骨髓纖維化與非霍奇金淋巴瘤骨髓病理學特征及預后
吳春萌 崔渤莉 費海榮 劉曉丹 孫玲潔 趙春亭

[摘要] 目的 探討骨髓纖維化(MF)與非霍奇金淋巴瘤(NHL)病人的骨髓病理特征及其對預后影響。方法對215例NHL病人(其中96例合并MF)的臨床資料回顧分析,觀察病人骨髓病理學特點,比較合并與未合并MF病人治療6個周期緩解率、總生存率(OS)及無疾病進展生存率(PFS)的差異。結果 Ⅲ期和Ⅳ期NHL病人較Ⅰ期和Ⅱ期更易合并MF(Z=-2.548,P<0.05)。合并MF的NHL病人脾大例數較未合并MF的NHL病人多,兩組比較差異有顯著性(χ2=13.019,P<0.05)。合并MF病人化療后3~4度骨髓抑制發生率較未合并MF者高(Z=-3.413,P<0.05)。合并和未合并MF的NHL病人治療6周期完全緩解率差異無顯著性(χ2=0.261,P>0.05)。壽命表分析顯示,合并MF的NHL病人1、2、3年PFS及OS分別為83%、68%、55%和86%、85%、60%,未合并MF的NHL病人的1、2、3年PFS及OS分別為94%、81%、81%和96%、95%、95%,兩組OS及PFS比較差異有顯著性(χ2=6.077、5.443,P<0.05)。COX回歸生存分析顯示,合并MF是影響淋巴瘤病人OS(RR=0.357,95%CI=0.136~0.933,P<0.05)和PFS (RR=0.459,95%CI=0.239~0.884,P<0.05)的獨立不良預后因素。結論 MF是NHL病人預后的危險因素,合并MF可降低NHL病人的PFS及OS,可能與化療后嚴重骨髓抑制引起早期死亡有關。
[關鍵詞] 淋巴瘤,非霍奇金;原發性骨髓纖維化;預后
[中圖分類號] R733.4 ?[文獻標志碼] A ?[文章編號] 2096-5532(2020)05-0544-05
doi:10.11712/jms.2096-5532.2020.56.145 [開放科學(資源服務)標識碼(OSID)]
[ABSTRACT] Objective To investigate the pathological features of bone marrow in patients with myelofibrosis (MF) and non-Hodgkin lymphoma (NHL) and their influence on prognosis. ?Methods A retrospective analysis was performed for the clinical data of 215 patients with NHL, among whom 96 patients had MF, and the pathological features of bone marrow were observed. Complete response (CR) rate, overall survival (OS) rate, and progression-free survival (PFS) rate were compared between the patients with MF and those without MF after 6 cycles of chemotherapy. ?Results The patients with stage Ⅲ/Ⅳ NHL were more likely to experience MF than those with stage Ⅰ/Ⅱ NHL (Z=-2.548,P<0.05). Compared with patients without secondary myelofibrosis, there are more symptoms of splenomegaly in patients with non-Hodgkins lymphoma with secondary myelofibrosis, the difference between the two is significant (χ2=13.019,P<0.05). The patients with MF had a significantly higher incidence rate of grade 3-4 myelosuppression than those without MF (Z=-3.413,P<0.05). There was no significant difference in CR rate after 6 cycles of treatment between NHL patients with MF and those without MF (χ2=0.261,P>0.05). The life table analysis showed that in the NHL patients with MF, the 1, 2, and 3 year PFS rates were 83%, 68%, and 55%, respectively, and the 1, 2, and 3 year OS rates were 86%, 85%, and 60%, respectively; in the NHL patients without MF, the 1, 2, and 3 year PFS rates were 94%, 81%, and 81%, respectively, and the 1, 2, and 3 year OS rates were 96%, 95%, and 95%, respectively; there were significant differences in OS and PFS rates between the two groups (χ2=6.077,5.443;P<0.05). The Cox regression survival analysis showed that in patients with NHL, MF was an independent negative prognostic factor for OS (RR=0.357,95%CI=0.136-0.933,P<0.05) and PFS (RR=0.459,95%CI=0.239-0.884,P<0.05). ?Conclusion MF is a risk factor for the prognosis of patients with NHL and can shorten PFS and OS of patients with NHL, which may be associated with early death caused by severe myelosuppression after chemotherapy.
[KEY WORDS] lymphoma, non-Hodgkin; primary myelofibrosis; prognosis
非霍奇金淋巴瘤(NHL)是一組異質性很大的淋巴增殖性疾病,絕大多數的NHL表現為淋巴結和(或)髓外淋巴組織受累。隨著NHL的進展,逐漸出現骨髓侵犯。而合并骨髓纖維化(MF)是否會對NHL的預后有影響,目前國內外相關研究較少。本研究通過對NHL伴有MF病人的臨床病理特征及其與預后的關系進行分析,探討NHL合并MF對疾病的預后影響,為其治療提供循證醫學依據。
1 資料和方法
1.1 研究對象
收集我院2016年6月—2018年6月收治的215例NHL病人的臨床資料。所有病人化療后骨髓抑制分度按照WHO規定的抗腫瘤藥物急性與亞急性毒副作用中血液學的分度標準;MF分級采用歐洲MF分級共識標準[1],MF-1級及以上即可判斷為MF;淋巴瘤分類按照2019年WHO淋巴瘤分類[2]。NHL合并MF(A組)的96例病人中,前驅淋巴性腫瘤(T淋巴母細胞淋巴瘤)1例,成熟B細胞淋巴瘤74例(其中彌漫大B細胞淋巴瘤49例,濾泡性淋巴瘤9例,結外黏膜相關淋巴組織邊緣帶B細胞淋巴瘤5例,淋巴漿細胞淋巴瘤3例,結內邊緣帶B細胞淋巴瘤及套細胞淋巴瘤各2例,脾邊緣帶淋巴瘤1例,未明確分型B細胞淋巴瘤3例),成熟T/NK細胞淋巴瘤21例(血管免疫母細胞T細胞淋巴瘤6例,外周T細胞淋巴瘤6例,結外NK/T細胞淋巴瘤5例,ALK陽性間變性大細胞淋巴瘤和ALK陰性間變性大細胞淋巴瘤各2例)。NHL非合并MF(B組)病人119例。所有病人診斷和治療均符合2018年版淋巴瘤診療規范[3]。
1.2 骨髓涂片和病理標本制備
骨髓涂片和骨髓活檢標本取材于病人髂前上棘或髂后上棘,在采集骨髓液涂片同時取骨髓活組織送檢,骨髓涂片采用瑞氏-吉姆薩染色,骨髓活檢組織切片采用蘇木精-吉姆薩-酸性品紅(HGF)染色,觀察病人骨髓病理學特點。
1.3 治療及隨訪
病人的治療采用以CHOP(環磷酰胺+長春新堿+表柔比星+潑尼松)為基礎的方案化療,平均治療6周期。所有病人均通過門診或電話隨訪至2018年12月,總生存時間為疾病確診時間至死亡或隨訪結束。收集病人性別、年齡、分期、病理分類、MF分級、血常規、基因檢測、治療方案、化療后骨髓抑制程度及轉歸等指標。
1.4 統計學分析
采用SPSS 17.0軟件進行統計分析,分期及骨髓抑制程度比較采用秩和檢驗,率的比較采用卡方檢驗,生存率分析采用壽命表法,單因素生存分析采用Log-rank檢驗,多因素分析采用COX回歸生存分析。P<0.05表示差異有統計學意義。
2 結 ?果
2.1 合并MF的NHL病人的臨床及病理特征
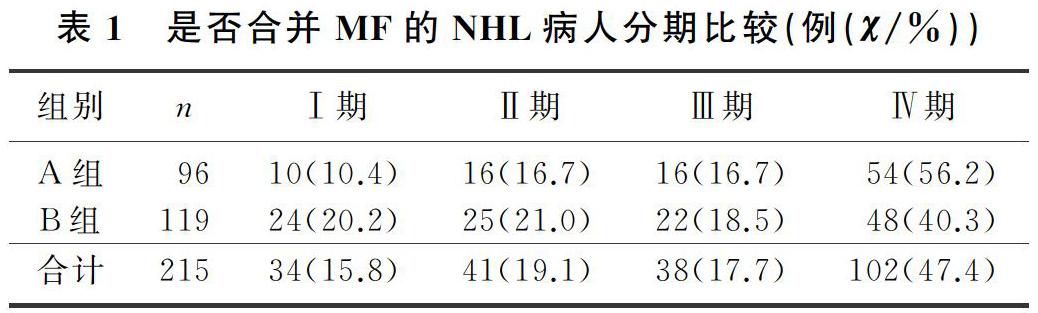
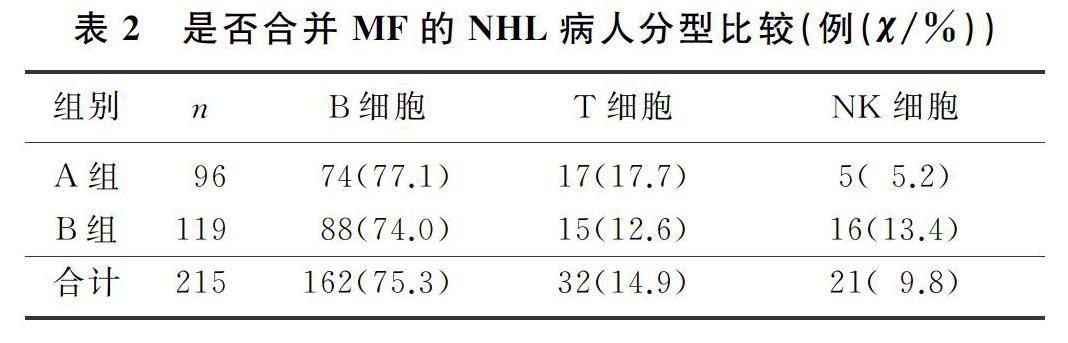
本文215例NHL病人中,男120例,女95例;年齡17~85歲,平均(57±13)歲。NHL分型:B細胞來源162例(75.3%),T細胞來源32例(14.9%),NK細胞來源21例(9.8%)。臨床分期(Ann Arbor分期):Ⅰ期34例(15.8%),Ⅱ期41例(19.1%),Ⅲ期38例(17.7%),Ⅳ期102例(47.4%)。合并MF的NHL病人有96例,其中27例(28.1%)脾大;未合并MF的NHL病人有119例,其中11例(9.2%)脾大,兩組脾大例數比較差異有統計學意義(χ2=13.019,P<0.05)。合并MF的NHL病人中,81例病人骨髓增生活躍或明顯活躍,15例病人增生欠活躍或增生低下,表現為粒系增生正常,以中、晚期粒細胞為主;紅系增生正常,以中、晚幼紅細胞為主;分裂象可見,成熟紅細胞大小不一。合并MF的NHL病人骨髓活檢可見纖維組織不同程度增生,MF-1級78例(81.2%),MF-2級為16例(16.7%),MF-3級2例(2.1%)。
2.2 是否合并MF的NHL病人分期與分型比較
各分期NHL病人均可合并MF,Ⅰ期和Ⅱ期NHL病人75例,其中26例(34.7%)合并MF;Ⅲ期和Ⅳ期NHL病人140例,其中70例(50.0%)合并MF,Ⅲ期和Ⅳ期較Ⅰ期和Ⅱ期更易合并MF,二者比較差異有顯著性(Z=-2.548,P<0.05)。215例NHL病人中,彌漫大B細胞淋巴瘤112例,其中合并MF者49例(43.7%);濾泡性淋巴瘤16例,其中合并MF者9例(56.3%);結外NK/T細胞淋巴瘤21例,其中合并MF者5例(23.8%),3種病理類型病人合并MF差異無統計學意義(χ2=4.314,P>0.05)。各種病理類型NHL均可合并MF,B細胞、T細胞、NK細胞來源NHL合并MF差異無顯著性(P>0.05)。見表1、2。
2.3 是否合并MF的NHL病人治療緩解率及骨髓抑制程度比較
本文215例NHL病人治療6周期后,合并MF的NHL病人達到完全緩解33例(34.4%),未合并MF的NHL病人達到完全緩解37例(31.1%),兩組比較差異無統計學意義(χ2=0.261,P>0.05)。合并MF的彌漫大B細胞淋巴瘤病人達到完全緩解23例(46.9%),未合并MF的彌漫大B細胞淋巴瘤病人達到完全緩解22例(34.9%),兩組比較差異無統計學意義(χ2=1.656,P>0.05);合并MF的B細胞來源淋巴瘤病人達到完全緩解33例(44.6%),未合并MF的B細胞來源淋巴瘤病人達到完全緩解34例(38.6%),兩組比較差異也無統計學意義(χ2=0.588,P>0.05)。
本文96例合并MF的NHL病人中,出現0~2度骨髓抑制23例(24.0%),3~4度骨髓抑制73例(76.0%);119例未合并MF的NHL病人中,出現0~2度骨髓抑制48例(40.3%),3~4度骨髓抑制71例(59.7%),合并MF的NHL病人更易發生較嚴重骨髓抑制,二者比較差異均具有顯著意義(Z=-3.413,P<0.05)。見表3。
[參考文獻]
[1] THIELE J, KVASNICKA H M, FACCHETTI F, et al. European consensus on grading bone marrow fibrosis and assessment of cellularity[J]. Haematologica, 2005,90(8):1128-1132.
[2] OTT G, KLAPPER W, FELLER A C, et al. Revised version of the 4th edition of the WHO classification of malignant lymphomas: what is new[J]? Pathologe, 2019,40(2):157-168.
[3] 中華人民共和國衛生和計劃生育委員會醫政醫管局,中華醫學會腫瘤學分會. 中國結直腸癌診療規范(2017年版)(摘編)[J]. 腫瘤綜合治療電子雜志, 2018,4(2):29-37.
[4] 肖志堅. 骨髓增生異常綜合征和骨髓增殖性腫瘤的診斷分型應重視的一些問題[J]. 中華血液學雜志, 2010,31(4):217-218.
[5] 董家薔,張劍,聶澤強,等. 80例繼發性骨髓纖維化患者臨床特點和骨髓活檢分析[J]. 哈爾濱醫科大學學報, 2009,43(6):603-608.
[6] 黃艷,孫嘉峰,楊佳,等. 繼發性與原發性骨髓纖維化骨髓組織形態學觀察及臨床意義[J]. 山西醫科大學學報, 2012,43(6):453-481.
[7] BHATT V R, BOCIEK R G, YUAN J, et al. Leukemic diffuse large B-cell lymphoma in a patient with myeloproliferative disorder[J]. J Natl Compr Cancer Netw: JNCCN, 2015,13(3):281-287.
[8] SHOUSE G, NIKOLAENKO L. Targeting the JAK/STAT pathway in T cell lymphoproliferative disorders[J]. Current Hematologic Malignancy Reports, 2019,14(6):570-576.
[9] WALDMANN T A, CHEN J. Disorders of the JAK/STAT pathway in T cell lymphoma pathogenesis: implications for immunotherapy[J]. Annu Rev Immunol, 2017,35:533-550.
[10] RUMI E, BARAT C, BENEVOLO G, et al. Myeloproli-ferative and lymphoproliferative disorders: state of the art[J]. Hematological Oncology, 2020,38(2):121-128.
[11] TEFFERI A. Myelofibrosis with myeloid metaplasia[J]. The New England Journal of Medicine, 2000,342(17):1255-1265.
[12] CHOI J, CHO H, KANG S, et al. Intravascular large B-cell lymphoma associated with myelofibrosis:a case report[J]. Molecular and Clinical Oncology, 2017.doi:10.3892/mco. 2017.1398.
[13] FU R, YU H, WU Y H, et al. Hodgkins lymphoma associa-ted with myelofibrosis: a case report[J]. Oncol Lett, 2015,10(3):1551-1554.
[14] OKABE S, MIYAZAWA K, IGUCHI T, et al. Peripheral T-cell lymphoma together with myelofibrosis with elevated plasma transforming growth factor-beta1[J]. Leuk Lymphoma, 2005,46(4):599-602.
[15] MATSUNAGA T, TAKEMOTO N, MIYAJIMA N, et al. Splenic marginal zone lymphoma presenting as myelofibrosis associated with bone marrow involvement of lymphoma cells which secrete a large amount of TGF-β[J]. Annals of Hematology, 2004,83(5):322-325.
[16] 張怡安,劉澎. 骨髓增殖性腫瘤與淋巴系統腫瘤[J]. 中國實用內科雜志, 2019,39(2):135-138.
[17] CERVANTES F, MARTINEZ-TRILLOS A. Myelofibrosis:an update on current pharmacotherapy and future directions[J]. Expert Opinion on Pharmacotherapy, 2013,14(7):873-884.
[18] MATSUI K, ADACHI M, TOMINAGA T, et al. Angioimmunoblastic T cell lymphoma associated with reversible mye-lofibrosis[J]. Intern Med Tokyo Jpn, 2008,47(21):1921-1924.
[19] 許雯,李曉霞. 以骨髓受累為首發表現的非霍奇金淋巴瘤的臨床特點[J]. 哈爾濱醫科大學學報, 2018,52(1):45-48.
[20] 張文娟,叢琳,肖靜,等. 以脾大為首發表現的惡性淋巴瘤24例臨床分析[J]. 中外醫學研究, 2014,12(12):15-16.
[21] 王文佳,董麗華,尹青松,等. 淋巴瘤合并骨髓纖維化五例臨床分析并文獻復習[J]. 中華血液學雜志, 2016,37(2):157-159.
[22] MASAROVA L, NEWBERRY K J, PIERCE S A, et al. Association of lymphoid malignancies and Philadelphia-chromosome negative myeloproliferative neoplasms: clinical characteristics, therapy and outcome[J]. Leuk Res, 2015,39(8):822-827.
[23] 劉亞琳,王雯娟,王曉寧. 非霍奇金淋巴瘤繼發骨髓纖維化的骨髓病理學特征及其與疾病預后的關系[J]. 中國實驗血液學雜志, 2015,23(3):674-678.
[24] 劉靜,薛梅,閻洪敏,等. B淋巴母細胞淋巴瘤合并骨髓纖維化一例并文獻復習[J]. 白血病·淋巴瘤, 2011,20(11):687-689.
[25] 祁明芳,郁知非. 繼發于非霍奇金淋巴瘤的可逆性骨髓纖維化一例[J]. 臨床血液學雜志, 1999,12(1):48.
[26] LARSON R A, HOCHHAUS A, HUGHES T P, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3 year follow-up [J]. Leukemia, 2012,26(10):2107-2203.
(本文編輯 黃建鄉)

