqueous solution to give L1 as a white powder(0.75 g,67.30%).m.p.>300 ℃;IR (KBr,cm-1):3 449,3 186,1 725,1 635,1 453,1 365,1 215,895.1H NMR(DMSO,500 MHz):δ7.89(s,2H),7.68(d,1H,J=9.1),7.42(s,1H),7.24(d,2H,J=8.6),7.11(d,2H,J=8.6),6.80(dd,1H,J1=9.1,J2=2.3),6.68(d,1H,J=2.3),4.81(s,2H),4.73(s,2H),4.15(s,2H),4.07(s,2H).Anal.Calcd.for C23H18N2O10Na2(%):C 52.28,H 3.43,N 5.30;Found(%):C 52.36,H 3.38,N 5.27.MS:m/z=528.2 for[M+].
1.4 Synthesis of 4′,7-bis((hydrazinocarbonyl)methoxy)isoflavone(L2)
Hydrazine hydrate solution (80%,10 mL)was added dropwise to a solution of compound 2(3.00 g,7 mmol)in anhydrous ethanol(60 mL)at room temperature,and the reaction mixture was then heated to reflux.The progress of the reaction was monitored by TLC(Vethylacetate∶Vpetroleumether=1∶2).After 12 h,the mixture was cooled to room temperature,and the solvents were removed under reduced pressure.The residue was washed with distilled water,and further stood over night.After the filtration,the crude solid was washed with distilled water and dried.The obtained solid was recrystallized with anhydrous ethanol to give L2 as a white powder(2.23 g,80.20%).m.p.132.2~133.1 ℃.IR (KBr,cm-1):3 452,3 315,2 927,1 640,1 626,1 572,1 365,1 240,920.1H NMR (DMSO,500 MHz):δ8.09(s,2H),7.68(d,1H,J=9.1),7.54(s,1H),7.31(d,2H,J=8.6),7.06(d,2H,J=8.6),6.78(dd,1H,J1=9.1,J2=2.3),6.67(d,1H,J=2.3),4.70(s,4H),2.0(s,4H).Anal.Calcd.for C19H18N4O6(%):C 57.28,H 4.55,N 14.06,Found(%):C 57.19,H 4.61,N 14.15.MS:m/z=398.1 for[M+].
1.5 Hydroxyl free radical scavenging assays
For the hydroxyl free radical scavenging experiments,thestock solution of samples(1 mmol·L-1)were prepared by dissolving each sample in DMSO.Stock solution of terephthalate(TA,2 mmol·L-1)wasprepared by dissolving terephthalate in PB buffer(phosphate buffer,pH=7.6),and the solution of H2O2(20 mmol·L-1)was prepared by diluting with distilled water.In order to optimize exposure time of the tested system,an experiment was performed as follows.In a 50 mL volumetric flask containing 1 mL of 20 mmol·L-1H2O2,4 mL of 2 mmol·L-1TA stock solution was added,and the mixture was diluted to 50 mL with distilled water.Fluorescence measurements of the system were carried out after different exposure time at 254 nm.The fluorescence intensity of the tested system showed obvious change in the condition of excitation at 312 nm,emission at 427 nm,UV irradiation for over 20 min at 254 nm and after standing for 20 min.Therefore,exposure time of the tested system was set as 20 min.The test solution was prepared by adding 0.2 mL H2O2stock solution,different concentrations of each sample,0.2 mL TA stock solution in order,then diluting with distilled water to 10 mL.The solution was irradiated at 254 nm for 20 min and further stood for another 20 min,then the fluorescence spectra were recorded with excitation at 312 nm,emission at 427 nm at once.We calculated the scavenging rates of the target compounds L1 and L2,the parent compound 1 and standard vitamin C on hydroxyl free radical by the following method at 25 ℃:Scavenging rate=(F0-F)/F0×100%,where F0represents the fluorescence intensity of the tested system without the target compounds L1 and L2,the parent compound 1 and vitamin C at 427 nm,and F represents the fluorescence intensity of the tested system with the target compounds,the parent compound 1 and vitamin Cat the same condition.
1.6 Superoxide anion free radical scavenging assays
For the superoxide anion free radical scavenging experiments,the stock solution of samples(10 mmol·L-1)were prepared by dissolving each sample in DMSO.The stock solution of HCl(10 mmol·L-1)was prepared by diluting with distilled water,and stock solution of pyrogallol(10 mmol·L-1)was prepared by dissolving pyrogallol in HCl(10 mmol·L-1).In order to select the optimal testing time and UV-Vis absorption wavelength of the tested system,the following procedures were performed.0.1 mL of pyrogallol stock solution(10 mmol·L-1)was added to the Tris-HCl buffer(0.1 mol·L-1,pH=8.2,5 mL)and then the mixture was diluted to 10 mL with distilled water at 30℃.After the mixture was shaken vigorously,and stood for over 3 min,the reaction mixture was repeatedly scanned(the time interval was 1 min),using a Cintra 10e UV-Vis Spectrophotometer,from 200 to 500 nm at 30℃.UVVis absorption of the system at 319 nm showed obviously increased with the change of time (0~16 min).Therefore,we set 4 min as testing time at 30℃and recorded UV-Vis absorption spectrum of the system at 319 nm.The test solution was prepared by adding 5 mL Tris-HCl buffer(0.1 mol·L-1,pH=8.2),different concentrations of each sample,0.1 mL pyrogallol stock solution in order at 30℃,then diluting with distilled water to 10 mL.After the mixture was shaken well and stood for 4 min at 30℃,the UV-Vis absorption spectra of the system were recorded at once.We calculated the scavenging rates of the target compounds L1,L2,the parent compound 1 and vitamin C on superoxide anion free radical using the following method:Scavenging rate=(A0-A)/A0×100%,where A0represents the UV absorbance of the tested system without the target compounds L1,L2,the parent compound 1 and vitamin C at 319 nm,and A represents the UV absorbance of the system with the tested compounds at the same wavelength.
1.7 Anti-hemolysis assays
For the antioxidative hemolysis experiments,the stock solution of samples(0.1 mmol·L-1)were prepared by dissolving each sample in DMSO.The stock solution of NaCl(0.15 mol·L-1)and AAPH(344 mmol·L-1)were prepared by diluting with distilled water,respectively.The human erythrocytes with anticoagulant were provided by Beijing Red Cross Blood Center and stored at 5℃before use.The erythrocytes were treated according to the following procedures:the erythrocytes were first separated by centrifugation at 2 000 r·min-1,then washed with PBSbuffer(phosphate buffer saline,0.15 mol·L-1,pH=7.2)for three times,and finally dissolved in PBSbuffer(0.15 mol·L-1,pH=7.2)to prepare a 5%suspension.In order to simulate the internal temperature of human body,all solutions were controlled strictly at 37℃.The reaction solution was prepared by adding 5 mL erythrocyte suspension,3 mL stock solution of each sample,1.5 mL AAPH stock solution in order at 37℃,then diluting with distilled water to 10 mL.The solution was further placed in the thermostatic bath to keep 37℃,and the test solution was prepared by diluting 1 mL reaction solution to 5 mL with NaCl stock solution,mixing it up,centrifugalizing the solution at 2 000 r·min-1for 10 min,and using the supernatant as testing solution.The UV-Vis absorption spectra were recorded at 540 nm every 30 min.We evaluated the anti-hemolysis activities of the tested compounds by the following method:Hemolysis rate=A/A0×100%,where A0represents the UV-Vis absorbance of the system at 540 nm without the tested compounds and with complete hemolysis of human erythrocytes,and A represents the UV-Vis absorbance of the system at the same wavelength with the tested compounds.
1.8 Cell toxicity assays
A549 cell was respectively treated with various concentrations (0,1,3,5 mmol·L-1)of the target compounds L1 and L2 for the same duration.The cell viability was evaluated with an MTT assay,and a cell growth curve was generated,which reflected the cytotoxicity of the target compounds L1 and L2 in vitro.
1.9 Statistical calculation
All the experiments were carried out independently at least three times to make the experimental error within 10%,and data were expressed as means±SD.The results were analyzed statistically using Origin 8.0 professional software.A value of P<0.05 was accepted as different,while value of P<0.01 was accepted as significantly different.
2 Results and discussion
2.1 Hydroxyl free radical scavenging activities of the target compounds L1 and L2
The hydroxyl free radical(·OH)in the cells can easily cross cell membranes at specific sites,react with most biomolecules and furthermore cause tissue damage and cell death[44]. Thus,removing excess hydroxyl free radical is very important for the protection of living systems and reducing autoimmune conflict for humans.Terephthalate is an ideal reagent for the evaluation of hydroxyl radical-scavenging activity,which allows one to measure sensitively concentration of hydroxyl free radical in aqueous solution.Compared with other chemical methods used for the measurement of hydroxyl radical, the terephthalate system performs better,particularly at low concentration of hydroxyl free radical levels[45].The system can emit strong fluorescence at 427 nm when excited by 312 nm light because of the formation of hydroxy-terephthalate.We used the following method to detect hydroxyl free radical scavenging activities of the target compounds L1,L2,the parent compound 1 and standard vitamin C in aqueous solution.The hydroxyl free radical was generated via the decomposition of hydrogen peroxide under the UV irradiation,which is a simple,fast and efficient way to produce hydroxyl free radical[46-48].The scavenging rate curves of the target compounds on hydroxyl free radical as presented in Fig.2 show that upon the increasing concentration of the target compounds from 0 to 4.5 μmol·L-1,the scavenging rate on hydroxyl free radical increased obviously.The IC50values were obtained by nonlinear fitting method using Origin nonlinear fitting program.The IC50values of the target compounds L1,L2,the parent compound 1 and vitamin C were(0.68±0.11) μmol·L-1,(0.43±0.10)μmol·L-1,(47±0.15)μmol·L-1and (1.3±0.21)mmol·L-1,respectively,which indicates that the compounds used in the study have much higher scavenging activities on hydroxyl free radical than the parent compound 1 and vitamin C scavenging activities.We postulate that the difference between the scavenging activities of the target compounds is the result of the different substituent on the nitrogen atom in the target compounds.The substituent group that can make nitrogen free radical stability increased will be propitious to scavenging activity increased on hydroxyl free radical,and the order of substituent group′s contribution to stability of nitrogen free radical was-NH2>-CH2COONa[49].If nitrogen radical stability increases,it will be helpful to increase the scavenging activity of the target compoundson hydroxyl free radical.The results of L1,L2 were analyzed statistically using Origin 8.0 professional software,P<0.05.Therefore,the scavenging activity of compound L2 on hydroxyl free radical is better than the scavenging activity of compound L1.

Fig.2 Scavenging rate curves on hydroxyl free radical of the target compounds L1 and L2 at different concentrations in PBSbuffer(pH=7.6)at 25℃
2.2 Superoxide anion free radical scavenging activities of the target compounds L1 and L2
Superoxide anion free radical(·O2-)has been implicated in initiating oxidation reactions associated with aging[50].It alsoplaysan important role in formation of other ROS,such as hydrogen peroxide,hydroxyl free radical,and singlet oxygen,which can damage human immune system and further initiate some immune diseases,such as rheumatoid arthritis[51].Therefore,scavenging superoxide anion free radical is alsocrucial toour health.Pyrogallol(1,2,3-benzenetriol)has long been known to autoxidize rapidly to produce superoxide anion free radical in alkaline solution[52]and the complicated mechanism can be briefly described in Fig.3.The reaction has been employed to examine superoxide anion free radical scavenging activity of the antioxidants[53].Herein,the pyrogallol autoxidation assay was performed to evaluate superoxide anion free radical scavenging activities of the target compounds by UV spectrum method.Fig.4 shows the scavenging rate curves of target compounds on superoxide anion free radical.The IC50values were obtained by nonlinear fitting method using Origin nonlinear fitting program.As the concentration of target compound increased from 0 to 32 μmol·L-1,the scavenging rate gradually increased,indicating that the target compounds can scavenge superoxide anion free radical well,and they showed concentrationdependent scavenging activity on superoxide anion free radical.The IC50values of L1,L2,the parent compound 1 and vitamin Cwere(11.2±0.10)μmol·L-1,(9.4±0.12)μmol·L-1,(59±0.15)μmol·L-1and (18±0.10)μmol·L-1respectively,The results of L1,L2 were analyzed statistically using Origin 8.0 professional software to gain P<0.05.Indicating that the antioxidant activity of target compounds on superoxide anion radical is a little better than that of the parent compound 1 and vitamin C.
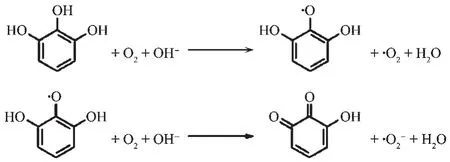
Fig.3 Sketch of autoxidation mechanism of pyrogallol in pH=8.2 Tris buffer

Fig.4 Scavenging rate curves on superoxide anion free radical of the target compounds L1 and L2 at different concentrations in Tris buffer(pH=8.2)
2.3 Anti-hemolysis activities of the target compounds L1 and L2
Increasing evidence suggests that oxidative damage to cell plays an important pathophysiological role in human diseases.The cells are particularly susceptible to oxidative damage owing to the high polyunsaturated fatty acid content in the cell membrane.Therefore,the inhibition of the cell membrane peroxidation is very important to prevent human diseases.Theoxidation of erythrocyte membrane serves as a model for the oxidative damage of biomembrane.It has been found that free radicals generated in the aqueous phase attack the membrane to induce the chain oxidations of lipids and proteins,and eventually cause hemolysis. The oxidative hemolysis of human erythrocytes induced by free radicals and its inhibition by antioxidants have been studied by the AAPH method[54].We studied antihemolysis activities of the target compounds,the parent compound 1 and vitamin C on human erythrocytes using the AAPH method.AAPH generates peroxyl radicals(ROO·)by decomposition in the presence of oxygen.The mechanism of generation of peroxyl radicals(ROO·)by the decomposition of the AAPH and the elimination mechanism of peroxyl radicals by the antioxidant are shown below:
Initiation
R-N=N-R → 2R·+N2
R·+O2→ ROO·
ROO·+LH → ROOH+L·
Propagation
L·+O2→ LOO·
LH+LOO·→ L·+LOOH
Termination
AH+LOO·→ A·+LOOH
LOO·+A·→ LOOA
where R=-C(CH3)2-C(NH2)=NH2+,LH=polyunsaturated lipids in human erythrocyte membranes,AH=antioxidant molecule
As an initiator,AAPH decomposes at physiological temperature (37℃)in aqueous solutions to generate alkyl radical(R·).In the presence of oxygen,the alkyl radical is converted to the corresponding propyl radicals(ROO·).At 37℃ in neutral water,the half-life of AAPH is about 175 h and generates radicals at a slow rate (1.3 μmol·L-1·s-1)[55].These peroxyl radicals can induce oxidation of LH causing a chain reaction known as lipid peroxidation.As a result of thislipid peroxidation,thehuman erythrocytemembrane undergoes quick damage and losses its integrity,leading to hemolysis.When the target compounds(antioxidant)are present,the peroxyl radical can be trapped and a new antioxidant radical A·produces.If A·is a stabilized radical,which will be advantageous to the hydrogen abstraction reaction,and promote termination a chain free radical reaction, the peroxidation can be inhibited.Thus,the hemolysis of the human erythrocyte can be inhibited effectively in the presence of the target compounds.Hemolysis rate curves of the tested system are shown in Fig.5.Compared to the blank sample,the hemolysis of human erythrocytes was inhibited significantly over 90%after the addition of target compounds,whilst only 40%reduction was observed after the addition of parent compound 1.For vitamin C,60%reduction could be observed in the same conditions.The experimental results indicate that the target compounds are effective in suppressing the hemolysis of human erythrocytes induced by AAPH at low concentration level(0.1 μmol·L-1).The target compounds′activity of suppressing the hemolysis of human erythrocytes is better than the activity of vitamin C and the parent compound 1 on suppressing the hemolysis of human erythrocytes in the same concentration condition(30 μmol·L-1).

Fig.5 Hemolysis rate curves of 5%human erythrocytes in the presence of compounds L1,L2,the parent compound 1,vitamin C,respectively,and in the absence of tested compounds
3 Conclusions
In conclusion,we have synthesized two new daidzein derivatives containing nitrogen and studied their antioxidant activities in vitro by the spectrum methods in aqueous solutions. Results of the experiments suggested that the target compounds have good antioxidant properties at 1 μmol·L-1level in physiological pH condition.Especially in the hydroxyl free radical scavenging activity and anti-hemolysis activity of human erythrocytes,the antioxidant activities of targeting compounds are better than that of vitamin C.The target compounds could be developed into a kind of new,high efficiency antioxidant or a kind of food additive potential for prevention of diseases related to free radicals.
Acknowledgements:This work was supported by Key Discipline Foundation of City of Beijing(Grant No.102-403101)and National Major Scientific and Technological Special Fund of China(Grants No.2014ZX 09507007-001,2014ZX09507007-003).

