基于民族融合的沈陽故宮文化衍生品創新設計
宋佳鑫,羅坤明,吳國榮,陳旭輝
基于民族融合的沈陽故宮文化衍生品創新設計
宋佳鑫1,羅坤明2,3,吳國榮1,陳旭輝4
(1.南昌大學 建筑與設計學院,南昌 330000;2.江西旅游商貿職業學院 藝術傳媒與計算機學院,南昌 330000;3.澳門城市大學 人文社會科學學院,澳門 999078; 4. 陜西科技大學 設計與藝術學院,西安 710021)
沈陽故宮作為世界文化遺產,擁有深厚的非物質文化資源,其建筑形制、空間布局、色彩裝飾上表現出滿、漢、蒙、藏等多民族特征。針對沈陽故宮的多民族融合特征進行文化衍生品創新設計,發揚和傳承沈陽故宮的優秀民族文化。首先將各民族特征元素進行層級化處理,并提取元素符號。使用層次分析法(AHP)構建評價矩陣,進行權重計算。確定最能引起消費者共鳴的民族文化元素符號,將其抽象變形融入到產品設計中,最終形成體現沈陽故宮民族文化特征的設計方案。通過提取元素符號與層次分析法相結合,排除主觀因素的影響,打造具有民族特色的沈陽故宮文化衍生品,對傳承優秀民族文化具有重要的啟示意義,也為相關類型文化衍生品創新設計提供借鑒意義。
沈陽故宮、文創設計、民族融合、AHP 層次分析法
作為連接歷史文化與藝術價值的載體,文化衍生品不僅能彰顯民族和地域歷史文化特色,而且具有增強中華傳統文化的傳播力和民族文化認同感的戰略意義[1]。沈陽故宮博物院文創產品應是在深刻理解沈陽故宮的歷史價值、藝術價值的基礎上,對其文化元素加以提煉和升華而形成。其根植于中國優秀的傳統文化,較之一般消費品具有更濃厚的人文情結[2]。通過文獻調研和實景勘察,并結合科學分析的方法對沈陽故宮的建筑形制、空間布局、色彩裝飾、多民族文化內涵進行深度解讀,為沈陽故宮博物院文化衍生產品提供設計思路,使之具有一定文化影響力,助力文化遺產保護和傳播事業。
1 沈陽故宮的歷史淵源及文化內涵
沈陽故宮分為東路、中路和西路3部分的建筑布局。后金天命十年努爾哈赤將都城遷至沈陽,并在沈陽城的中心修建了大政殿和十王亭,這便是沈陽故宮的東路建筑。其中大政殿大致位于中軸線上,十王亭呈八字排列于大政殿的兩側,這種極具滿族特色的建筑布局源于后金開國初期的八旗制度。
沈陽故宮中路建筑與中原王朝宮殿的建筑風格有較大相似之處,采用中軸對稱式布局,各個建筑由南向北依次遞進,使宮殿建筑群有機連成一體。凸顯出“君命天授、皇權至上”的帝王思想[3]。隨著漢族、蒙古族及其他少數民族人民的歸順,各民族文化交流日趨頻繁,中路建筑呈現出多民族融合的視覺效果。乾隆十一年,乾隆皇帝在盛京皇宮中路兩側增建了東西所行宮,是當今所見的沈陽故宮中路建筑群的前身。東西所行宮在建筑布局、色彩裝飾上已經呈現出明顯的中原建筑形制,與皇太極時期的中路建筑風格迥異,體現出濃郁的多民族融合特色。乾隆中后期,乾隆皇帝在盛京皇宮西路增建了一批具有江南文化特色的宮殿建筑。沈陽故宮西路建筑與清新雅致的中路建筑和豪放大氣的清早期建筑風格迥異,為這座充滿濃郁民族特色的宮殿增添了中原氣息。
沈陽故宮所蘊含的深厚的民族文化內涵能夠帶給人們豐富的視覺體驗和深刻的精神思考置身其中有穿梭于歷史長河之感,這種奇妙的錯位感源于沈陽故宮獨特的建筑形制和多民族融合的特征。因此讓消費者通過文化衍生品感受到文化價值,是沈陽故宮文創產品設計所強調的重點。提取沈陽故宮的文化符號并融于產品設計中,做到文化融入產品,產品傳承文化,這樣可以使產品的設計內涵得到升華,滿足消費者的精神需求。可以有效推動中華優秀傳統文化的創造性轉化和創新性發展,真正做到“古為今用”,彰顯其民族特色。
2 沈陽故宮文創產品需求分析
調研現有文化衍生品,分析其優缺點從而提煉消費者需求,并根據提煉出來的需求編制Kano問卷。選擇合適的人群進行問卷調查并按照題目特征進行數據統計,將整理好的數據按需求分類對照表進行歸類,并確定其需求歸屬項,為沈陽故宮文化衍生品創新設計提供指導參考。
2.1 同類產品分析
博物館文創產品的發展歷經3個階段——旅游紀念品時代、文化創意時代及文創融合時代[4]。21世紀初,臺北故宮博物院作為領頭羊,帶領博物館走進文化創意時代。隨著產品創意附加值的重要性日益凸顯,文創產品開發在市場定位、產品設計等常規環節之外更增加了創意賦能等關鍵環節[5]。文化衍生品的多元化和年輕化為文化產業注入了新活力,但是仍然相對膚淺和保守。總體上看,由于受到博物館等級、所處地域、藏品質量和營銷宣傳方式等因素的影響,各地區博物館的文化衍生品發展水平差距明顯[6]。通過分析市面上各個博物館的文化衍生品(如表1所示),尋找博物館文創設計的市場痛點和優勢劣勢,為接下來的沈陽故宮文化衍生品創新設計提供方向。
表1 同類產品調研

Tab.1 Research on similar products

續表1
通過情感化設計理論將“本能、行為、反思”3個層面視為3種情感要素,運用至博物館文化衍生品設計中,從情感要素構成對博物館文化衍生品用戶的影響出發,可歸納為“外觀、行為、心理”3方面[7]。通過對同類產品分析,發現市面流行的大多數文化衍生品設計語言停留在本能層和行為層兩方面,沒有挖掘產品背后的文化內涵。針對此現象,對文化衍生品中的民族文化特征進行重點設計尤為重要,以期在反思層面上滿足消費者的情感需求。
2.2 消費者需求分析
20世紀70年代,Noriaki Kano學者首次提出著名的Kano模型。該模型認為產品質量要素與用戶滿意度呈現某種非線性關系,根據產品質量要素對提升用戶滿意度的影響程度將其分成:魅力要素(A)、期望要素(O)、必備要素(M)、無差異要素(I)及反向要素(R)[8],其中縱坐標代表用戶滿意度,橫坐標代表功能的具備程度,即用戶需求滿足率,見圖1。以問卷的形式調研消費者對文創產品的期望,并利用Kano模型對調研結果進行研究,在進行調查問卷的設計時,需要對同一個需求進行正反面兩個角度的提問。
2.2.1 問卷設計
為了解目標用戶對沈陽故宮文化衍生品的需求,根據前期調研情況,提煉出8種需求,分別是:實用功能、造型特征、色彩協調、用戶體驗、紀念意義、文化內涵、民族特色和情感共鳴,以編制Kano問卷。組織南昌大學64名教師和學生成立評價小組,采取線下采訪和線上調查的方式讓消費者根據自己真實感受進行相應的評價。每個問題設置5個量級,分別是:喜歡、理應如此、無所謂、可以忍受、不喜歡,最后再根據Kano模型需求分類對照表對消費者的需求屬性進行歸類,見表2。以“紀念意義”這一需求屬性為例,可以設置正向問題:你購買的沈陽故宮文化衍生品可以作為紀念品。并設置對應的5個量級。同樣道理,反向問題可以設置為:你購買的沈陽故宮文化衍生品不具有紀念意義。根據調研結果對比需求分類對照表得出歸屬項。

圖1 Kano模型
表2 需求分類對照

Tab.2 Requirements classification comparison
2.2.2 數據收集
依據Kano需求分類對照表(如表2所示),從調查對象中篩選出共計50份有效樣,對問卷中的有效數據進行對照統計,即針對各需求項在全部問卷中所屬的不同Kano類別進行數量統計,并將統計數據最大的需求屬性劃分成該需求項所屬的Kano質量類別[9]。由此得出沈陽故宮文化衍生品需求屬性分類表,見表3。
表3 需求屬性分類

Tab.3 Requirement attribute classification
通過上述分析結果我們發現,其中紀念意義、文化內涵、情感共鳴為產品的期望型需求,用戶體驗和民族特色為魅力型需求,而實用功能、造型特征、色彩協調則屬于無差異需求。因此在進行文化衍生品設計時,應重點考慮其紀念意義和文化內涵,與消費者產生深層次的情感共鳴。
3 沈陽故宮民族文化元素提取
沈陽故宮在建筑形制、空間布局、色彩裝飾上表現出濃郁的多民族特征,體現了各民族文化從相互影響融合到逐漸同化的過程。通過對沈陽故宮民族文化的研究,對滿、漢、蒙、藏多家建筑裝飾藝術元素的提取,挖掘蘊藏在其中的歷史淵源和文化內涵,使民族文化元素以更具象的形式深入到人們的日常生活中,打造城市文化新形象。
3.1 建筑造型元素提取
3.1.1 充滿藏傳佛教特征的東路建筑
大政殿是東路建筑的焦點,也是整個沈陽故宮的代表性建筑。大政殿同時融合了滿、漢、蒙、藏多家建筑裝飾技法,是多民族融合風格的獨特典范。大政殿為八面體造型,屋頂采用八角重檐攢尖頂,這種屋頂形式源于滿族游牧民族的帳幄造型,是具有代表性的民族文化元素。殿頂的每條彩脊上都屈身立著一個蒙古力士,手握鎖鏈面向殿頂寶瓶火焰珠,八條鎖鏈的盡頭是由寶珠、相輪、寶瓶組成的寶頂。這種琉璃寶頂是極具佛教特色的民族文化元素,是將佛教風格融合于宮殿建筑的一大典范。
大政殿的外檐柱頭為獸面裝飾,獸面寬鼻環眼,犄角卷曲,似羊似獅,頗具神秘色彩。這種獸面裝飾不僅在外檐柱頭有所體現,在沈陽故宮的寶頂、石雕上也均有體現,它雖形似藏族獸面但又融入了滿族特征,是具有滿藏兩民族風格的文化元素,因此呈現出莊重神秘的特殊韻味。大政殿外檐兩根檐柱上部的蟠龍裝飾與山西太原晉祠的圣母殿頗有淵源。這種蟠龍柱發展到清初,已經有了很大的變化[10]。與圣母殿的蟠龍柱相比,大政殿的蟠龍更加飽滿威風,龍身變得更加粗壯,龍鱗更加明顯,龍角間距加寬向后飛揚,五爪張開有飛天之勢,極具動感和威懾力。
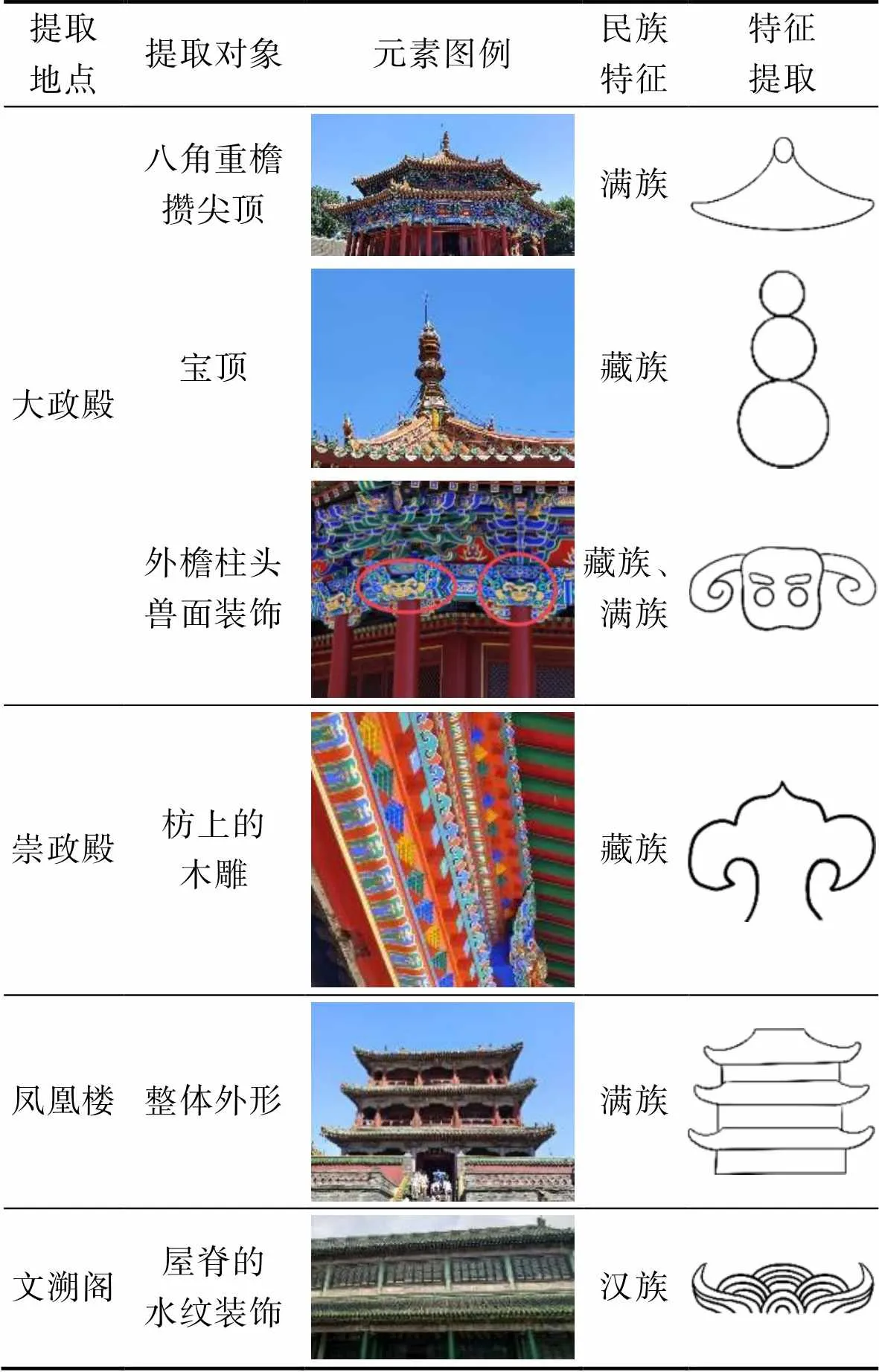
建筑造型方面,東路建筑提取八角重檐攢尖頂、寶頂、外檐柱頭獸面裝飾作為代表性的文化元素,風格上表現出滿族、藏族的建筑裝飾形式。
3.1.2 多民族特征完美融合的中路建筑
沈陽故宮中路建筑始于皇太極時期,空間布局不同于開放的東路建筑,蘊藏于中路建筑中的中央集權思想尤為強烈。縱觀中路建筑群,借鑒漢族的木結構宮殿建筑樣式,建筑形制上呈現出多民族融合的傾向。崇政殿的枋上并未安裝檐檀,而是在其上飾以雕有如意、蜂窩坊、蓮花等圖案的木雕,每組木雕造型由下而上愈漸突出,呈倒三角形狀,且里外對稱。這種裝飾藝術常見于藏族寺廟,屬于喇嘛教的建筑形式,是頗具藏族特征的民族文化元素。鳳凰樓建于四米高的臺基之上,是滿族殿低宮高建筑形式的具體表現。鳳凰樓以北為高臺五宮,五宮中心的清寧宮是典型的東北民居形式,為前后廊硬山式建筑,極具滿族風情。清寧宮的煙囪同樣采用滿族民居的建造方式,并非建于屋頂,而是置于屋側的地面上。這種建造方式主要是為了防火。因為滿族民居屋頂多為苫房草鋪就,如果煙囪設在房頂上,屋頂的苫房草容易被冒出的火星點燃。中路建筑以崇政殿、鳳凰樓和清寧宮為代表性建筑,分別提取崇政殿枋上的木雕和鳳凰樓整體外形作為中路建筑的代表性民族文化元素。
3.1.3 體現漢族宮殿風貌的西路建筑
乾隆中晚期的西路建筑,在空間布局和建筑形制上嚴格按照官式做法進行營建。與盛京皇宮早期的功能性建筑不同,沈陽故宮西路建筑更具娛樂氣息和文化氛圍。戲臺位于西路建筑的南端,戲臺北側的嘉蔭堂為皇室賞戲之地,兩側為連接南北的走廊,中間形成天井,這屬于北方的圍合院落形式。文溯閣位于西路中央,是皇家藏書之地。其中文溯閣的屋脊為水紋裝飾,造型優美,具有典型的官式特征,表達了以水壓火的美好寓意,提取水紋裝飾作為漢族文化元素,進行抽象變形。
3.1.4 建筑造型元素特征提取
通過分析沈陽故宮東路、中路、西路的建筑造型,提取其具有代表性的民族文化元素,并進行特征提取,見表4。
表4 民族文化元素提取——建筑造型
Tab.4 National cultural elements extraction: architectural modeling
3.2 室內裝飾元素提取
3.2.1 華貴神秘的大政殿穹頂
殿內穹頂正中是圓形的木雕金漆降龍藻井,藻井中心處沿圓周均勻分布八個篆書漢字圖案,分別為“福、壽、祿、喜、萬”,其中祿、萬、喜重復出現。藻井外環由八塊五井天花組成,每塊五井天花上面兩塊繪有雙龍,下面兩塊繪有雙鳳,中間為梵文天花。上下的龍鳳天花形狀均為等腰梯形,而中間的梵文天花則為正方形,可見梵文天花是這組五井天花的重點。梵文天花是藏傳佛教裝飾藝術的典型代表,可以看出清初時期藏傳佛教有著舉足輕重的地位,而龍鳳圖案以及福祿壽喜等文字天花呈現濃厚的漢族文化特色。大政殿穹頂融合了多民族的裝飾形式,使大政殿不僅華麗高貴又增添一抹神秘莊嚴的氣氛,因此提取穹頂圖案作為東路建筑的代表性民族文化元素。
3.2.2 充滿薩滿教氣氛的清寧宮
滿族傳統建筑的高度普遍偏矮且屋頂坡度較緩,清寧宮也不例外,建筑的屋頂形式多以硬山為主,這與中原地區中國傳統建筑森嚴的等級制度有很大區別[11]。清寧宮的宮門不居中設在東側,為典型的“口袋房”布局,目的是適應東北的嚴寒天氣[12]。走進清寧宮,北面設下兩口大鍋,為制作祭品之用,這源于薩滿教的祭祀傳統,東側間為暖閣,西四間是宮廷內舉行祭祀宴會的地點,屋內砌有3面相連的“萬字炕”,其中南北炕是生活起居之用,西炕設神像和放置祭品的桌案。滿族有“以南為大”“以西為尊”的思想,3面大炕之間的空地是薩滿教舉行宗教儀式的場地。清寧宮是典型的滿族民居,因此提取清寧宮內的裝飾為文化元素,可以體現其滿族特征。
3.2.3 室內裝飾元素特征提取
重點分析沈陽故宮大政殿穹頂、清寧宮的室內裝飾,提取其具有代表性的民族文化元素,并進行特征提取,見表5。
表5 民族文化元素提取——室內裝飾

Tab.5 National cultural elements extraction: interior decoration
3.3 院落陳設元素提取
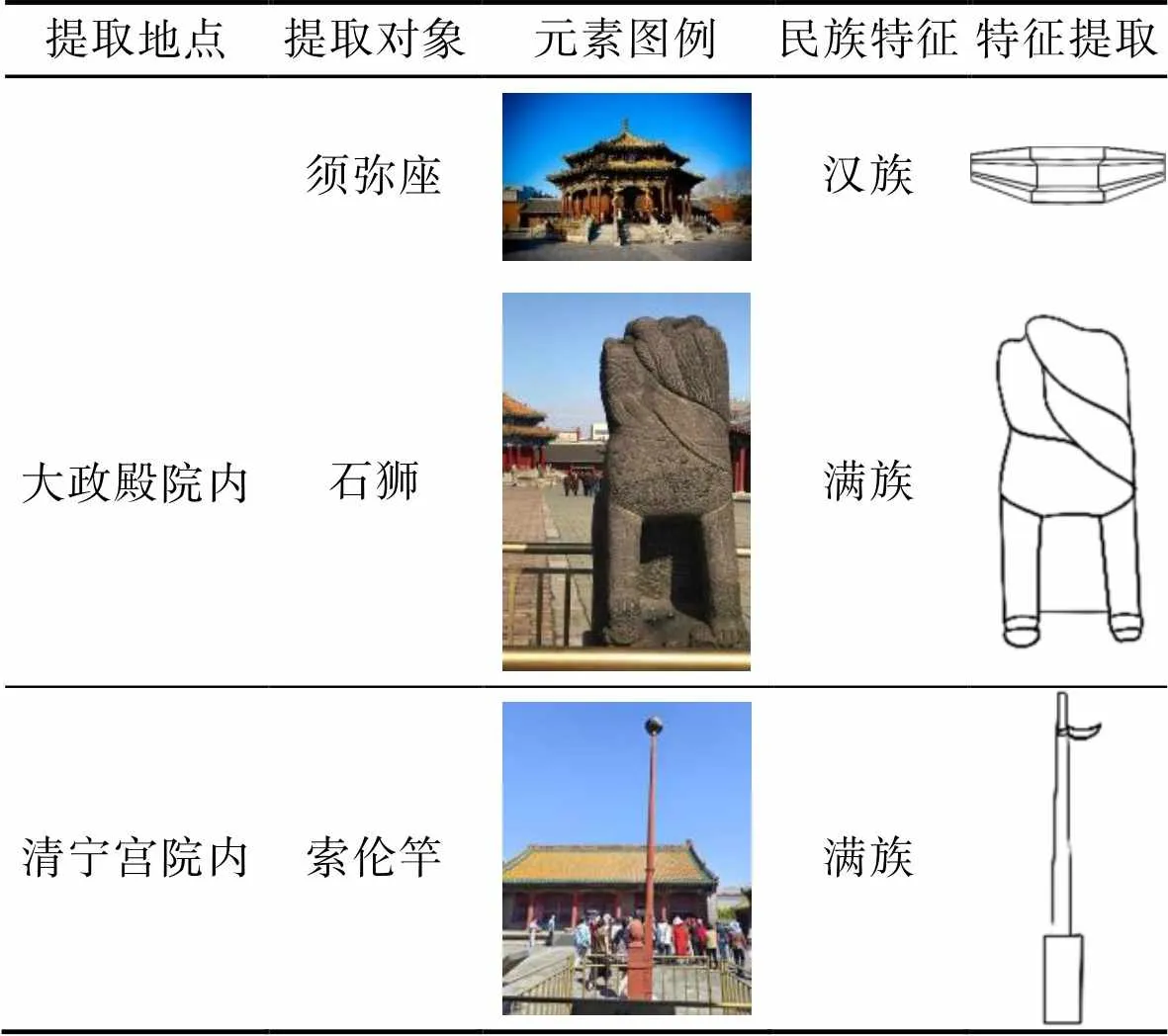
大政殿的石雕藝術有著濃郁的民族特征,其欄板、望柱、須彌座上雕刻著紋飾,紋飾圖案以花卉為主,大政殿前立有1對石獅,獅子整體造型圓潤,莊重大方,和中原地區的石獅子外形相差很大。在清寧宮前的院落內,有1個紅色的木制竿子,竿頂處有1只錫碗,滿族人稱其為索倫竿,這是薩滿教在舉行祭天典禮時使用的,祭祀時將碎米、雜肉放于碗內,供神鵲食用,以答謝天神之意。將須彌座、石獅、索倫竿作為代表性的院落民族文化元素,并進行特征提取,見表6。
3.4 整體色彩元素提取
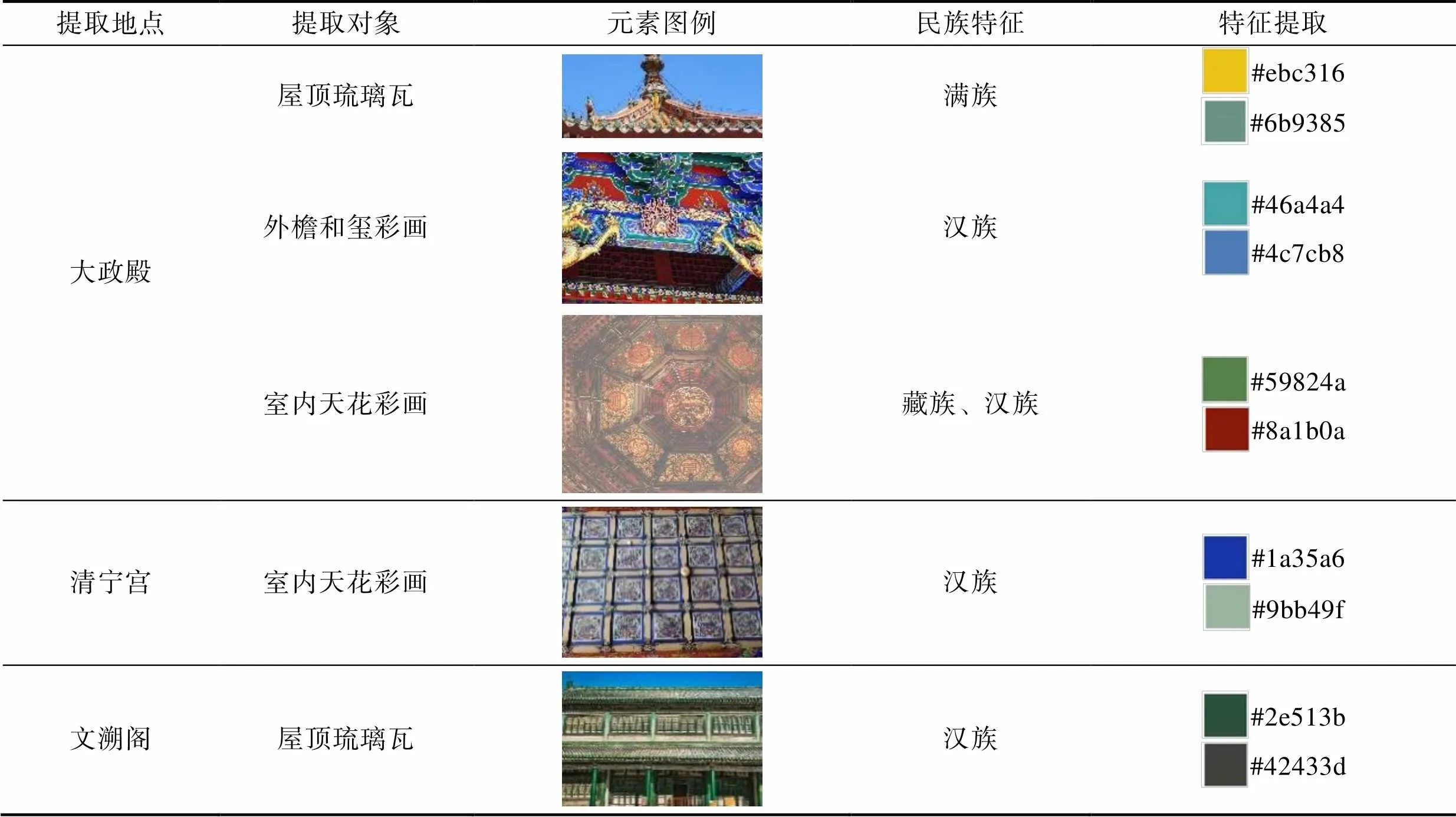
沈陽故宮的整體色彩具有滿、漢、蒙、藏多民族融合的特征,這種民族融合體現在屋頂、彩畫、天花的色彩運用上。沈陽故宮的琉璃瓦屋面中黃琉璃瓦綠剪邊類型居多。黃色琉璃瓦在我國宮殿建筑中通常用于皇家建筑。采用黃琉璃瓦符合皇家宮殿至高無上的地位,綠剪邊則體現了其鮮明的民族特征,反映出草原游牧民族的生活習慣。像戲臺、翔鳳閣等建筑則采用灰色琉璃瓦。西路建筑的文溯閣閣頂琉璃瓦為黑色琉璃瓦鑲綠色剪邊,這種異于常規的色彩主要是有防火之意,黑色代表水,水能克火。
大政殿的斗拱裝飾也獨具一格。在色彩運用方面,斗拱各個部分的邊沿線均采用貼金裝飾,打破死板沉悶,顯得熠熠生輝,盡顯皇家建筑的威嚴高貴。斗與升的顏色青綠相間,斗拱間顏色青綠兩色間隔變化。大政殿外檐為和璽彩畫,主要為青綠、紅、金三色組合。大政殿室內的天花彩畫是清代早期的做法,特征鮮明,描繪出當時多民族文化融合的社會現狀。彩畫的中心位置是藏傳佛教的梵文,體現出藏傳佛教在清初統治中的重要地位。其彩畫色彩的冷暖色調約各占一半,整體色彩豐富,具有濃郁的滿蒙風情[13]。清寧宮內天花彩畫風格為蘇式彩畫,色調淡雅,主要以青綠色為主。分別提取大政殿、清寧宮、文溯閣的色彩元素,見表7。
表6 民族文化元素提取——院落陳設
Tab.6 National cultural elements extraction: courtyard furnishings
表7 民族文化元素提取——整體色彩
Tab.7 National cultural elements extraction: overall color
4 AHP層次分析各功能需求要素權重
4.1 構建層次分析模型
采用層次分析法(AHP)比較故宮建筑中各個元素的影響程度,借此方法為后續文化衍生品設計中各類元素的占比提供數據支撐。首先將各民族特征元素進行層級化處理,將其分類并細化具體物象,提取元素符號。使用層次分析法進行科學的權重計算,并通過評估一致性檢驗。針對分析后的結果,確定最能引起消費者共鳴的民族文化元素符號,將其抽象變形融入到產品設計中,最終形成最符合故宮民族性文化特征的設計方案,見圖2。
為了體現沈陽故宮的民族文化特征,科學地構建沈陽故宮文創設計需求層次模型,考慮建筑形制、空間布局、色彩裝飾、藝術表現等因素,根據上文提取的民族元素特征并結合層次分析法對民族文化素材進行層級整理。將沈陽故宮建筑提取4類特征,分別為:整體色彩元素(1)、院落陳設元素(2)、室內裝飾元素(3)、建筑造型元素(4)。在制定文創設計方案中,把每類特征細分成更多的三級具象特征,作為設計方案素材來源的實物依據,見圖3。

圖2 設計流程

圖3 沈陽故宮民族文化元素層次結構
根據層次分析法對每一層級項進行判斷標定,其中,要素與的重要度比為b,反之b=1/b,標定值范圍為1~6,當兩者重要程度等同時,標定值為1;當其中一項遠比另一項重要時,標定值為6,判斷矩陣標定表見表8。根據判斷標定對設計方案中4個一級準則層級和19個二級素材項目進行兩兩比較得到各層級的權重值,根據層次分析法對數據進行處理。為避免設計師主觀決策結果,保證沈陽故宮文化衍生品的設計效果能完美地表現其民族特征,與消費者產生情感共鳴,選取在校大學生15人,專家教師5人組成評定小組對每個文化元素的民族特征表現進行打分,以期得到最能體現其民族特征的文化元素。依據評定小組打分結果,對層次分析模型中同一層級的素材元素中進行兩兩比較,構建判斷矩陣。
表8 1~6級標定法

Tab.8 Scaling method of levels 1-6
4.2 構建判斷矩陣及方案有效性檢驗
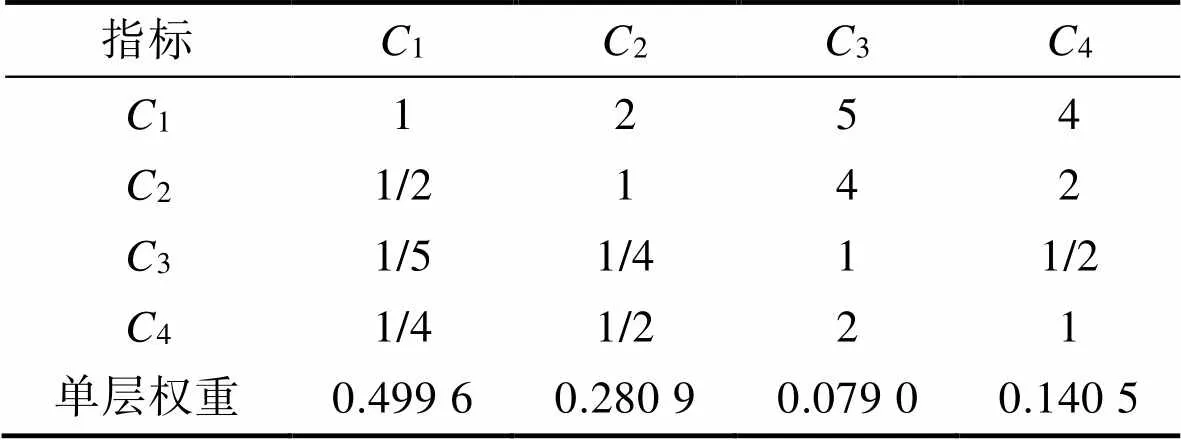
量化各個特征元素的權重是后續設計實踐的基礎,沈陽故宮文化衍生品作為層次分析模型目標任務,依據層次分析法對各層級項指標進行兩兩評價,見表9。
表9 沈陽故宮民族文化元素判斷矩陣
Tab.9 National culture element judgment matrix of Shenyang Imperial Palace
解判斷矩陣的特征向量,采用方根法計算矩陣特征向量的近似值,判斷矩陣最大特征根,見式(1)。

求對判斷矩陣的一致性進行檢驗。判斷矩陣偏離一致性指標()的計算,見式(2)。

越大,判斷矩陣一致性越差,為0時,判斷矩陣具有完全一致性。一致性比率()的計算,見式(3)。

式中:為平均隨機一致性指標,當<0.1時可以認為判斷矩陣的一致性檢驗通過。
根據式(1),計算出故宮文化衍生品設計方案判斷矩陣的最大特征根max=4.010。查詢判斷矩陣的隨機一致性指標值,并對此判斷矩陣按式(2)—(3)進行一致性檢驗,計算得到=0.003,=0.004,均小于0.1,故此判斷矩陣通過一致性檢驗,此方案有效。
要確定此設計方案最優解,需依照沈陽故宮文化衍生品設計素材來源層級架構對每一個二級素材項目進行層次分析法檢驗。特征1、2、3、4的值分別為0.016、0.019、0.009、0.026,均小于0.1,通過一次性檢驗。通過將一級權重值與二級權重值相乘,可計算出沈陽故宮各民族文化元素的綜合權重值,對綜合權重進行從大到小排序,見表10。
表10 總需求矩陣權重

Tab.10 Total demand matrix weight
5 沈陽故宮文化衍生品設計
基于層次分析法的文化元素提取與應用并非簡單地分解與重組,而需綜合考量文化符號與承接載體結合的視覺效果、使用效果以及文化傳達效果等[14]。因此在立足于沈陽故宮民族性文化特征的同時,還要考慮產品的美觀性、實用性、創新性,只有平衡好產品功能與文化創新的關系才能設計出優秀的文創產品。通過上文對沈陽故宮民族特征元素的提取,可以看到民族與宗教是密不可分的,無論是沈陽故宮的建筑形制還是色彩裝飾,都可以看到濃郁的宗教氣息。
通過層次分析法對沈陽故宮文化衍生品設計元素進行權重分析,可得出對于此類文化衍生品來說,針對建筑造型元素進行創新設計更能反映出沈陽故宮的民族性特征,室內裝飾、整體色彩、院落陳設位列其次。對子準則層進行分析計算,可以得出沈陽故宮最具文化識別度的民族特征元素,文化識別度是建立在設計者與消費者之間能夠引起最大共鳴的標準,文化識別度越高的文化因子越能夠激發消費者認知記憶,引起消費者情感共鳴[15]。其中八角重檐攢尖頂元素、大政殿寶頂、藻井上的篆書漢字等是最能體現沈陽故宮民族文化的裝飾元素。將上述文化元素符號進行再設計,設計出一款具有沈陽故宮鮮明特色的文化衍生品。
中國香文化源遠流長,香爐是能夠代表民族造物文化的器具之一。近年來,隨著人們生活節奏的加快,香道再一次走進人們的生活,成為大家體味自然、釋放壓力的方式,它所反映的返璞歸真的審美意境與現代人所追求的精神境界相吻合。因此設計一款以沈陽故宮民族性為主題的香爐,將民族文化融入香爐設計中,不僅產品屬性符合沈陽故宮的民族性基調,其傳遞出的哲學思想還可以與消費者產生深層次的情感共鳴。
香爐整體形狀圓潤,線條流暢。爐蓋的外形輪廓來源于大政殿殿頂形狀,爐身是對清寧宮兩口大鍋形狀的再設計,底座八角形設計靈感源于大政殿須彌座。分別將其造型元素抽象變形,并進行組合,形成香爐的基本形態,見圖4。爐蓋的花紋取自篆書漢字圖案、屋脊的水紋裝飾、枋上的木雕,其中篆書漢字:福、祿、壽、喜、萬取自大政殿藻井,整個爐蓋充滿民族氣息,見圖5。

圖4 香爐基本形態推演

圖5 壺蓋紋樣元素來源
香爐爐體為弟窯粉青,香爐爐身通體青色,色彩來源于大政殿外檐和璽彩畫,青色釉質光澤度高,與銅質爐蓋的蓮花造型相契合,如圖6。爐蓋為銅合金材質,呈金色,與大政殿屋頂金黃琉璃瓦相呼應,鏤空及花邊造型均勻對稱分布在蓋體,生產中可采用沖壓成型工藝。將蓋體拆分為兩部分,分別為蓋體和蓋帽。根據沖壓工藝相關知識[16-18]及沖壓工藝手冊,設計蓋體生產工藝為落料,拉深,沖孔3道主工序。由于蓋體具有較多鏤空特征,一道工序直接成型可能會導致銅板料失穩變形,故拉伸,沖孔工序中應設置不少于兩道子工序,逐個對稱成型蓋體特征。蓋帽芯部采用拉延工藝,制成封閉水滴形狀外觀,包覆芯部的外部“花瓣”“狀葉片”采用一塊板料通過級進模具沖壓生產。待所有零件生產完畢,通過鉚接工藝將其組裝,整個生產過程成型質量好,生產成本低,使得產品具有較大利潤空間。

圖6 香爐設計
6 結語
隨著經濟的高速發展,各民族文化逐漸走向融合。在此背景下,民族文化的傳播顯得尤為重要,設計的民族化已然成為當今文化產業發展的趨勢。本文采用Kano模型進行用戶需求分析,對沈陽故宮文化元素進行特征符號提取,借助層次分析法對各個民族文化元素進行分析,確定最能與消費者產生共鳴的元素符號,將其抽象變形后融入到文化衍生品的設計中。文創衍生品作為文化之間相互交流的載體,是文化產業的重要組成部分,通過沈陽故宮文化衍生品的創新設計,發展沈陽故宮文化產業,增強民族傳統文化的傳播力和影響力。
[1] 董旸, 劉威, 蘆博文. 基于沈陽故宮歷史文化的文創產品設計研究[J]. 包裝工程, 2017, 38(4): 11-16.DONG Yang, LIU Wei, LU Bo-wen. Cultural Creative Product Design Based on Shenyang Imperial Palace History and Culture[J]. Packaging Engineering, 2017, 38(4): 11-16.
[2] 錢倩. 基于市場環境下的博物館文創發展途徑探索[J]. 中國博物館, 2020, 37(1): 3-7.QIAN Qian. Exploration of the Development Approaches for Museum Cultural Creation Based on the Market Environment[J]. Chinese Museum, 2020, 37(1): 3-7.
[3] 欒曄, 李理. 從沈陽故宮宮殿建筑看滿漢文化的交融[J]. 沈陽建筑大學學報(社會科學版), 2010, 12(2): 166-171.LUAN Ye, LI Li. From the Architectural Styles of Shenyang Imperial Palace to See the Han, Manchu Cultural Exchange between the Two Communities[J]. Journal of Shenyang Jianzhu University (Social Science), 2010, 12(2): 166-171.
[4] 吳方. 湖南省博物館文化創意產品開發研究[D]. 長沙: 湖南大學, 2018.WU Fang. Research on Cultural and Creative Product Development of Hunan Museum[D]. Changsha: Hunan University, 2018.
[5] 王璐瑤, 周雨卉, 李永春. 基于層次分析法的博物館文創設計研究[J]. 包裝工程, 2022, 43(18): 320-326.WANG Lu-yao, ZHOU Yu-hui, LI Yong-chun. Museum Cultural and Creative Design Based on Analytic Hierarchy Process[J]. Packaging Engineering, 2022, 43(18): 320-326.
[6] 李紅超, 王昕宇, 李維鈺. 基于文化元素的故宮博物院文創產品設計研究[J]. 包裝工程, 2022, 43(2): 325-332.LI Hong-chao, WANG Xin-yu, LI Wei-yu. Cultural and Creative Product Design of the Palace Museum Based on Cultural Elements[J]. Packaging Engineering, 2022, 43(2): 325-332.
[7] 李帥, 易姍姍, 鄭仁華, 等. 博物館文創產品情感化設計研究[J]. 包裝工程, 2022, 43(16): 372-379.LI Shuai, YI Shan-shan, ZHENG Ren-hua, et al. Emotional Design of Cultural and Creative Products in Museums[J]. Packaging Engineering, 2022, 43(16): 372- 379.
[8] KANO N, SERAKU N, TAKAHASHI F, et al. Attractive Quality and Must-be Quality[J].Journal of the Japanese Society for Quality Control, 1984, 14(2): 147-156.
[9] 喬歆新, 丁婷婷, 應源山. 基于Kano模型的社區高血壓移動醫療服務需求研究[J]. 包裝工程, 2017, 38(22): 32-36.QIAO Xin-xin, DING Ting-ting, YING Yuan-shan. Mobile Medical Service Requirement of Community Hypertension Based on Kano Model[J]. Packaging Engineering, 2017, 38(22): 32-36.
[10] 武斌. 沈陽故宮博物院[M]. 沈陽: 萬卷出版公司, 2006.WU Bin. Shenyang Palace Museum[M]. Shenyang: Rolls Publishing Company, 2006.
[11] 王麗暉, 陳佳俊. 從民間到宮廷, 滿族文化在中國傳統建筑中的演變研究——以沈陽故宮為例[J]. 藝術工作, 2022(1): 109-111.WANG Li-hui, CHEN Jia-jun. A Study of the Evolution of Manchu Culture in Traditional Chinese Architecture-Take the Shenyang Palace Museum for Example [J]. Art Work, 2022(1): 109-111.
[12] 吳國榮, 李泳星. 文化視閾下的滿族傳統民居建筑裝飾研究[J]. 貴州民族研究, 2017, 38(6): 87-90.WU Guo-rong, LI Yong-xing. Study on the Decoration of Manchu Traditional Dwellings in the Cultural Perspective[J]. Guizhou Ethnic Studies, 2017, 38(6): 87-90.
[13] 趙乃欣. 沈陽故宮色彩文化研究與設計應用[D]. 北京: 北京服裝學院, 2020.ZHAO Nai-xin. Shenyang Imperial Palace Color Culture Research and Design Application [D], Beijing: Beijing Institute Of Fashion Technology, 2020.
[14] 李娟, 陳香. 地域文化符號融入博物館文創產品的設計策略[J]. 包裝工程, 2020, 41(8): 160-165.LI Juan, CHEN Xiang. Strategies of Regional Cultural Symbols into the Design of Museum Cultural Creation Products[J]. Packaging Engineering, 2020, 41(8): 160-165.
[15] 劉暢, 雷青. 文化場域視角下蒙古包文化基因圖譜構建與設計轉譯[J]. 包裝工程, 2023, 44(6): 286-301.LIU Chang, LEI Qing. Gene Map Construction and Design Translation of Yurts Culture from the Perspective of Cultural Field[J]. Packaging Engineering, 2023, 44(6): 286-301.
[16] 趙躍文. 端蓋沖壓工藝及模具設計[J]. 模具制造, 2017, 17(11): 21-24.ZHAO Yue-wen. Stamping Process and Die Design for the End Cover[J]. Die & Mould Manufacture, 2017, 17(11): 21-24.
[17] 桂中祥, 朱彬, 張宜生. 適用于熱沖壓成形工藝的零件結構設計原則[J]. 鍛壓技術, 2016, 41(8): 24-28.GUI Zhong-xiang, ZHU Bin, ZHANG Yi-sheng. Principles of Part Geometry Design Applied for Hot Stamping Process[J]. Forging & Stamping Technology, 2016, 41(8): 24-28.
[18] 張宜生, 王子健, 王梁. 高強鋼熱沖壓成形工藝及裝備進展[J]. 塑性工程學報, 2018, 25(5): 11-23.ZHANG Yi-sheng, WANG Zi-jian, WANG Liang. Progress in Hot Stamping Process and Equipment for High Strength Steel Sheet[J]. Journal of Plasticity Engineering, 2018, 25(5): 11-23.
Innovative Design of Cultural Derivatives of Shenyang Imperial Palace Based on Ethnic Fusion
SONG Jia-xin1, LUO Kun-ming2,3, WU Guo-rong1, CHEN Xu-hui4
(1. School of Architecture and Design, Nanchang University, Nanchang 330000, China; 2. College of Art Media and Computer Science, Jiangxi Tourism & Commerce Vocational College, Nanchang 330000, China; 3. School of Humanities and Social Sciences, City University of Macau, Macau 999078, China; 4. College of Art and Design, Shanxi University of Science & Technology, Xi'an 710021,China)
As a world cultural heritage, Shenyang Imperial Palace has profound intangible cultural heritage resources. Its architectural form, spatial layout, color, decoration displayed the Han, Mongolian, Tibetan ethnic features. The work aims to conduct creative design of cultural derivatives based on the ethnic fusion of Shenyang Imperial Palace to carry forward the excellent national culture and heritage of Shenyang Imperial Palace. Firstly, each ethnic characteristic element was processed hierarchically and element symbols were extracted. The Analytic Hierarchy Process (AHP) was used to construct the evaluation matrix and calculate the weight. The symbols of national cultural elements that could most arouse the resonance of consumers were determined, and their abstract deformation was integrated into the product design. Finally, the design scheme reflecting the national cultural characteristics of Shenyang Imperial Palace was formed. Through the combination of element symbol extraction and hierarchical analysis, the influence of subjective factors is excluded, and the cultural derivative of Shenyang Imperial Palace with national characteristics is created, which has important enlightenment significance for the inheritance of excellent national culture. It also provides reference for innovative design of related types of cultural derivatives.
Shenyang Imperial Palace; cultural and creative design; ethnic fusion; AHP
TB482
A
1001-3563(2023)14-0390-11
10.19554/j.cnki.1001-3563.2023.14.044
2023–02–19
2022年度江西省教育廳科學技術研究項目(GJJ2205508)
宋佳鑫(2000—),女,碩士生,主攻工業設計。
吳國榮(1973—),男,碩士,副教授,主要研究方向為產品設計。
責任編輯:藍英僑

