一個基于[Cu4(μ2-OH)2N12]的10-連接3D配合物的合成、結構和光催化性質
劉天寶 彭艷芬
(池州學院材料與環境工程學院,微納粉體與先進能源材料安徽普通高校重點實驗室(池州學院),池州 247000)
0 Introduction
In recent years,the design and construction of functional coordination polymers,containing metal ions or metal clusters and bridging organic ligands,have developed rapidly because of their potential applications in magnetism,photoluminescence,catalysis and interesting topological structure[1-4].
Topology is a very important subject and a useful tool in the crystal engineering,which can simplify the complicated structure of coordination polymers(CPs).The simple 3-,4-,and 6-connected nets are very common and can not meet the demand of complicated network topology.So,the study on synthesis,properties of complexes containing highly connected nodes is more attractive.But,up to now,only a few examples of 9-,10-and 12-connected networks have been reported because that the central metal ions have limited coordination sites and most organic ligands have steric hindrance.In order to solve this problem,using metal clusters instead of transition metal ions as nodes can provide more coordination sites[5-11].Most of the clusters in coordination polymers are synthesized by oxo-centered metal clusters.For example,[Cu4(μ3-OH)2]tetranuclear copper (Ⅱ) clustersare observed in severalcompounds[12-14].But,in some coordination polymers,hydroxyl group and carboxyl or triazole group induce the aggregation of some clusters with special properties,simultaneously,such as[Co4(μ2-H2O)2(COO)6]cluster[7],[Cu4(μ2-H2O)2N20]cluster[15].
N-donor ligands play an important role in synthesis of CPs or metal-organic frameworks(MOFs).A large number of coordination polymers have been synthesized based on pyridine,imidazole and triazole derivative ligands in the past decades,such as 1,2-di(4-pyridyl)ethylene (bpe),1,2-bis(imidazol-1-ylmethyl)benzene(bimb)[16],1,4-bis(2-methyl-imidazol-1-yl)butane)(bib)[17-18],1,2-bis(1,2,4-triazol-1-yl)ethane(bte)[19],1,4-bis(1,2,4-triazol-1-yl)butane(btb)[20],1,4-bis(1,2,4-triazol-1-ylmethyl)-2,3,4,5-tetramethyl benzene(tmtz)[21]and 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene(bbtz)[22].Comparatively,the 4-substituted-1.2.4-triazole derivatives have more coordination sites.We have synthesized some coordination polymers based on the rigid 4-substituted-bis(1,2,4-triazole)ligand 1,4-bis(4H-1,2,4-triazole)benzene(btx)[23],the flexible ligand 1,2-bis(4H-1,2,4-triazole)ethane(btre)[24-26],1,4-bis(4H-1,2,4-triazole)benzene(btrb)[27]and a long and semi-rigid ligand bis(4-(4H-1,2,4-triazole)phenyl)methane(btpm)[28].
With this background information,one new coordination polymer{[Cu2(OH)(btre)1.5(1,2,4-btc)]·13H2O}n(1·13H2O)was synthesized(1,2,4-btc=1,2,4-benzenetricarboxylate).Compound 1 shows an unusual 10-connected three-dimensional metal-organic framework based on the tetranuclear copper clusters[Cu4(μ2-OH)2N12].
1 Experimental
1.1 Materials and methods
The ligand btre was synthesized according to the literature method[29].All other reagents(such as CuCl2·2H2O,1,2,4-benzenetricarboxylic acid,methanol)were of analytical grade and used without further purification.Elemental analyses for C,H and N was performed on a Perkin-Elmer 240C analyzer.The IR spectra was obtained using KBr pellets on a Nicolet iS10 spectrophotometer in the 4 000~400 cm?1region.Powder X-ray diffraction(PXRD)were performed on a D/MAX-3C diffractometer with the CuKαradiation(λ=0.154 06 nm,U=40 kV,I=40 mA)over the 2θrange of 5°~50°at room temperature.TGA was carried out using a Thermal Analyst 2100 TA Instrument and SDT 2960 Simultaneous TGA-DTA Instrument in flowing dinitrogen at a heating rate of 10 ℃·min?1.UV-Vis spectrum was measured using a U-3900 spectrophotometer.
1.2 Preparation of{[Cu2(OH)(btre)1.5(1,2,4-btc)]·13H2O}n(1·13H2O)
A solution of 1,2,4-benzenetricarboxylic acid(0.2 mmol)in 5 mL H2O was adjusted to pH 6 with 1.0 mol·L?1NaOH solution and btre(0.2 mmol)dissolved in 2 mL H2O was added.The mixture was placed at the bottom of glass tube.CuCl2·2H2O(0.4 mmol)dissolved in 5 mL methanol was placed on top of glass tube.The ligands(1,2,4-btc,btre)and CuCl2were separated by the mixture of methanol and H2O(1∶1,V/V).Two weeks later,the blue single crystals of 1·13H2O were obtained(0.090 8 g,Yield:82%based on btre).Anal.Calcd.for[C18H16Cu2N9O7(1)+13H2O](%):C,25.97;H,5.05;N,15.15.Found(%):C,26.01;H,4.98;N,15.16.IR data(cm?1):3 429s,3 121w,1 582m,1 487w,1 384m,1 198w,1 170w,1 128w,1 079w,1 023w,772w,647w.
1.3 Crystal structure determination
The diffraction data of 1·13H2O was collected on Rigaku Mercury CCD diffractometer with graphite monochromated MoKα(λ=0.071 073 nm)radiation.Intensities were collected by theφ-ωscan technique.The structure was solved and refined by the SHELXTL package[30].All non-hydrogen atoms were refined anisotropically and hydrogen atoms were determined with theoretical calculations and refined isotropically.The lattice water of 1·13H2O is highly disordered and could not be modeled properly and was removed by the SQUEEZE routine in PLATON[31].The number of lattice water molecules for 1·13H2O was deduced from the TGA and elemental analysis.The parameters of the crystal data collection and refinement of 1 are given in Table 1.Selected bond lengths and bond angles of 1 are listed in Table 2.
CCDC:1526760.

Table 1 Crystallographic collection and refinement parameters of 1

Table 2 Selected bond distances(nm)and angles(°)for 1
2 Results and discussion
2.1 Crystal structure description of[Cu2(OH)(btre)1.5(1,2,4-btc)]n(1)
The asymmetric unit of 1 consists of two kinds of Cu (Ⅱ) ions(Cu(1)and Cu(2)),oneμ2-OH group(O(7)),one 1,2,4-btc,one and half of btre ligands.Each Cu (Ⅱ)ion is five-coordinated by one carboxylate oxygen atom from 1,2,4-btc and oneμ2-OH(O(7))and three triazole nitrogen atoms from three btre ligands,and adopts a square-pyramidal geometry with a[CuO2N3]chromophore.N(8)A and N(5)C are located at vertex positions of the square-pyramidal geometry of Cu(1)and Cu(2),respectively.The Cu-O bond lengths are in a range of 0.191 9(3)~0.199 2(4)nm and the Cu-N bond lengths vary from 0.197 9(4)to 0.238 2(5)nm,which is similar to those observed in the other Cu-O/N compound constructed by btre ligand[12](Fig.1).
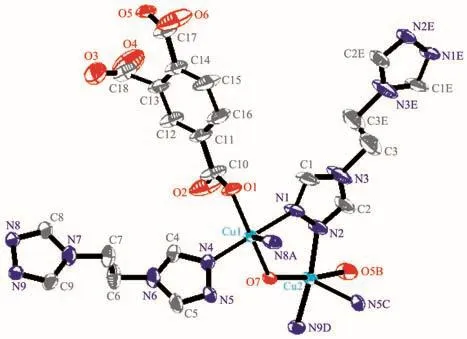
Fig.1 Coordination environment of the Cu (Ⅱ)atom in 1
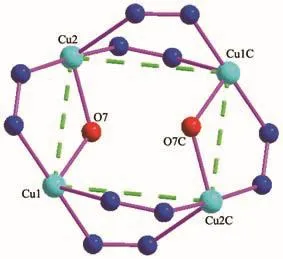
Fig.2 Tetranuclear copper cluster in 1
The Cu(1)and Cu(2)are bridged by oneμ2-OH group(O(7))and oneμ2-triazole ring of btre ligand to form a dinuclear[Cu2(μ2-OH)N2]cluster.Two dinuclear[Cu2(μ2-OH)N2]units are bridged by four triazole rings giving a tetranuclear[Cu4(μ2-OH)2N12](Fig.2).In tetranuclear cluster,theμ2-OH group(O(7))connects two different crystallographic Cu (Ⅱ)(Cu(1),Cu(2))with bond lengths of 0.191 9(3)and 0.192 2(3)nm,respectively,and deviates from the Cu (Ⅱ)ions plane(Cu(1),Cu(2),Cu(1)C,Cu(2)C)over 0.055 58 nm.The Cu(1)-O(7)-Cu(2)bond angle is 125.011(1)°.The distance between Cu(1)and Cu(2)is 0.340 74(7)nm.Cu(1)and Cu(2)C are bridged by two triazole groups with a distance of 0.395 37(7)nm.
There is one kind of 1,2,4-btc ligand in 1.1,4-Carboxylate groups(O(1)O(2),O(5)O(6))of 1,2,4-btc ligand bridge two Cu (Ⅱ)ions from two different tetranuclear Cu (Ⅱ)clusters.The third carboxylate group(O(3)O(4))is free.Each btre ligand exhibits the anti-conformation and bridges two tetranuclear copper (Ⅱ)clusters.Each tetranuclear[Cu4(μ2-OH)2N12]cluster connects ten adjacent clusters through four 1,2,4-btc and six btre ligands to yield an unusual 3D framework,as shown in Fig.3 and 4.
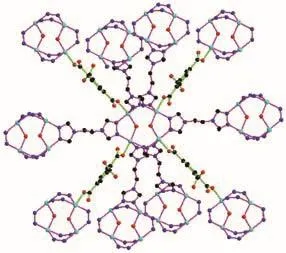
Fig.3 Tetranuclear copper cluster surrounded by ten neighboring tetranuclear copper clusters through six btre ligands and four 1,2,4-btc ligands
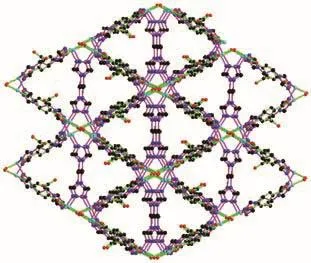
Fig.4 Three dimensional framework in 1
As depicted in Fig.3,each tetranuclear copper cluster is connected to ten adjacent tetranuclear copper clusters through four 1,2,4-btc and six btre ligands.Topologically,the tetranuclear copper cluster is simplified as one 10-connected node.The 1,2,4-btc and btre ligands are 2-connected linkers.The structure of 1 can be described as a 10-conneced 3D network with the 312·428·55topology(Fig.5).This network is identified by the codebct.The same topology has been reported in the literature[8].In addition,some of other 10-connected complexes have different topological struc-tures with Schl?fli symbol of 36·434·53·62[7,32-33],44·832·129[34].
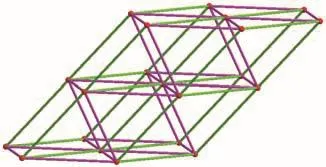
Fig.5 Three 10-connected bct topological network in 1
2.2 Thermogravimetric analysis of 1·13H2O
In order to characterize the purity of compound 1·13H2O,the as-synthesized sample was measured by X-ray powder diffraction at room temperature.As shown in Fig.6,the peak positions of the measured patterns are in good agreement with the simulated patterns,indicating the high purity of the sample.In order to characterize the thermal stability of compound 1·13H2O,the thermal behavior was investigated by TG in dry nitrogen atmosphere from room temperature to 600℃with a heating rate of 10 ℃ ·min?1.In the TG curve of 1·13H2O(Fig.7),the weight lost in the range of 40~121℃is attributed to the release of lattice water molecules(Calcd.28.19%,Obsd.28.18%).The anhydrous substance was stable upon heating to 211℃.Then the weight decrease happened rapidly and did not end until to 425℃.The framework of 1 was collapsed from 211 to 425℃.The residue might be CuO(Calcd.:19.15%,Obsd.19.16%).
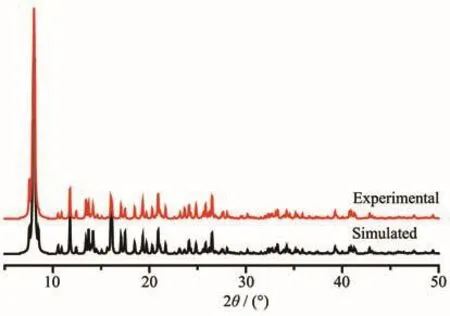
Fig.6 Simulated and experimental PXRD patterns of 1·13H2O
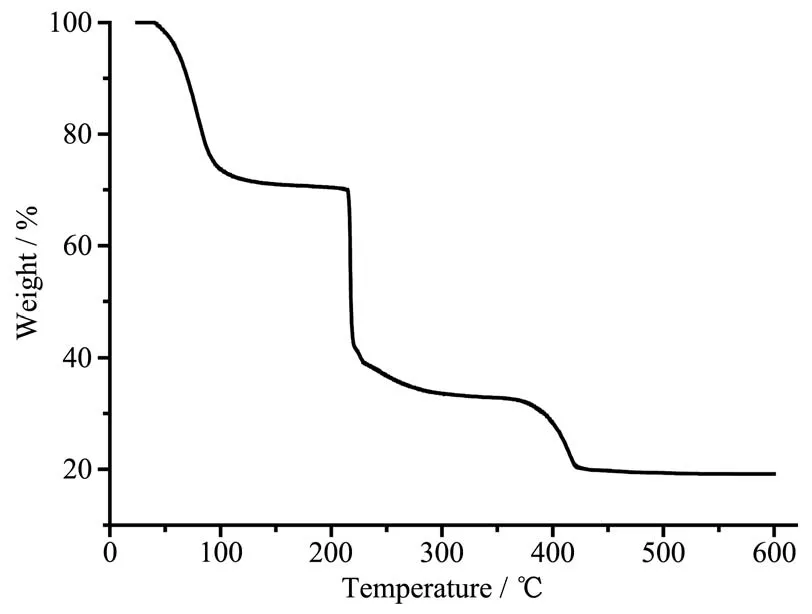
Fig.7 TG curve of 1·13H2O
2.3 Catalytic properties of 1·13H2O
Photocatalytic degradation has attracted more and more research interest because of its potential application in the purification of wastewater[4,35-41].The photocatalysis activity of compound 1·13H2O was evaluated in purifying waste water in the presence of small H2O2(0.20 mL 30%H2O2in 100 mL aqueous solution)by measuring the concentration of the models dye contaminant methyl orange(MO)under UV illumination.The photocatalytic reactions were carried out in a typical process.The degradation experiments of MO were tracked by spectroscopy(Fig.8).The degradation efficiencies for MO were 11.6%in the presence of only H2O2,and 19%in the presence of only catalyst 1·13H2O after 105 min.However,the degradation efficiencies of MO reached 86.7%for 1·13H2O in the presence of H2O2after 105 min(Fig.9).Obviously,com-pound 1·13H2O shows good catalytic activity for the degradation of MO.The reaction mechanism should be well-known Fenton type mechanism[37].UV light can induce Cu (Ⅱ)atoms with unsaturated square-pyramidal configuration to react with H2O2to generate hydroxyl radicals.The·OH radicals are highly active oxidizing species and can effectively decompose MO to complete the photocatalytic process[4,42-44].
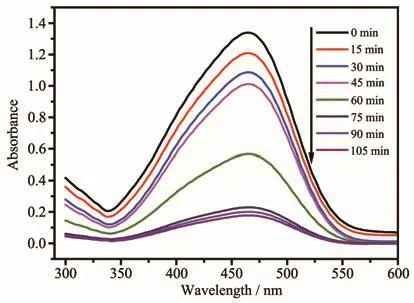
Fig.8 UV-Vis absorption spectra of MO solution degraded by photocatalyst 1·13H2O under irradiation at different time intervals
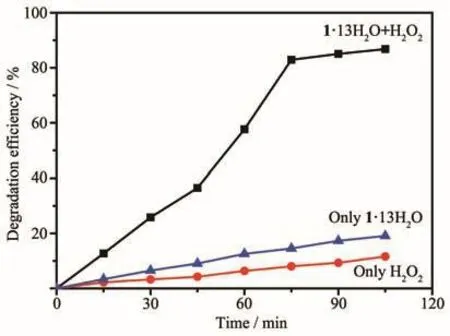
Fig.9 Photocatalytic degradation of MO solution under UV light using catalyst 1·13H2O in the presence or absence of H2O2,and only H2O2
3 Conclusions
In summary,we successfully synthesized one unusual coordination polymer 1 using flexible 1,2-bis(4H-1,2,4-triazole)ethane and rigid multicarboxylate ligands.1 exhibits an unusual 10-connected threedimensional metal-organic framework based on[Cu4(μ2-OH)2N12].1·13H2O shows good photocatalytic activity in the degradation of methyl orange.