杉木凋落物及其生物質炭對土壤原有有機碳礦化的影響*
盧曉蓉,尹 艷,馮競仙,馬紅亮,高 人,尹云鋒
杉木凋落物及其生物質炭對土壤原有有機碳礦化的影響*
盧曉蓉,尹 艷,馮競仙,馬紅亮,高 人,尹云鋒?
(濕潤亞熱帶山地生態國家重點實驗室培育基地,福建師范大學地理科學學院,福州 350007)
利用13C同位素技術和培養實驗,研究不同添加量(0、10、20、30、40、50 g?kg–1)杉木凋落物和生物質炭(Biochar,BC,350℃熱解)對土壤原有有機碳(原SOC)礦化及外源碳自身分解的影響。實驗進行28 d,培養溫度為25℃,水分保持為60%土壤持水量(Water holding capacity,WHC)。結果表明:凋落物及BC添加顯著提高了土壤總CO2累積排放量(<0.05),且凋落物的影響更為明顯;來源于外源碳及原SOC的CO2累積排放量均隨添加量的增加而增加。培養結束時,凋落物碳(LR-C)分解率為5.71%~13.68%,生物質炭碳(BC-C)分解率僅0.34%~0.50%,凋落物和BC處理下原SOC分解率分別為對照土壤的6.42倍~13.58倍與2.06倍~3.94倍。回歸分析發現,2種外源碳處理下原SOC分解率與添加量均呈極顯著的線性關系(<0.01);LR-C分解率亦隨添加量的增加而增加;但BC-C分解率則與添加量呈顯著的開口向上的拋物線關系(<0.05),并在10 g?kg–1添加量時達到最大。相關分析表明,短期內BC分解受微生物活動的影響較小,在土壤中更加穩定。因此,將凋落物制備為BC后施入更有利于杉木人工林土壤有機碳固存。
凋落物;生物質炭;原有有機碳;分解率;人工林
土壤有機碳(SOC)礦化是陸地生態系統碳循環過程中的重要環節,也是溫室氣體的主要來源之一[1]。因此,研究SOC礦化過程,對于了解生態系統碳循環及全球變化具有重要意義[2]。諸多研究表明,影響SOC礦化的因素主要包括土壤性質(有機質含量、pH、質地、溫度、濕度等)、土地利用方式、外源碳的質量和數量以及微生物活性等[3-4]。在森林生態系統中,凋落物是SOC的重要來源,其分解及相關過程均會影響大氣中CO2濃度和土壤碳庫穩定[5]。凋落物通過微生物的分解作用產生CO2,同時亦對土壤原有有機碳(原SOC)的礦化產生重要影響[6]。如史學軍等[5]利用室內91 d培養實驗發現,4種凋落物(青岡櫟oerst.、麻櫟carr.、馬尾松lamb.、狗牙根pers.)添加均顯著促進了黃棕壤有機碳的礦化。而王曉峰等[7]利用81 d的培養實驗發現,杉木凋落物顯著改變了土壤有機碳的分解速率,從而產生激發效應,并且深層(40~45 cm)土壤有機碳分解對杉木凋落物的響應高于表層(0~5 cm)土壤。研究表明,大氣CO2濃度升高,氮沉降等全球變化將會提高植物凈初級生產力[8],相應地進入土壤中的凋落物亦會增加。
生物質炭(Biochar,BC)是生物質在厭氧或者部分厭氧條件下經高溫裂解產生的多孔芳香類物質,具有惰性,可在土壤中存在成百上千年[9]。BC輸入可改善土壤肥力,減少溫室氣體排放和增加土壤碳固持[10],國際上已將施用BC作為土壤改良的有效措施及碳封存的重要技術[11]。王富華等[12]研究表明BC(油菜秸稈,500℃熱解)與秸稈配施顯著提高紫色土團聚體有機碳含量,且隨著施用量增加,有機碳含量呈升高趨勢。在我國亞熱帶地區,杉木是一種重要的速生造林樹種,但由于杉木特有的生態學特性和連栽等措施導致人工林土壤肥力下降[13]。如在人工林經營初期,若將采伐剩余物和凋落物部分制成BC,并將其返還土壤,可避免直接火燒造成的環境污染和水肥流失等問題,亦有可能改善人工林土壤肥力,提高人工林土壤的固碳潛力[14]。凋落物為易分解碳源,而BC為較難分解碳源,杉木人工林原SOC礦化對2種類型外源碳輸入的響應有何異同?而原SOC礦化與添加量之間是否存在一定的數量關系?基于上述問題,本研究以杉木人工林土壤為研究對象,利用13C標記的杉木凋落物及其制備的BC作為2種不同類型外源碳,采用室內培養實驗結合穩定性同位素技術,研究凋落物和BC不同添加量對其自身分解及土壤原SOC礦化的影響,為人工林土壤固碳管理與生物質資源有效利用提供科學依據。
1 材料與方法
1.1 供試材料
土壤樣品取自福建省建甌市萬木林自然保護區內杉木人工林,樹齡約36 a,海拔350 m,西北坡向,坡度27°。該區屬中亞熱帶季風氣候,年均氣溫19.4℃,年均降水量1 731 mm,土壤為花崗巖發育的普通山地紅壤。根據“S”型多點取樣,去除表層凋落物后取0~20 cm土壤,剔除石塊和可見植物殘體與根系,新鮮土壤樣品過2 mm篩置于冰箱4℃冷藏備用。
利用13C脈沖標記的杉木凋落物作為供試材料,標記方法參照尹云鋒等[15]。去離子水沖洗干凈后60℃烘干,粉碎機粉碎過2 mm篩后用于培養和BC制備。BC生產設備采用管式爐(O-KTF1200,江蘇前錦爐業),將凋落物適量置于錫箔紙中放置爐中封閉,制備溫度為350℃,通入氮氣厭氧熱解2 h,冷卻的BC樣品過2 mm篩后充分混勻備用。供試土壤、杉木凋落物及BC基本性質參見表1。
1.2 實驗設計
采用雙因素實驗設計,外源碳類型(凋落物、BC)和添加量(0、10、20、30、40、50 g?kg–1)。實驗處理:土壤(S)、土壤+10 g?kg–1凋落物(SL1)、土壤+20 g?kg–1凋落物(SL2)、土壤+30 g?kg–1凋落物(SL3)、土壤+40 g?kg–1凋落物(SL4)、土壤+50 g?kg–1凋落物(SL5)、土壤+10 g?kg–1BC(SB1)、土壤+20 g?kg–1BC(SB2)、土壤+30 g?kg–1BC(SB3)、土壤+40 g?kg–1BC(SB4)、土壤+50 g?kg–1BC(SB5),每個處理4個重復。

表1 供試土壤、凋落物、生物質炭的基本性質
為避免不同處理間13CO2相互污染問題,本實驗對培養過程中產生的CO2收集方法做了改進,采用堿吸收結合采氣法,在不同培養時段更換堿液可以保證所有樣品均在密閉好氧條件下進行,而在特定時間采集氣體可以方便CO2濃度及其13C豐度的測定。具體步驟為:稱取過2 mm篩的新鮮土壤(相當于40.00 g烘干土)和相應添加量的凋落物或BC,分別混合均勻置于60 mL塑料杯中,另取2個60 mL塑料杯分別內置20 mL去離子水和20 mL 1 mol?L–1NaOH溶液,將3個塑料杯置于1 L帶有橡膠塞培養瓶中,培養溫度為25℃。由于BC分解緩慢且其顯著變化主要體現在培養前期(2周或4周內),故培養時間選擇28 d。在培養第0、1、3、5、7、14、21、28 d采集氣體。氣體采集時,將裝有土壤樣品的塑料杯轉移至1 L采氣瓶中,同時設置4個空白采氣瓶測定CO2背景值,采氣瓶瓶口帶有橡皮塞、玻璃管、膠塞等裝置便于采集氣體,連接處均涂抹704硅膠密封。2 h后用注射器抽取20 mL氣體注入已抽真空的20 mL采氣瓶中密封保存。采氣結束后通過稱重法調節水分維持60%土壤持水量(WHC),然后將裝有土壤樣品的塑料杯轉移至原來的培養瓶中,更換新的堿液后繼續密閉培養。
1.3 分析方法
pH利用便攜式pH計(STARTER 300,美國)測定,土壤pH測定的水土比例為2.5︰1,凋落物和BC的pH測定選擇水與凋落物或BC的比例為15︰1;土壤、凋落物和BC的碳、氮含量采用元素分析儀(Elementar Vario EL III,Elementar,德國)測定,溶解性有機碳(DOC)含量采用總碳分析儀(TOC-V CPH,日本)測定;BC的碳酸鹽測定采用容量滴定法;CO2濃度使用氣相色譜儀(GC-2010,Shimadzu,日本)測定;樣品13C豐度利用穩定同位素質譜儀(MAT 253,Thermo Fisher,德國)測定;土壤微生物含量采用磷脂脂肪酸(Phospholipid fatty acid,PLFA)法,用氣相色譜儀(Agilent 6890 N,美國)結合微生物識別系統(MIDI Inc.,Newark,DE,美國)測定提取的甲基酯化脂肪酸(Fatty acid methyl eaters,FAME)含量[16]。
1.4 計算方法
CO2排放速率計算方法參照Lang等[17],具體如下:
=V×(s–b)×M×(273/(273+T)×22.4)//(1)
式中,代表CO2排放速率,mg?kg–1?h–1;V為采氣瓶中氣體體積,L;s和b分別代表土樣和空白采氣瓶中CO2濃度,mg?kg–1;T為培養溫度,℃;M代表CO2分子量,g?mol–1;代表土樣質量(干物質量),g;代表采氣瓶密封時間,h。
區分CO2中2種碳源方法參照Keith等[18],具體如下:
a=t×(t–s)/(a–s) (2)
s=t–a(3)
式中,a代表來源于凋落物或BC的CO2排放速率,mg?kg–1?h–1;s為來源于原SOC的CO2排放速率,mg?kg–1?h–1;t為土壤總CO2排放速率,mg?kg–1?h–1;t為不同處理CO2的13C值,‰;s代表SOC的δ13C值,‰;a為凋落物或BC的δ13C值,‰。
CO2累積排放量計算方法如下:
=∑(F-1+F)/2×(t–t–1)×24 (4)
式中,代表CO2累積排放量,mg?kg–1;F–1為第–1次采樣時的CO2排放速率,mg?kg–1? h–1;F代表第次采樣時的CO2排放速率,mg?kg–1?h–1;t為第次采樣時間,d;t1是第–1次采樣時間,d。
分解率計算方法如下:
=×m×(12/44)/×100% (5)
式中,代表外源碳或原SOC的分解率,%;為來源于外源碳或原SOC的CO2累積排放量,mg?kg–1;m代表土樣質量,kg;代表外源碳或者原SOC的總碳量,mg。
土壤微生物PLFA含量測定用峰面積和內標曲線法,以正十九烷酸甲酯(19︰0)為內標,參照劉波等[19]方法。
1.5 數據統計
實驗所有數據用Excel 2013進行整理,統計學分析和作圖采用SPSS 19.0、OriginPro 9.0軟件,處理間差異采用鄧肯(Duncan)多重比較法,圖表數據均為平均值±標準差(=4)。
2 結 果
2.1 不同處理對不同來源CO2累積排放量的影響
如圖1所示,相較對照土壤(S),凋落物及BC添加顯著提高了土壤總CO2累積排放量(∑CO2),且前者的作用更明顯。分析不同來源∑CO2可知,來源于凋落物的∑CO2(LR-CO2)顯著大于其對應BC處理(BC-CO2)的,為其8.29倍~25.41倍。此外,2種外源碳添加條件下來源于原SOC的∑CO2(SOC-CO2)均顯著大于S處理的,凋落物處理為S處理的6.50倍~13.74倍,而BC處理為S處理的2.08倍~3.98倍。回歸分析顯示,2種來源的∑CO2與添加量均呈極顯著線性關系(<0.01),且隨著添加量的增加,來源于凋落物(或BC)分解與原SOC礦化的∑CO2均呈升高的趨勢。并且,凋落物處理的回歸方程斜率遠大于相應BC處理的(圖2)。
2.2 不同處理對凋落物碳、生物質炭碳和原SOC分解率的影響
培養結束時,凋落物碳(LR-C)分解率介于5.71%~13.68%之間,而生物質炭碳(BC-C)分解率僅為0.34%~0.50%(表2),并且LR-C分解率均顯著高于相應BC-C的(<0.01)。此外,凋落物和BC添加亦顯著促進了原SOC分解,分別為對照處理的6.42倍~13.58倍與2.06倍~3.94倍。回歸分析表明,添加量與BC-C分解率呈顯著開口向上的拋物線關系(<0.05),與LR-C分解率則呈極顯著的線性關系(<0.01);而在2種外源碳添加條件下,添加量與原SOC分解率也呈現極顯著的線性關系(<0.01),且凋落物處理的回歸方程斜率明顯高于BC處理(圖3)。
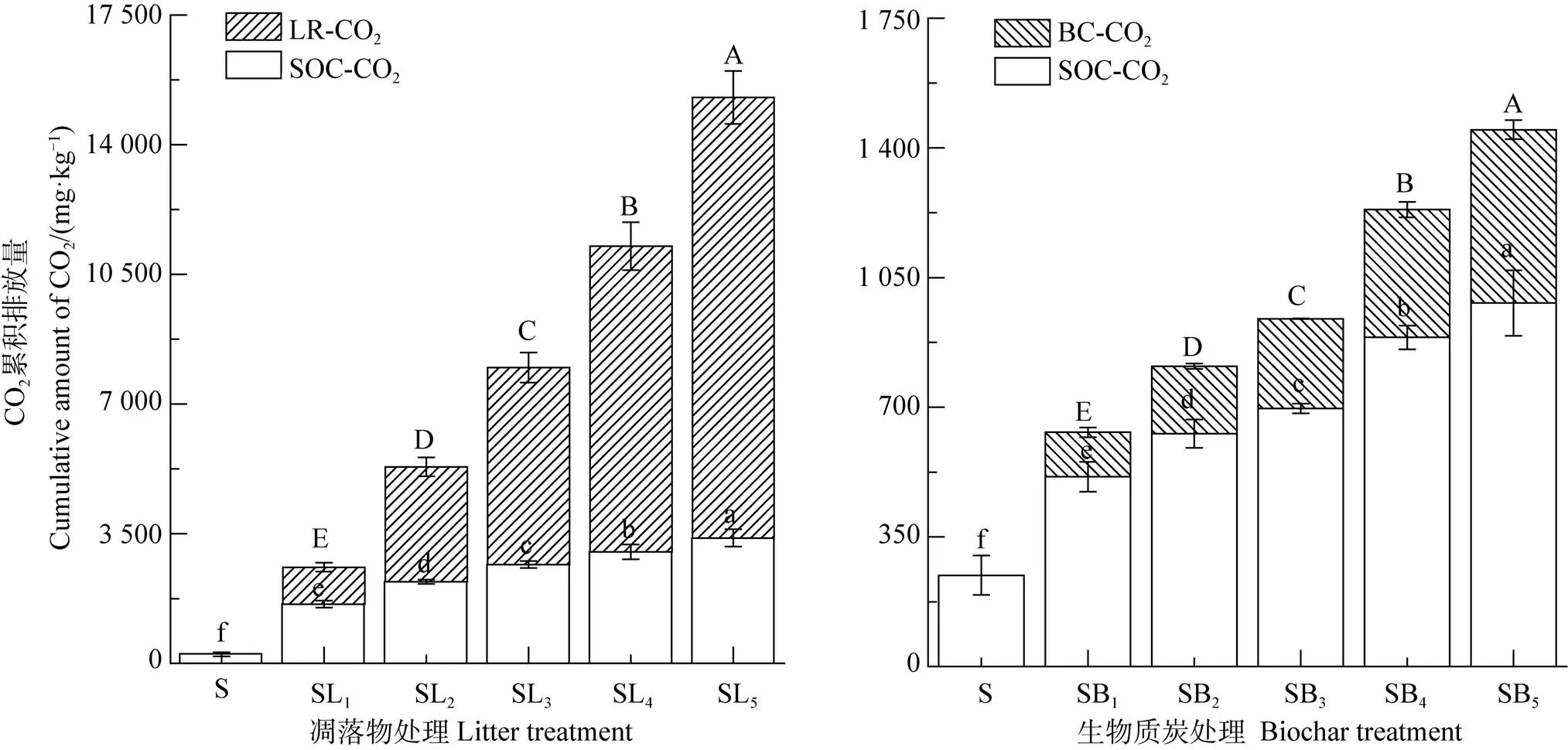
注:不同大寫字母表示處理間CO2累積排放量來源于凋落物或生物質炭的差異顯著(P<0.05);不同小寫字母表示處理間CO2累積排放量來源于原SOC的差異顯著(P<0.05)。 S:土壤;SL1:土壤+10 g?kg–1凋落物;SL2:土壤+20 g?kg–1凋落物;SL3:土壤+30 g?kg–1凋落物;SL4:土壤+40 g?kg–1凋落物;SL5:土壤+50 g?kg–1凋落物;SB1:土壤+10 g?kg–1BC;SB2:土壤+20 g?kg–1BC;SB3:土壤+30 g?kg–1BC;SB4:土壤+40 g?kg–1BC;SB5:土壤+50 g?kg–1BC;LR-CO2:來源于凋落物的CO2累積釋放量;BC-CO2:來源于生物質炭的CO2累積釋放量;SOC-CO2:來源于原SOC的CO2累積釋放量。下同。Note:Different uppercase letters indicate significant difference at P<0.05 level between treatments in cumulative CO2 emission from litter or biochar;Different lowercase letters indicate significant difference at P<0.05 level between treatments in cumulative CO2 emission from native SOC. S stands for control(soil without any addition);SL1,for soil added with 10 g?kg–1 litter;SL2,for soil added with 20 g?kg–1 litter;SL3,for soil added with 30 g?kg–1 litter;SL4,for soil added with 40 g?kg–1 litter;SL5,for soil added with 50 g?kg–1 litter;SB1,for soil added with 10 g?kg–1 BC;SB2,for soil added with 20 g?kg–1 BC;SB3,for soil added with 30 g?kg–1 BC;SB4,for soil added with 40 g?kg–1 BC;SB5,for soil added with 50 g?kg–1 BC;LR-CO2,for cumulative amount of the CO2 derived from litter;BC-CO2,for cumulative amount of the CO2 derived from biochar,and SOC-CO2,for cumulative amount of the CO2 derived from native SOC. The same below.
2.3 不同來源的CO2累積排放量和分解率的影響因素
如圖4所示,2種外源碳添加條件下土壤微生物PLFA總量均高于對照S處理,且凋落物處理顯著大于相對應BC處理的。由表3可見,相關分析表明,凋落物處理中來源于凋落物的CO2累積排放量及LR-C分解率與土壤pH、全碳、全氮、C/N比以及土壤PLFA總量均呈極顯著正相關(<0.01),而來源于原SOC的CO2累積排放量及原SOC分解率除與pH相關性不顯著外,與其他指標均呈極顯著的正相關(<0.01);而BC處理中來源于原SOC的CO2累積排放量和原SOC分解率與土壤基本性質均呈極顯著正相關(<0.01),來源于BC的CO2累積排放量與土壤pH、全碳、全氮、C/N比呈極顯著正相關(<0.01),而BC-C分解率則與上述指標呈顯著或極顯著負相關(表3),但兩者與土壤PLFA總量的關系均未達顯著水平。
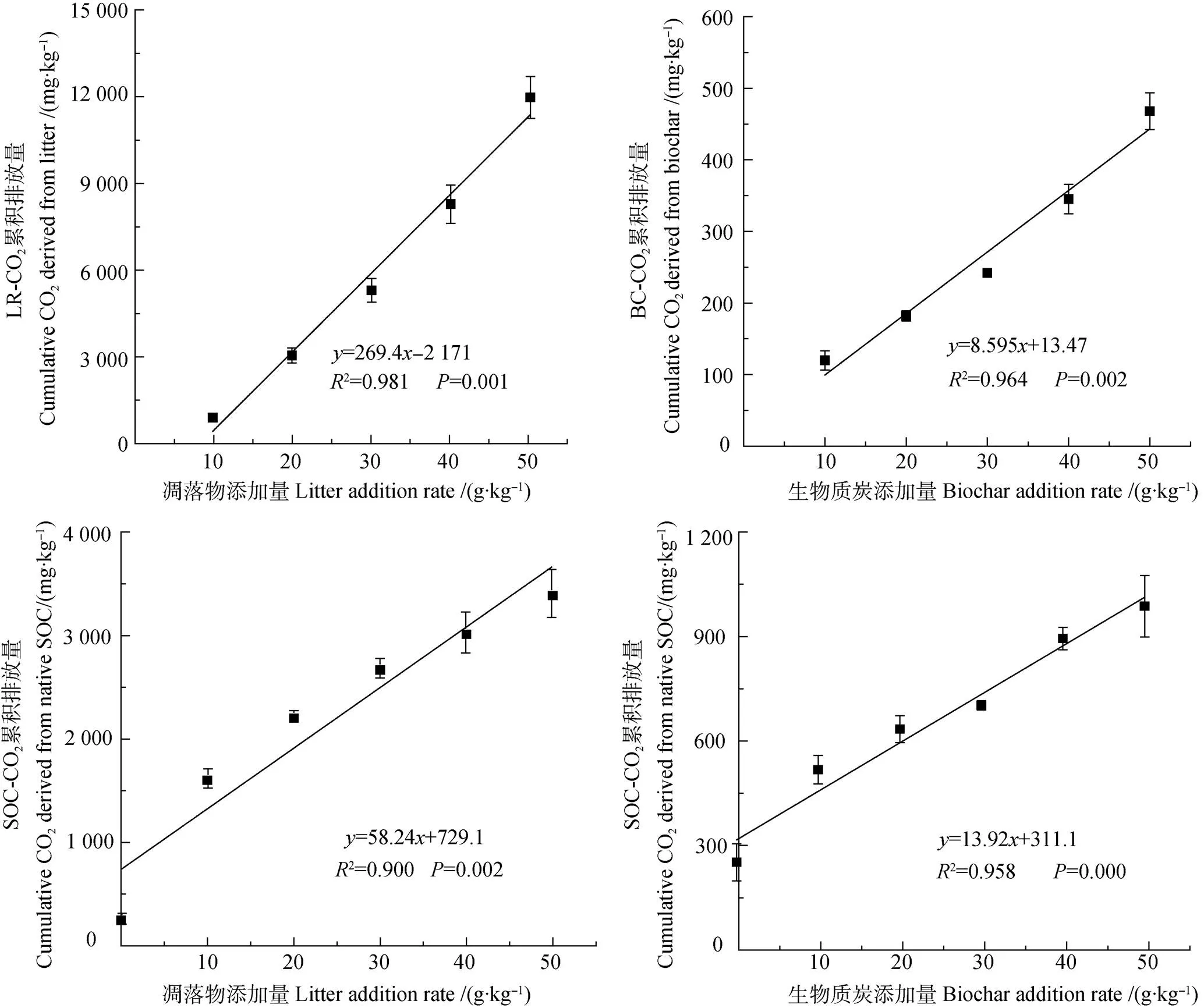
圖2 不同來源CO2累積排放量與凋落物碳、生物質炭碳添加量之間的關系
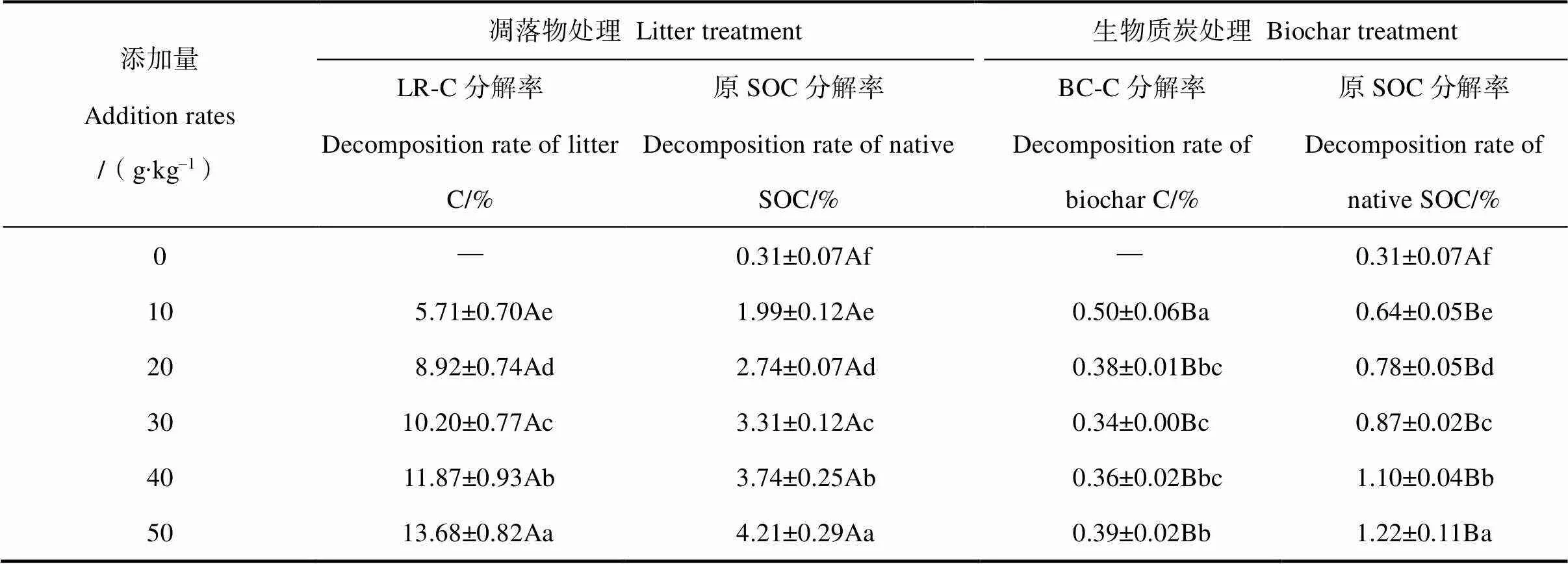
表2 凋落物碳、生物質炭和土壤原SOC分解率
注:同列不同小寫字母表示不同添加量處理差異顯著(<0.05),同行不同大寫字母表示凋落物和生物質炭處理間差異顯著(<0.01)。Note:Different lowercase letters within the same column indicate significant difference among different addition rates at<0.05 level. Different uppercase letters within the same line indicate significant difference between litter and biochar treatments at<0.01 level.

圖3 凋落物碳和生物質炭碳分解率及原SOC分解率與添加量的關系
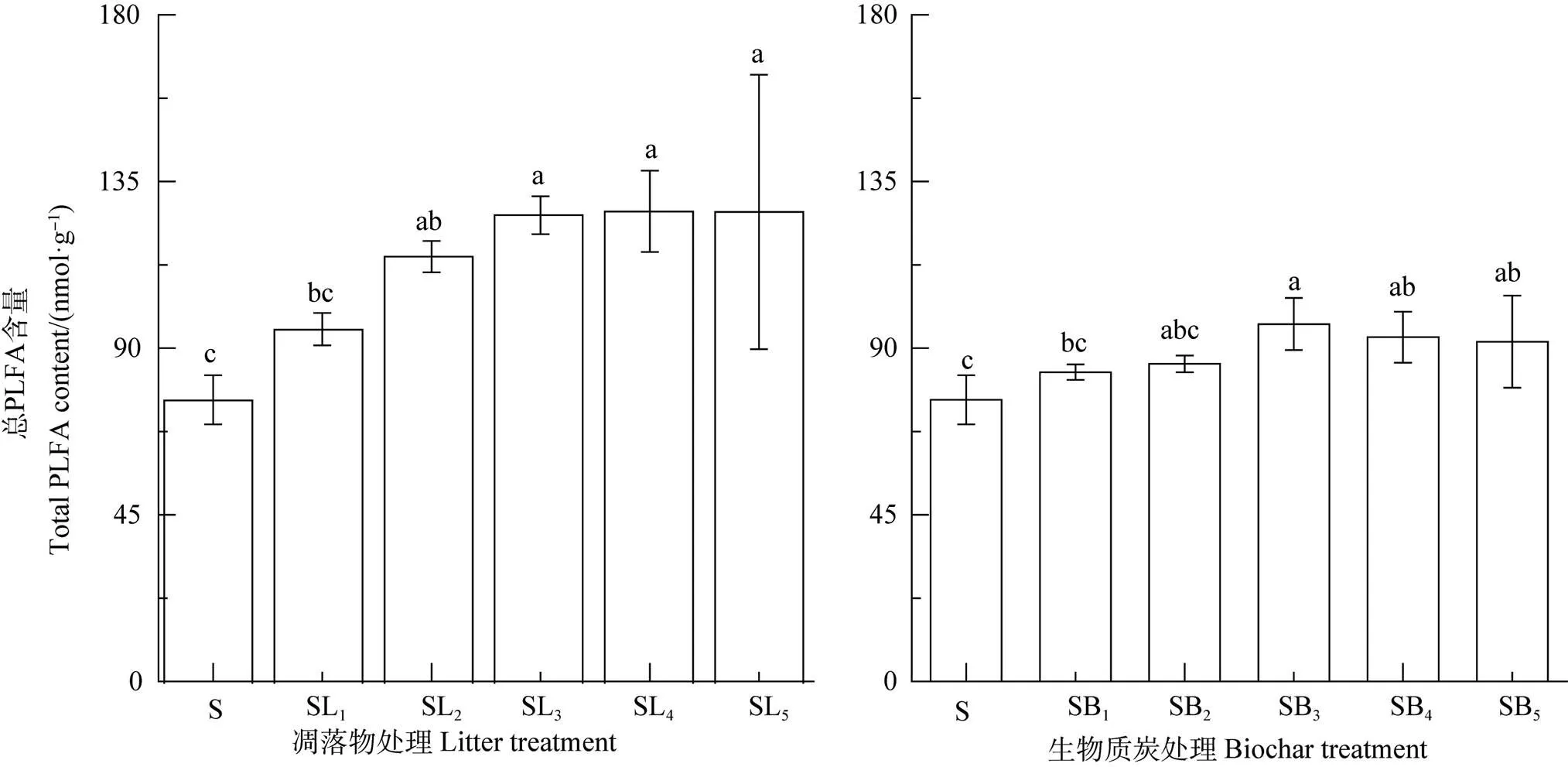
圖4 不同處理對土壤微生物磷脂脂肪酸(PLFA)總量的影響

表3 CO2累積排放量、分解率與土壤基本性質的相關系數
**<0.01,*<0.05. ①Decomposition rate of litter carbon,②Decomposition rate of native SOC,③Decomposition rate of biochar carbon.
3 討 論
3.1 不同處理對凋落物和BC分解的影響
目前,對來源于新鮮有機物質及BC的CO2累積排放量與其添加量的關系研究亦有報道:如Shahzad等[20]添加玉米秸稈(0、3、6、12、24 g?kg–1)到土壤中,發現來源于秸稈的CO2累積排放量隨添加量增加呈上升趨勢;而Smith等[21]研究表明來源于BC(500℃熱解柳枝稷,添加量為0、11.2、22.4、44.8 t?hm–2)的CO2累積排放量亦隨添加量增加而增加。在本研究中,來源于凋落物或BC的CO2累積排放量呈現了類似趨勢,并且來源于凋落物的CO2累積排放量顯著大于BC的,其原因在于凋落物含有更多易分解物質,對微生物刺激作用更為明顯(圖4),進而促進了凋落物的分解。來源于凋落物的CO2累積排放量和LR-C分解率均與微生物PLFA總量呈極顯著正相關,進一步證實凋落物的分解主要與微生物活動有關;但本研究中BC-C分解率與土壤pH、TC、TN、C/N均呈顯著負相關,而來源于BC的CO2累積排放量與土壤微生物PLFA總量的相關性也不顯著,其原因在于BC處理的CO2累積排放量并非完全來自微生物的降解,也可能來自無機碳(表1)。BC中無機碳主要以碳酸鹽形式存在[22],具有較強的堿性,與土壤中酸性物質反應,進而釋放CO2[23]。
外源碳的分解速率與其數量和質量以及土壤性質、微生物等因素有關[24]。Maestrini等[25]將450℃熱解黑麥草產生的BC添加至森林土壤中,培養5個月后BC分解率為4.3%。而Naisse等[26]經過336 d的室內培養,發現黑麥草碳分解率為25%,但BC僅為0.4%~0.6%。本實驗結果與上述研究基本一致,培養28 d后凋落物碳分解率(5.71%~13.68%)亦顯著高于BC分解率(0.34%~0.50%)。其原因在于BC高度碳化且芳香環和烷基結構緊密堆積,這種穩定性機制使其不易被微生物分解,并且BC可以與土壤黏粒或粉粒形成團聚體,從而提高其穩定性[27]。Jiang等[28]量化了BC分解率與其添加量關系,將BC(550℃,橡木)以0、10、50、100、200 g?kg–1土壤質量比添加至4種土壤中進行30個月的培養,發現BC的損失均隨著添加量增加而增加。本研究也證實,凋落物碳分解率隨添加量增加而增加,但BC分解率卻未呈現這一趨勢,在10 g?kg–1添加量時即達到最大,其原因在于BC具有大量的孔隙結構能有效隔離微生物及其產生的胞外酶[29],添加量增加時大量BC的“隔離微生物”起主要作用,使其分解率下降。同時,添加量增加后土壤pH、全碳和全氮含量也相應提高,這不利于BC分解(表3)。
3.2 不同處理對原SOC礦化的影響
研究表明,外源碳輸入對土壤原SOC礦化存在抑制作用(負激發效應)、促進作用(正激發效應)或無影響[30]。導致這種截然不同結論與土壤性質、培養條件、植物類型以及外源碳的類型、數量與質量有關。苗淑杰等[31]利用35 d的培養實驗發現,玉米秸稈不同添加量(10、50、90 g?kg–1)均顯著抑制了原SOC的礦化,且在50 g?kg–1添加量時抑制作用最強,其原因在于外加大量玉米秸稈釋放出較多易被分解的碳抑制了微生物對原SOC的利用。尹艷等[14]將不同溫度制備的BC(350、550和750℃,杉木與木荷凋落物)添加至杉木人工林土壤中培養112 d后發現,所有BC處理均顯著抑制了土壤原SOC礦化。這種抑制作用的機制可能是BC具有大量的孔隙結構與巨大的比表面積,將土壤有機質吸附至其孔隙內或外表面上,可降低有機質的可利用率[29]。但Farrell等[32]通過80 d的培養實驗發現,2種BC(450℃,小麥秸稈和桉樹枝)的添加均促進了土壤原SOC礦化。Cui等[33]將水稻秸稈及其制備的BC(400℃)分別添加至土壤中進行200 d的培養后,發現秸稈及BC均促進了原SOC礦化,且秸稈處理來源于原SOC的CO2排放量大于BC處理的。本研究結論與其一致,相較對照土壤,凋落物及BC處理均提高了原SOC的礦化,且凋落物促進效果更明顯。隨著添加量增加,促進效果越明顯,原因是原SOC的分解與微生物活性呈顯著正相關(表3)。凋落物或BC的添加提高了土壤微生物活性(圖4),且凋落物對土壤微生物PLFA的影響更為明顯,而微生物產生的胞外酶存在土壤中,在其催化作用下降解外源碳的同時也可能降解了土壤原SOC[34],引發“共代謝”作用。
王曉峰等[7]通過13C標記杉木凋落物進行室內培養實驗,81 d培養結束時SOC分解率為3.6%~6.0%。而Zhang等[35]通過Meta分析表明在添加外源碳(植物殘體及小分子物質)的實驗中,SOC平均分解率增加26.5%。本實驗則發現,凋落物及BC添加條件下SOC分解率為0.64%~4.21%,并且隨著添加量的增加而增加,進一步表明外源碳輸入并未減緩微生物對原SOC的利用,反而會促進其分解。原因可能在于凋落物與BC的易分解物質為微生物提供了生長所需的碳源及其他養分[23],BC的多孔結構還為其提供了適宜的場所,增加了原SOC的微生物可利用性,從而加速原SOC礦化。
4 結 論
利用短期實驗結合同位素13C示蹤技術發現,相較于對照土壤,凋落物或BC單獨添加對原SOC礦化的促進作用隨著添加量的增加而增加,且前者的影響更為明顯。杉木人工林原SOC的分解率與添加量之間均呈線性關系;凋落物碳分解率與添加量之間呈線性關系,但生物質炭分解率并未呈現這一趨勢,在添加量為10 g?kg–1時即達到最大值。同凋落物相比,短期內BC的分解受微生物活動的影響較小,生物有效性更低,在土壤中更穩定,有關的微生物學機制尚需進一步研究。
[1] Post W M,Emanuel W R,Zinke P J,et al. Soil carbon pools and world life zones[J]. Nature,1982,298(5870):156—159.
[2] Trumbore S. Carbon respired by terrestrial ecosystems - recent progress and challenges[J]. Global Change Biology,2006,12(2):141—153.
[3] Tan Y L,Chen J,Yan L M,et al. Mass loss and nutrient dynamics during litter decomposition under three mixing treatments in a typical steppe in Inner Mongolia[J]. Plant and Soil,2013,366(1/2):107—118.
[4] Andersson S,Nilsson S. Influence of pH and temperature on microbial activity,substrate availability of soil-solution bacteria and leaching of dissolved organic carbon in a mor humus[J]. Soil Biology and Biochemistry,2001,33(9):1181—1191.
[5] Shi X J,Pan J J,Chen J Y,et al. Effects of different types of litters on soil organic carbon mineralization[J]. Environmental Science,2009,30(6):1832—1837. [史學軍,潘劍君,陳錦盈,等. 不同類型凋落物對土壤有機碳礦化的影響[J]. 環境科學,2009,30(6):1832—1837.]
[6] Yu Z P,Wan X H,Hu Z H,et al. Contrasting responses of soil respiration to litter manipulation in subtropicalandplantations[J]. Acta Ecologica Sinica,2014,34(10):2529—2538. [余再鵬,萬曉華,胡振宏,等. 亞熱帶杉木和米老排人工林土壤呼吸對凋落物去除和交換的響應[J]. 生態學報,2014,34(10):2529—2538.]
[7] Wang X F,Wang S L,Zhang W D. Effects of Chinese fir litter on soil organic carbon decomposition and microbial biomass carbon[J]. Chinese Journal of Applied Ecology,2013,24(9):2393—2398. [王曉峰,汪思龍,張偉東. 杉木凋落物對土壤有機碳分解及微生物生物量碳的影響[J]. 應用生態學報,2013,24(9):2393—2398.]
[8] Uselman S M,Qualls R,Thomas R. Effects of increased atmospheric CO2,temperature,and soil N availability on root exudation of dissolved organic carbon by a N-fixing tree(L.)[J]. Plant and Soil,2000,222(1/2):191—202.
[9] Leng L J,Huang H J,Li H,et al. Biochar stability assessment methods:A review[J]. Science of the Total Environment,2019,647:210—222.
[10] Xie Z B,Liu Q,Xu Y P,et al. Advances and perspectives of biochar research[J]. Soils,2011,43(6):857—861. [謝祖彬,劉琦,許燕萍,等. 生物炭研究進展及其研究方向[J]. 土壤,2011,43(6):857—861.]
[11] Kang X L,Zhang X H,Zhang S S,et al. Effects of biochar application history on soil:Effect of moisture regime on dynamics of soil organic carbon mineralization[J]. Soils,2016,48(1):152—158. [康熙龍,張旭輝,張碩碩,等. 旱地土壤施用生物質炭的后效應——水分條件對土壤有機碳礦化的影響[J]. 土壤,2016,48(1):152—158.]
[12] Wang F H,Huang R,Gao M,et al. Effect of combined application of biochar and straw on organic carbon content in purple soil aggregates[J]. Acta Pedologica Sinica,2019,56(4):929—939. [王富華,黃容,高明,等. 生物質炭與秸稈配施對紫色土團聚體中有機碳含量的影響[J]. 土壤學報,2019,56(4):929—939.]
[13] Zheng L J,Huang Z Q,He Z M,et al. Influence of forest and foliar ages on the composition of stable carbon and nitrogen isotope ofin subtropic China[J]. Scientia Silvae Sinicae,2015,51(1):22—28. [鄭璐嘉,黃志群,何宗明,等. 林齡、葉齡對亞熱帶杉木人工林碳氮穩定同位素組成的影響[J]. 林業科學,2015,51(1):22—28.]
[14] Yin Y,Liu Y,Yin Y F,et al. Effects of biochar addition on the mineralization of native soil organic carbon inplantation[J]. Chinese Journal of Applied Ecology,2018,29(5):1389—1396. [尹艷,劉巖,尹云鋒,等. 生物質炭添加對杉木人工林土壤原有有機碳礦化的影響[J]. 應用生態學報,2018,29(5):1389—1396.]
[15] Yin Y F,Yang Y S,Gao R,et al. A preliminary study on phyto-enrichment13C labeling technique [J]. Acta Pedologica Sinica,2010,47(4):790—793. [尹云鋒,楊玉盛,高人,等. 植物富集13C標記技術的初步研究[J]. 土壤學報,2010,47(4):790—793.]
[16] Gai X P,Liu H B,Zhai L M,et al. Temporal fluctuations of impacts of corn-stover biochar on nutrients and microbial community structure in a neutral paddy soil[J]. Journal of Agro-Environment Science,2016,35(4):719—728. [蓋霞普,劉宏斌,翟麗梅,等. 生物炭對中性水稻土養分和微生物群落結構影響的時間尺度變化研究[J]. 農業環境科學學報,2016,35(4):719—728.]
[17] Lang M,Cai Z C,Chang S X. Effects of land use type and incubation temperature on greenhouse gas emissions from Chinese and Canadian soils[J]. Journal of Soils and Sediments,2011,11(1):15—24.
[18] Keith A,Singh B,Singh B P. Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil[J]. Environmental Science & Technology,2011,45(22):9611—9618.
[19] Liu B,Hu G P,Zheng X F,et al. Analysis on microbial diversity in the rhizosphere of rice by phospholipid fatty acids biomarkers[J]. Chinese Journal of Rice Science,2010,24(3):278—288. [劉波,胡桂萍,鄭雪芳,等. 利用磷脂脂肪酸(PLFAs)生物標記法分析水稻根際土壤微生物多樣性[J]. 中國水稻科學,2010,24(3):278—288.]
[20] Shahzad T,Anwar F,Hussain S,et al. Carbon dynamics in surface and deep soil in response to increasing litter addition rates in an agro-ecosystem[J]. Geoderma,2019,333:1—9.
[21] Smith J L,Collins H P,Bailey V L. The effect of young biochar on soil respiration[J]. Soil Biology and Biochemistry,2010,42(12):2345—2347.
[22] Mitchell P J,Simpson A J,Soong R,et al. Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil[J]. Soil Biology and Biochemistry,2015,81:244—254.
[23] Jones D L,Murphy D V,Khalid M,et al. Short-term biochar-induced increase in soil CO2release is both biotically and abiotically mediated[J]. Soil Biology and Biochemistry,2011,43(8):1723—1731.
[24] Butnan S,Deenik J L,Toomsan B,et al. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy[J]. Geoderma,2015,237/238:105—116.
[25] Maestrini B,Herrmann A M,Nannipieri P,et al. Ryegrass-derived pyrogenic organic matter changes organic carbon and nitrogen mineralization in a temperate forest soil[J]. Soil Biology and Biochemistry,2014,69:291—301.
[26] Naisse C,Girardin C,Davasse B,et al. Effect of biochar addition on C mineralisation and soil organic matter priming in two subsoil horizons[J]. Journal of Soils and Sediments,2015,15(4):825—832.
[27] Chen Y,Liu Y X,Chen C J,et al. Priming effect of biochar on the minerialization of native soil organic carbon and the mechanisms:A review[J]. Chinese Journal of Applied Ecology,2018,29(1):314—320. [陳穎,劉玉學,陳重軍,等. 生物炭對土壤有機碳礦化的激發效應及其機理研究進展[J]. 應用生態學報,2018,29(1):314—320.]
[28] Jiang X Y,Denef K,Stewart C E,et al. Controls and dynamics of biochar decomposition and soil microbial abundance,composition,and carbon use efficiency during long-term biochar-amended soil incubations[J]. Biology and Fertility of Soils,2016,52(1):1—14.
[29] Kaiser K,Guggenberger G. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils[J]. Organic Geochemistry,2000,31(7/8):711—725.
[30] Huang W Z,Zhao X L,Zhu J G,et al. Priming effect of soil carbon pools[J]. Chinese Journal of Soil Science,2007,38(1):149—154. [黃文昭,趙秀蘭,朱建國,等. 土壤碳庫激發效應研究[J]. 土壤通報,2007,38(1):149—154.]
[31] Miao S J,Qiao Y F,Wang W T,et al. Priming effect of maize straw addition on soil organic matter in yellow-brown soil[J]. Soils,2019,51(3):622—626. [苗淑杰,喬云發,王文濤,等. 添加玉米秸稈對黃棕壤有機質的激發效應[J]. 土壤,2019,51(3):622—626.]
[32] Farrell M,Kuhn T K,MacDonald L M,et al. Microbial utilisation of biochar-derived carbon[J]. Science of the Total Environment,2013,465:288—297.
[33] Cui J,Ge T D,Kuzyakov Y,et al. Interactions between biochar and litter priming:A three-source 14C and δ13C partitioning study[J]. Soil Biology and Biochemistry,2017,104:49—58.
[34] Kuzyakov Y,Friedel J,Stahr K. Review of mechanisms and quantification of priming effects[J]. Soil Biology and Biochemistry,2000,32(11/12):1485—1498.
[35] Zhang W D,Wang X F,Wang S L. Addition of external organic carbon and native soil organic carbon decomposition:A meta-analysis[J]. PLoS One,2013,8(2):e54779. https://doi.org/10.1371/journal.pone. 0054779.
Effects of Chinese Fir Litter and Its Biochar Addition on Mineralization of Native Soil Organic Carbon
LU Xiaorong, YIN Yan, FENG Jingxian, MA Hongliang, GAO Ren, YIN Yunfeng?
(State Key Laboratory for Subtropical Mountain Ecology of the Ministry of Science and Technology and Fujian Province, School of Geographical Sciences, Fujian Normal University, Fuzhou 350007, China)
【】Understanding of how soil organic carbon (SOC) mineralization response to management practice and environment change is crucial for mitigating greenhouse gases emission and minimizing the impacts of forest ecosystems on climate. However, there is little information available about relationship of SOC mineralization with quantity of extraneous carbon. In this experiment, effects of addition of Chinese fir litter and its biochar (BC), on native SOC mineralization were investigated to provide certain scientific basis for building up soil fertility in Chinese fir plantations and utilizing biomass resources efficiently in subtropical China. 【】In this study, soil samples (0~20 cm topsoil layer) were collected from theplantation at the Wanmulin Nature Reserve of Fujian Province.13C-labeled litter (Chinese fir) and its biochar (pyrolysed at 350°C) were used as two different types of extraneous carbon (an easily decomposable carbon and a relatively stable carbon) in the indoor incubation experiment. The experiment consisted of 11 treatments, i.e. S (soil without any addition), SL1(soil added with 10 g?kg–1litter), SL2(soil added with 20 g?kg–1litter), SL3(soil added with 30 g?kg–1litter), SL4(soil added with 40 g?kg–1litter), SL5(soil added with 50 g?kg–1litter), SB1(soil added with 10 g?kg–1BC), SB2(soil added with 20 g?kg–1BC), SB3(soil added with 30 g?kg–1BC), SB4(soil added with 40 g?kg–1BC) and SB5(soil added with 50 g?kg–1BC). The carbon derived from different sources was distinguished with the13C isotope technique. The soil samples were incubated at 25°C with water holding capacity kept at 60% for 28 days. 【】Results showed that the addition of either litter or BC significantly increased the cumulative amount of CO2, and the effect of the litter treatments was more obvious than that of their corresponding BC treatments. Cumulative CO2derived from added litter or BC and native SOC increased with increasing quantity of the extraneous carbon. After 28 days of incubation, 5.71% –13.68% of the litter carbon (LR-C)was decomposed, whereas only 0.34%–0.50% of the biochar carbon (BC-C) was, and 6.42–13.58 times and 2.06–3.94 times as much of the native SOC in the litter and BC treatments, respectively, as that in Treatment S was decomposed. Regression analysis shows that there was a significant linear relationship between native SOC decomposition rate and quantity of the extraneous carbon added (<0.01). LR-C decomposition rate was positively related to addition rate of the extraneous carbon, while BC-C decomposition rate displayed a significant parabolic relationship (<0.05) and peaked when the addition rate was 10 g?kg–1(Treatment SB1). 【】Addition of litter and BC accelerated mineralization of native SOC and the effect increased with addition rates. Compared with decomposition of litter, that of BC was less affected by microbial activity due to its lower bioavailability and higher stability in the tested soil during the short incubation period. Therefore, it may be a management practice more conducive to enhancing soil carbon sequestration of the plantations in this region, to return biochar into the soil.
Litter; Biochar; Native soil organic carbon; Decomposition rate; Plantation
S714
A
10.11766/trxb201906040226
盧曉蓉,尹艷,馮競仙,馬紅亮,高人,尹云鋒. 杉木凋落物及其生物質炭對土壤原有有機碳礦化的影響[J]. 土壤學報,2020,57(4):943–953.
LU Xiaorong, YIN Yan, FENG Jingxian, MA Hongliang, GAO Ren, YIN Yunfeng.Effects of Chinese Fir Litter and Its Biochar Addition on Mineralization of Native Soil Organic Carbon[J]. Acta Pedologica Sinica,2020,57(4):943–953.
* 國家自然科學基金項目(31470628,31770659)和教育部科學技術研究項目(213019A)資助Supported by the National Natural Science Foundation of China(Nos. 31470628 and 31770659)and the Research Project of Chinese Ministry of Education(No. 213019A)
,E-mail:yunfengyin@163.com
盧曉蓉(1994—),女,山西臨汾人,碩士研究生,主要從事森林生態系統碳循環研究。E-mail:1270859070@qq.com
2019–06–04;
2019–09–09;
2019–10–29
(責任編輯:陳榮府)

