Two Nitronyl Nitroxide Biradical-Bridged Lanthanide One-Dimensional Chains:Crystal Structure,Magnetic Properties and Luminescent Behavior
LI Hong-Dao ZHAI Li-Jun SONG Yong-Bo NIU Yu-Lan( 030008)
Abstract:The rational design of 2p-4f chains,which are made of 4f ions and nitronyl nitroxide biradical,has been presented.The reaction of Ln(hfac)3·2H2O (hfac=hexafluoroacetylacetonate)and nitronyl nitroxide biradical BNPhOEt(BNPhOEt=1,2-(bis-2,2′-(4,4,5,5-tetramethylimidazolyl-1-oxyl-3-oxide)phenoxy)ethane)afforded two isostructural chains of the formula[Ln(hfac)3(BNPhOEt)]·C6H14(Ln=Tb(1),Ho(2)).Direct-current magnetic susceptibility studies show ferromagnetic 4f-radical interaction in Tb complex while antiferromagnetic interaction in Ho derivative.In addition,the luminescence emission spectra of two complexes vary depending on lanthanide ion.CCDC:1906230,1;1906231,2.
Keywords:biradical;lanthanide chains;magnetic properties;luminescent behavior
In recent years,rare-earth complexes,exhibiting slow relaxation of magnetization on molecular level,attract a lot of attention on account of their latent adhibition in molecular spintronics and quantum computing[1-4].Therefore,great efforts in this field have been devoted to search novel 4f-based complexes[5].In 2003,Ishikawa and his colleagues obtained the first SMMs(single-molecule-magnets)based on the Tbion initiating a span-new chapter in molecular magnetism[6].More recently,the remarkable magnetic reversal barrier(Ueff)of 1 541 cm-1as well as magnetic hysteresis around 80K had been achieved with a mononuclear Dymetallocene complex[(η5-Cp*)Dy(η5-CpiPr5)][B(C6F5)4][7].
On the other hand,to design and construct Lnbased complexes with diverse structural topologies and intriguing magnetic properties,the option of suitable organic ligands is vital,among which nitronyl nitroxides are very efficient building blocks.Nitronyl nitroxides are very well suited to bind 4fions and provide strong magnetic coupling with lanthanide metal,on account of the direct overlap of orbitals containing unpaired electrons.Accordingly,a number of 4fmagnetic complexes based on nitronyl nitroxide radicals,including single-chain magnets[8]and single-molecule magnets[9],had been reported.For instance,a family of nitronyl nitroxide-lanthanide one-dimensional(1D)chains[Ln(hfac)3(NITPhOPh)](Ln=Tb,Dy,Ho,Tm;hfac=hexafluoroacetylacetonate)behave as SCMs(singlechain-magnets)[10].Pyridine-substituted nitronyl nitroxide-bridged ring-like Dy-SMMs had been isolated[11].In 2010,one tri-spin Dy-nitronyl nitroxide biradical compound,presenting SMM behavior[12],was synthesized.Previously,we also designed nitronyl nitroxide biradical(NITPhImbis)bridged 1D lanthanide chains[13]and two lanthanide complexes involving BNPhOEt biradical[Ln(hfac)3(BNPhOEt)]·C6H14(Ln=Gd,Dy;BNPhOEt=1,2-(bis-2,2′-(4,4,5,5-tetramethylimidazolyl-1-oxyl-3-oxide)phenoxy)ethane)[14],but the investigation of the coupling between 4fions and radical is limited.Base on this,we continue the above work on nitronyl nitroxide biradical(BNPhOEt)for lucubrating the magnetic coupling between lanthanide metal and nitronyl nitroxide radical and fluorescent properties.Here,we use BNPhOEt ligand to react with Tb/Hoions for constructing two 4f-complexes,namely[Ln(hfac)3(BNPhOEt)]·C6H14(Ln=Tb(1),Ho(2))(Scheme 1).
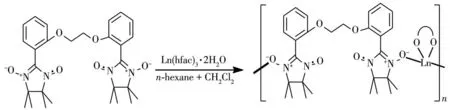
Scheme 1 Schematic representation for synthesis of 1 and 2
1 Experimental
1.1 Materials and instrum ents
All reagents are commercially achieved and used without more purification.Elemental analyses(C,H and N)were performed by Perkin-Elmer 240 elemental analyzer.Magnetic measurements were recorded on a Quantum Design SQUID VSM magnetometer.Measured values were corrected for the sample holder and the diamagnetism deduced from Pascal′s constants.Fluorescent spectra of complexes 1 and 2 were gathered via F-7000 fluorescence spectrophotometer.
1.2 Syntheses of[Ln(h fac)3(BNPhOEt)]·C6H14(Ln=Tb(1),Ho(2))
A solution of Ln(hfac)3·2H2O(Ln=Tb(1),Ho(2))(0.01 mmol)in 16 mL dryn-hexane was refluxed for 1.5 h with constant stirring.After cooling to 53℃,a solution of BNPhOEt(0.005 3 g,0.01 mmol)in CH2Cl2(4 mL)was added in one portion with refluxing for 6 min followed by filtration.The resultant filtrate was left at ambient temperature to evaporate without any disturbance,giving red crystals suitable for X-ray structure analysis over 8 d.For 1:FT-IR(KBr,cm-1):1 796(s),1 358(m),1 181(s),1 159(s),1 074(s),948(s),858(m),547(s) cm-1. Elemental Anal. Calcd for C49H53F18TbN4O12(%):C,42.31;H,3.84;N,4.03.Found(%):C,42.05;H,3.31;N,3.89.For 2:FT-IR(KBr,cm-1):1 795(s),1 357(m),1 179(s),1 160(s),1 073(s),947(s),857(m),546(s)cm-1.Elemental Anal.Calcd.for C49H53F18HoN4O12(%):C,42.13;H,3.82;N,4.01.Found(%):C,42.02;H,3.49;N,4.23.
1.3 Crystal structure determination
Crystal data of complexes 1 and 2 were collected at 113(2)K on a Rigaku Saturn CCD diffractometer with MoKαradiation(λ=0.071 073 nm).SADABS[15]was applied to empirical absorption correction.The structures of two complexes were solved by direct methods and refined by the full-matrix least squares method with a suite of SHELX programs[16].Anisotropic parameters were assigned to non-hydrogen atoms.Meanwhile,H atoms were set in calculated positions and refined isotropically by a riding mode.Several severely disorderedn-hexane molecules in the unit cell of both complexes were treated with SQUEEZE routine[17]during the structural refinement.Data collection and refinement parameters are summarized in Table 1,and selected bond distances and bond angles are given in Table 2 and S1(Supporting information).
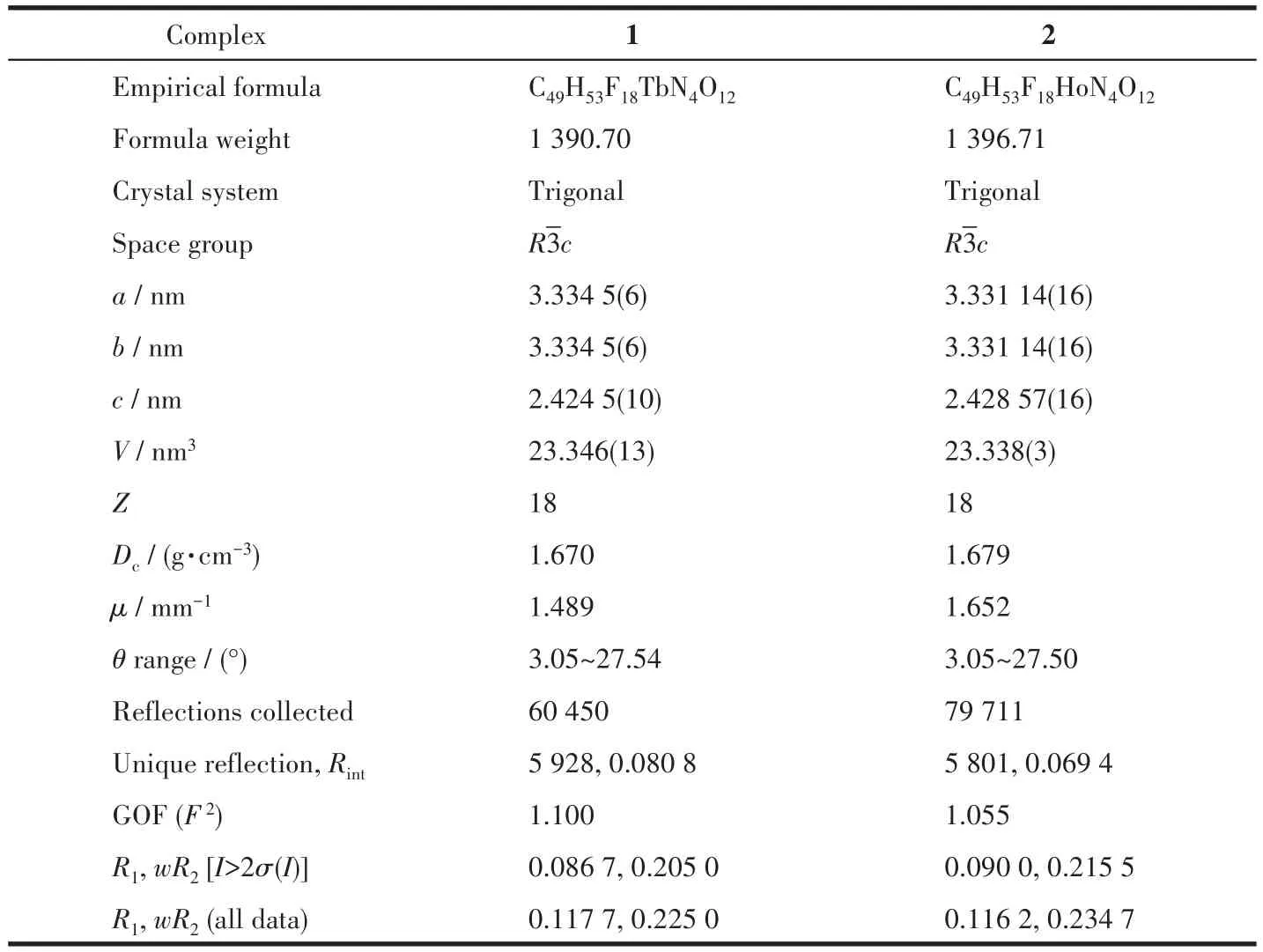
Table 1 Crystallographic data and structure refinement for 1 and 2
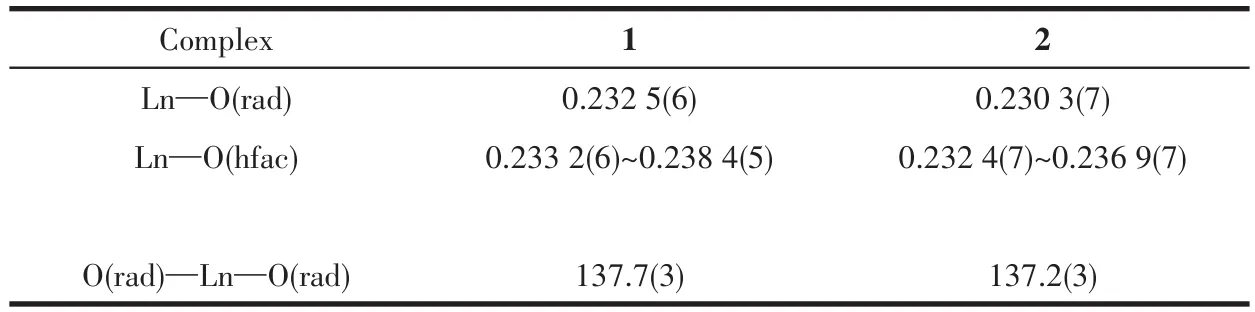
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2
CCDC:1906230,1;1906231,2.
2 Results and discussion
2.1 Description of crystal structures
As revealed by single-crystal X-ray crystallography,complexes 1 and 2 are isostructural and crystallize in the trigonalRcspace group.As shown in Fig.1 and S1,each BNPhOEt biradical behaves as a bidentate ligand to bind two Lnions(Tb/Ho)in theμ2-η1∶η0∶η0∶η1mode via nitroxide groups in the chain.The Lncenter is eight-coordinated,surrounded by six oxygen atoms from three chelated hfac-ligands and remaining oxygen atoms descending from nitroxide groups.To determine the coordination sphere of Lncenters,the continuous shape measure parameters(CShMs)were calculated by SHAPE software[18],indicating the distorted dodecahedron with triangular faces coordination geometry(D2d)for 1 and 2(Fig.2 and S2,Table 3).
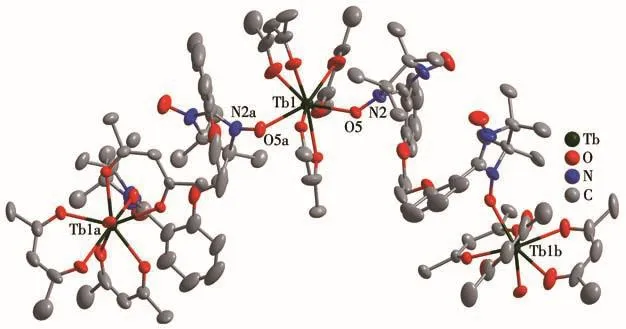
Fig.1 Crystal structure of complex 1

Fig.2 Local coordination geometry of Tbion
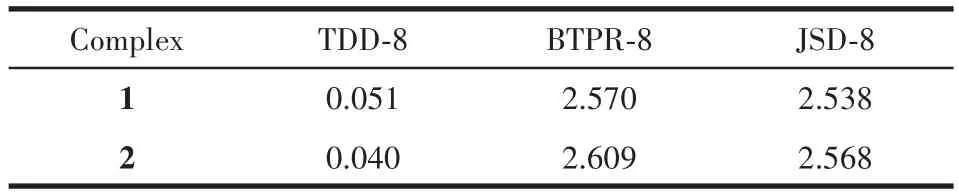
Table 3 SHAPE analysis for 4f metal of complexes 1 and 2
Ln—Ohfacbond lengths(0.233 2(6)~0.238 4(5)nm for 1 and 0.232 3(7)~0.237 0(7)nm for 2)and Ln—Oradicaldistances(0.232 5(6)nm for 1 and 0.230 2(7)nm for 2)are similar to those of reported 4f-radical complexes[19].Packing of these chains is shown in Fig.3 and S3.The intrachain distances between both Lnions are 1.113 nm for 1 and 1.115 nm for 2,while the nearest interchain Tb…Tb and Ho…Ho separations are found to be 1.094 4 and 1.094 3 nm,respectively.The shortest interchain contacts between uncoordinated nitroxide groups are equal to 0.834 1 and 0.825 1 nm for complexes 1 and 2,respectively.
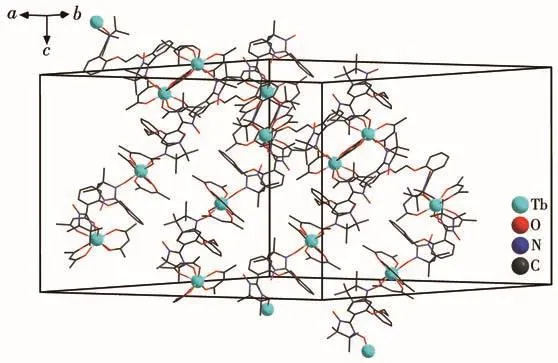
Fig.3 Packing diagram of complex 1
2.2 Magnetic properties
The direct current(dc)magnetic susceptibilities of complexes 1 and 2 were recorded under an external field of 1 000 Oe in a temperature range of 2~300 K.As shown in Fig.4,theχMTproducts at 300 K were 12.60 cm3·K·mol-1for 1 and 14.85 cm3·K·mol-1for 2,which were slightly higher than theoretical values of 12.57 and 14.82 cm3·K·mol-1for one uncoupled Lnion(Tb:7F6,S=3,L=3,g=3/2,C=11.82 cm3·K·mol-1;Ho:5I8,S=2,L=6,g=5/4,C=14.07 cm3·K·mol-1)and one biradical(mono radical:S=1/2,g=2.0,C=0.375 cm3·K·mol-1).For complex 1,on lowering temperature,the value ofχMTstayed relatively unchanged until about 49 K.Then,χMTproduct fell sharply.For complex 2,χMTvalue continuously decreased from room temperature to 5.649 cm3·K·mol-1at 2 K.
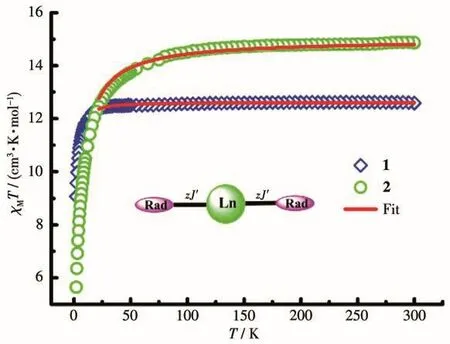
Fig.4 χM T vs T plots of complexes 1 and 2
For both complexes,there are two effective magnetic couplings:(a)the interaction between Tb/Hoion and coordinated nitroxide group;(b)magnetic coupling between both NO groups through Tb/Hoion.The magnetic interaction between two mono-radicals within the biradical is expected to be very weak.To achieve a rough quantitative analysis,based on the large anisotropy of Tband Ho,we suppose that the total magnetic susceptibility(χtotal)is the aggregation of the isolated lanthanide ion and two monoradicals(Eq.1).Tb/Hoion may be assumed to show a splitting of themjenergy levels in an axial crystal field[20].Δrepresents the zero-field-splitting parameter.Thus,Eq.2~4 can be used to describeχTb,χHoandχrad,respectively.The magnetic coupling between 4fion and mono-radical is introduced by the mean-field,zJ′(Eq.5).



The best fitting parametersg=1.51,Δ=0.11 cm-1,zJ′=0.022 cm-1were given for complex 1 in a range of 20~300 K and the determinedzJ′value manifests that there is ferromagnetic interaction between Tbion and the coordinated mono-radical.For 2,g=1.26,Δ=-0.02 cm-1,zJ′=-0.06 cm-1in the same temperature range.The negative value ofzJ′indicates the antiferromagnetic Ho-radical interaction.These values are consistent with values previously observed for Ln-rad complexes[21](Table 4).
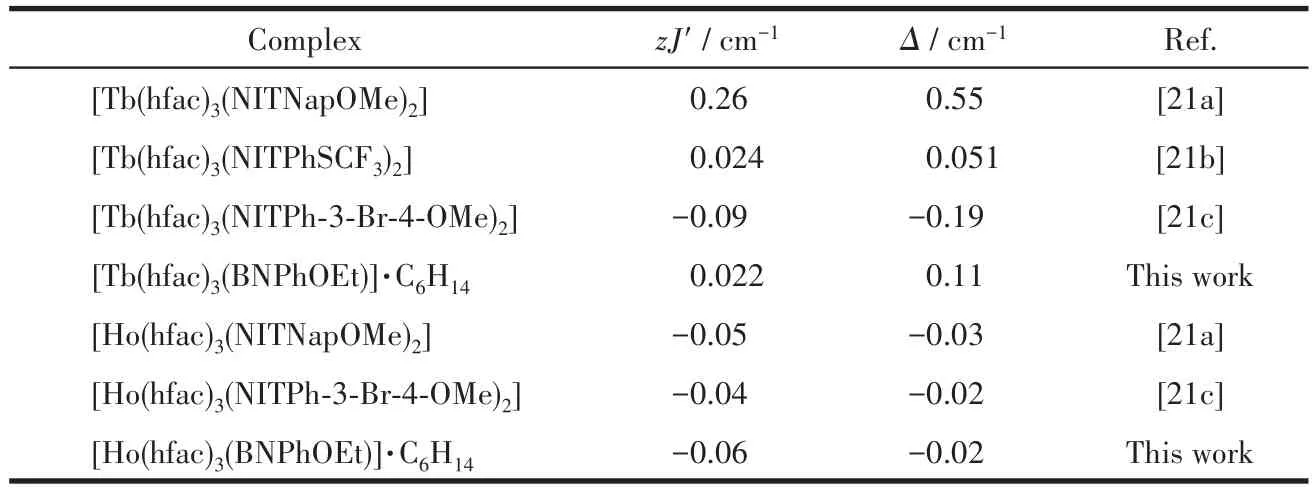
Table 4 Magnetic parameters for Ln-rad complexes with tri-spin units
The magnetization as a function of applied field was determined at 1.8 K in a field range of 0~70 kOe(Fig.5).TheMversusHplots of complexes 1 and 2 displayed thatMvalues increased precipitously at low fields,then the magnetization increased gently and did not reach saturation values at 70 kOe.The behavior of both complexes manifests the presence of low-lying excited states and/or significant magnetic anisotropy.
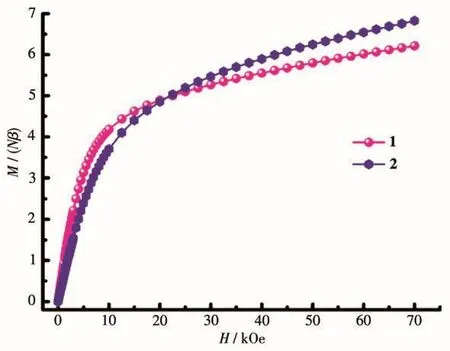
Fig.5 Field dependence of magnetization at 2 K for complexes 1 and 2
To study the dynamic magnetism of complex 1,temperature-dependent alternating-current(ac)susceptibility data were collected,but no out-of-phase signal could be observed under zero direct-current(dc)field(Fig.6).To restrain possible quantum tunneling process(QTM),3 kOe dc field was applied to probe dynamic magnetic behavior.As depicted in Fig.S5,the out-ofphase susceptibility curves showed weak frequency dependent,revealing the presence of slow relaxation magnetization.
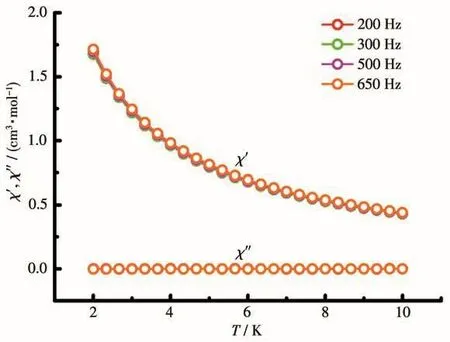
Fig.6 Temperature dependence of in-phase and out-of-phase of ac magnetic susceptibilities for 1 in zero dc field with an oscillation of 3 Oe
2.3 Fluorescent properties
The fluorescent spectra of Tb and Ho complexes in CH2Cl2(10 μmol·L-1)was researched at room temperature.Characteristic fluorescent emissive peaks of Tbion were observed with four narrow emission bands at 491,547,581 and 622 nm,which correspond to the5D4-7F6,5D4-7F5,5D4-7F4and5D4-7F3transitions of Tbion.The stronger emission intensity of the5D4-7F5transition manifests that biradical BNPhOEt is propitious to sensitize green light of Tbion(Fig.7,left).Complex 2 displayed emission spectra at 339,411 and 470 nm,assigned to the characteristic emission of the5I8-5G4+3F7transition of the Ho3+center[22](Fig.7,right).
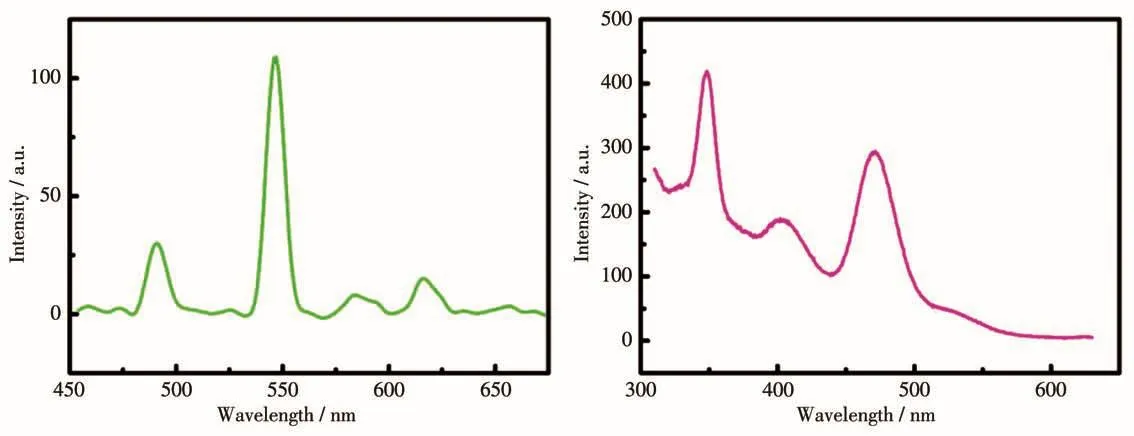
Fig.7 Emission spectra of complexes 1(left)and 2(right)
3 Conclusions
In summary,two one-dimensional biradicalbridged lanthanide complexes[Ln(hfac)3(BNPhOEt)]·C6H14(Ln=Tb(1),Ho(2))have been successfully designed and synthesized,in which 4fions are connected by biradcial ligands through the NO groups of two mono-radicals.The magnetic studies indicate that there are ferromagnetic 4f-radical coupling in 1 and antiferromagnetic interaction in 2.Tb complex displayed slow relaxation magnetization.Moreover,the fluorescent emission spectra of[Ln(hfac)3(BNPhOEt)]·C6H14exhibited typical 4f-centered luminescence.This work not only enables us to understand the optical behavior and magnetic interactions between lanthanide ion and nitronyl nitroxide radical,but also provides valuable insight into the chemistry of 2p-4fcomplexes.
Acknowledgements:This work was supported by Science and Technology Innovation Project of Shanxi Province(Grant No.2020L0650).
Supporting information is available at http://www.wjhxxb.cn
- 無機(jī)化學(xué)學(xué)報的其它文章
- Synthesis and Characterization of Metal-Organic Framework Based on 2,6-Bis(4-carboxybenzylidene)cyclohexanone
- Two Metal-Organic Frameworks Built from 2,2′-Dimethyl-4,4′-biphenyldicarboxylic Acid
- Three Photochromic Co-crystals Based on Viologen Moiety
- Effect of Mass Ratio of Ni and Co in Initial Solution on Oxygen Evolution Reaction Performance of Ni-Co-S-O/NF Catalyst in Alkaline Water Electrolysis
- Structure and Fluorescence Properties of Three 1D/2D/3D Zn/Cocomplexes Based on Flexible Tetracarboxylic Acid
- Synthesis and Visible-Light-Driven Photocatalytic Properties of Floating BiFeO3/Expanding Perlite Photocatalysts

