基于MrgrpX2的雙黃連注射劑中潛在成分虛擬篩選
陳莉 季文君 戴國英 陶淵達 周浩澤 楊袁
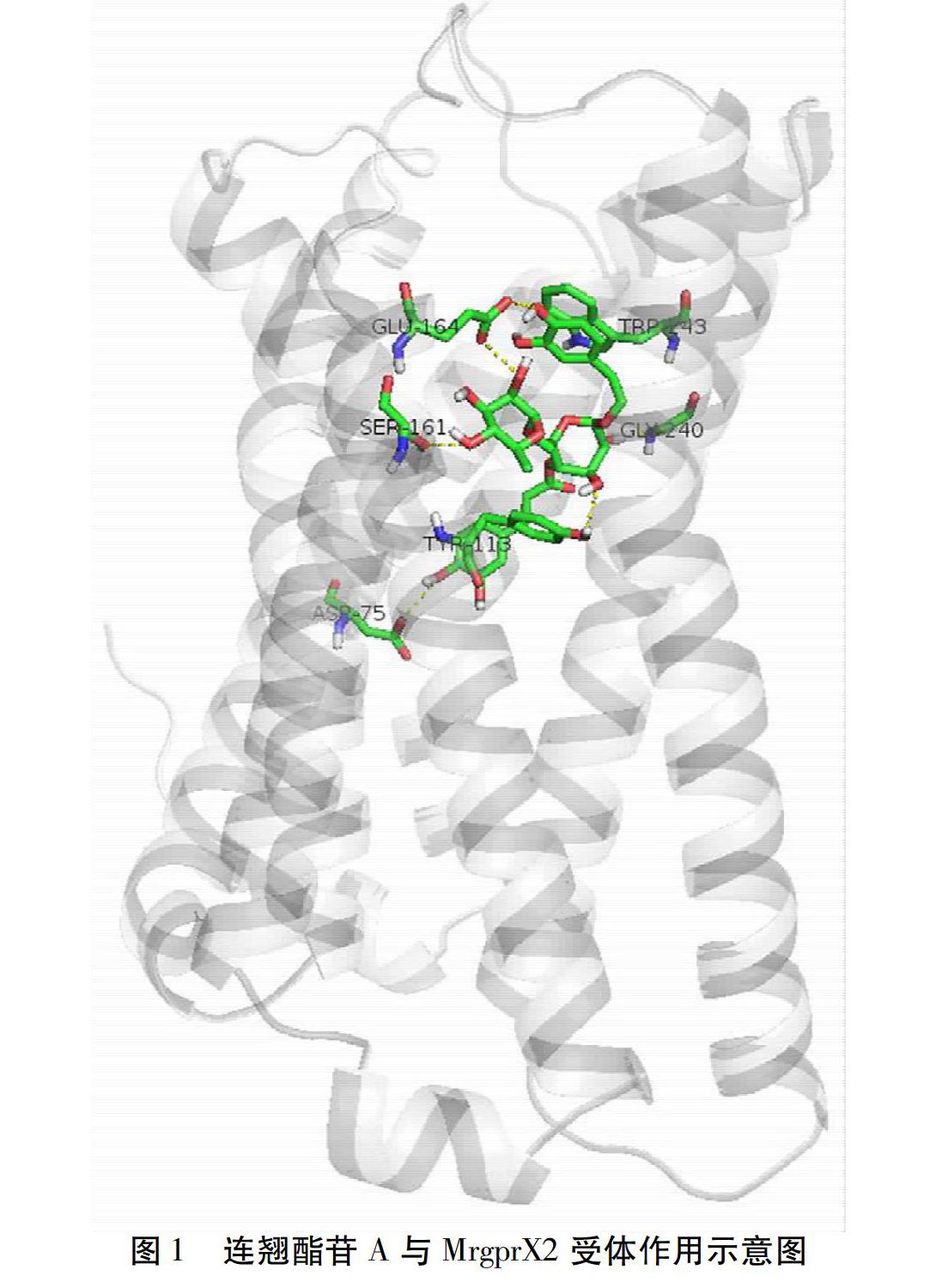
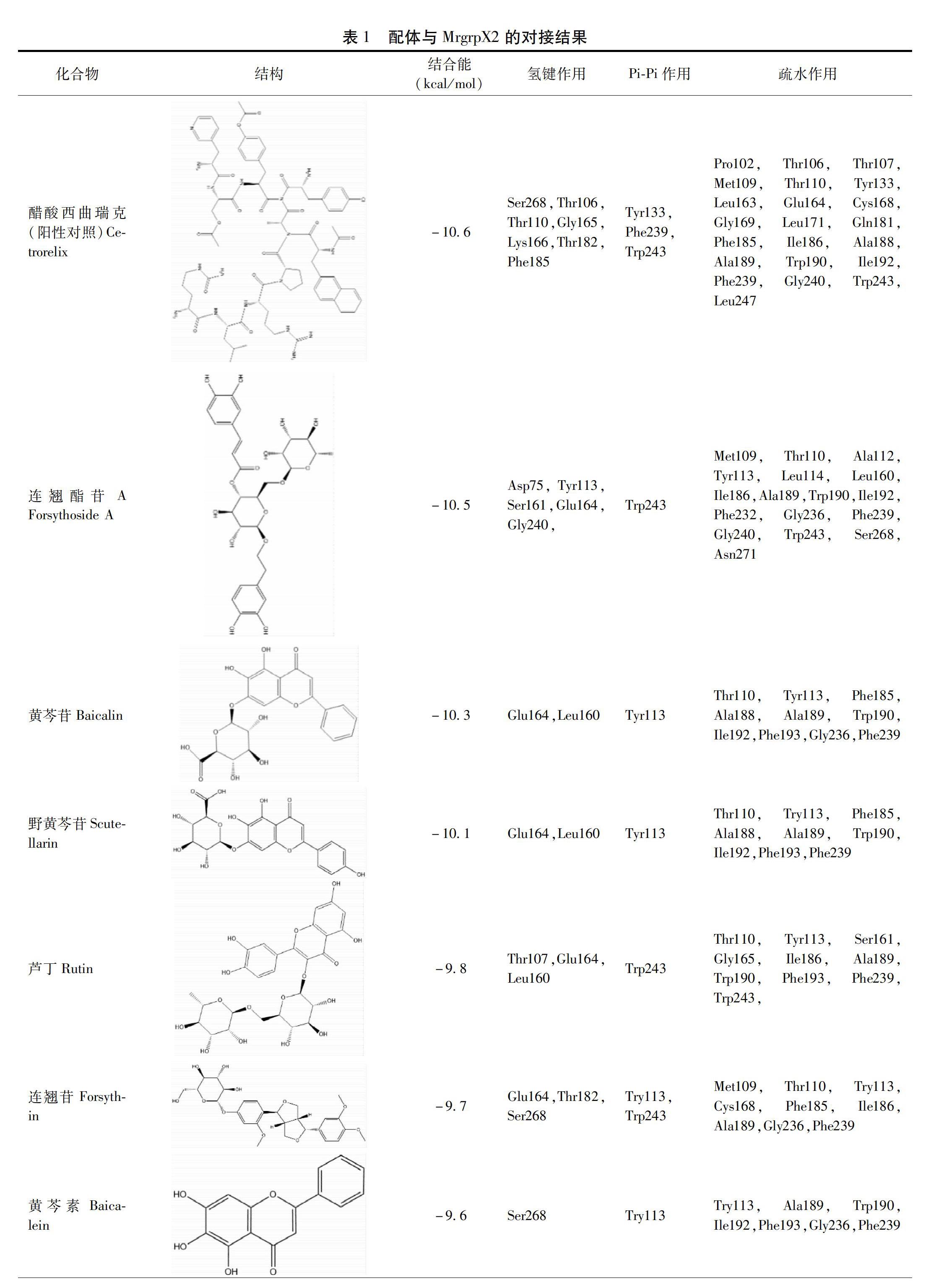
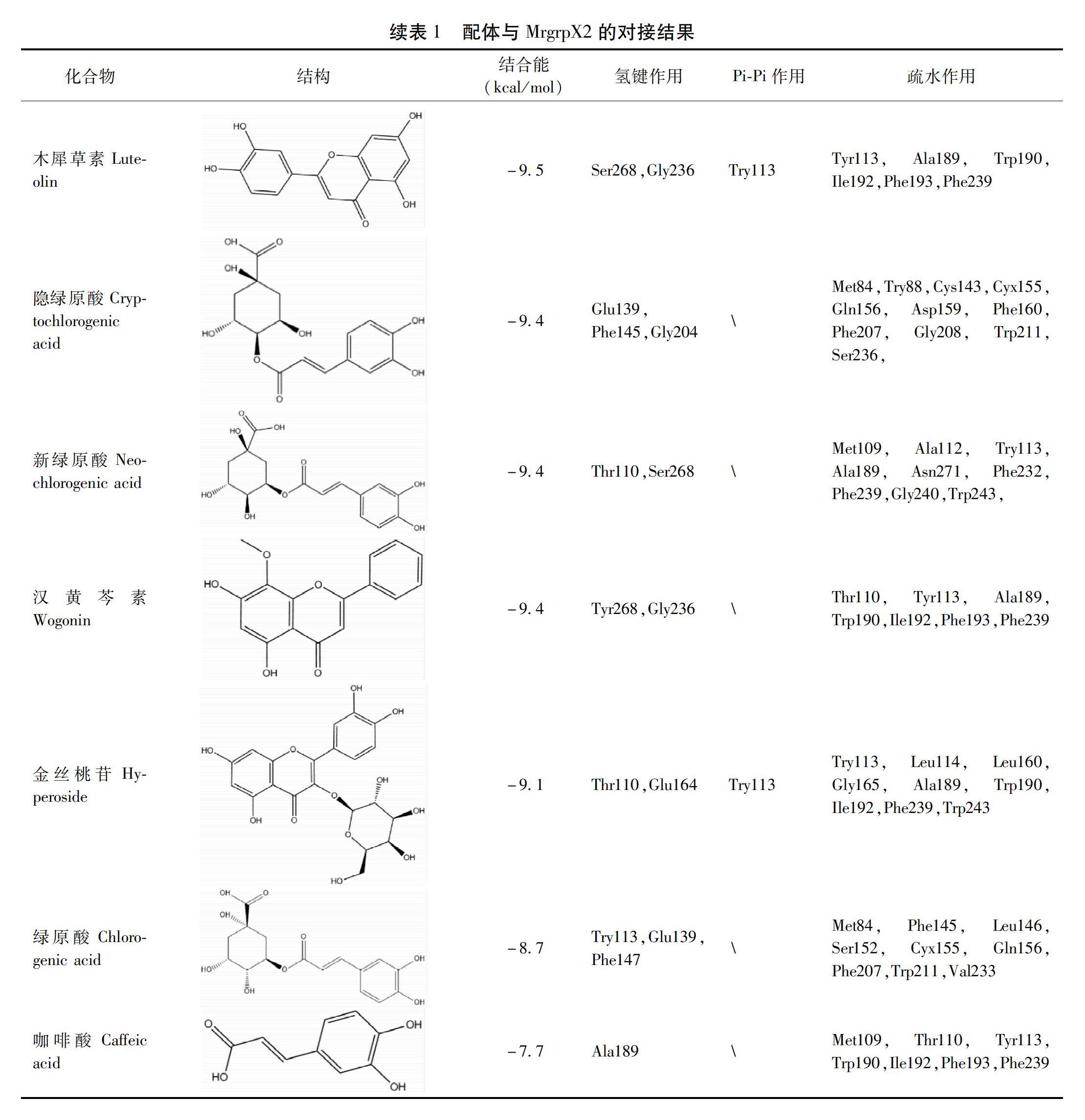
摘要 目的:運用計算機虛擬篩選技術,建立基于MrgrpX2的潛在類過敏成分的篩選模型,對雙黃連注射液中13種成分進行虛擬篩選。方法:采用AutoDock Vina軟件及LigPlot+軟件,以MrgrpX2作為目標蛋白,對各單體成分進行虛擬分子對接篩選,計算受體和配體間的結合能并分析分子間作用類型。結果:分子對接的結果顯示,13種單體成分的結合能依次為連翹酯苷A、黃芩苷、野黃芩苷、蘆丁、連翹苷、黃芩素、木犀草素、隱綠原酸、新綠原酸、漢黃芩素、金絲桃苷、綠原酸和咖啡酸,分子間作用以Pi-Pi作用力、氫鍵作用和疏水作用為主。結論:建立的基于MrgrpX2的篩選模型可用于輔助潛在類過敏成分的初步篩選,篩選出的雙黃連注射劑中的連翹酯苷A在這13種單體成分中與類過敏關鍵受體MrgrpX2具有更高的親和力,提示可能有更高的致類過敏風險,值得深入研究。
關鍵詞 類過敏;MrgrpX2;分子對接;雙黃連注射劑;連翹酯苷A;隱綠原酸;新綠原酸;綠原酸
Virtual Screening Potential Anaphylactogens in Shuanghuanglian Injection Based on Mrgrpx2
CHEN Li,JI Wenjun,DAI Guoying,TAO Yuanda,ZHOU Haoze,YANG Yuan
(Department of Pharmacology,Suzhou Institute for Drug Control of Jiangsu Province,Suzhou 215104,China)
Abstract Objective:Using computer virtual screening technology,to establish a screening model of potential allergic components based on MrgrpX2 and to screen 13 components in Shuanghuanglian injection were virtually.Methods:AutoDock Vina software and LigPlot+software was used,with MrgrpX2 as the target protein.Virtual molecular docking screening of each monomer component was carried out,and the binding energy between the receptor and the ligand was calculated.The type of intermolecular interaction was analyzed.Results:The results of molecular docking showed that the binding energies of 13 monomer components were forsythin A,baicalin,scutellarin,rutin,forsythin,baicalein,luteolin,cryptochlorogenic acid,and neochlorogen.Acid,wogonin,hyperoside,chlorogenic acid and caffeic acid,the intermolecular interactions were mainly Pi-Pi interaction,hydrogen bond interaction and hydrophobic interaction.Conclusion:The established screening model based on MrgrpX2 can be used to assist the preliminary screening of potential allergy-like components.The selected forsythoside A in Shuanghuanglian Injection has higher levels of forsythin A in these 13 monomer components than the key allergic receptor MrgrpX2.Affinity indicates that there may be a higher risk of allergy-causing,and it is worthy of in-depth study.
Keywords Anaphylactoid reaction; MrgrpX2; Molecular docking; Shuanghuanglian Injection; Forsythoside A; Cryptochlorogenic acid; Neochlorogenic acid; Chlorogenic acid
中圖分類號:R242;R283文獻標識碼:Adoi:10.3969/j.issn.1673-7202.2021.03.027
中藥注射劑的急性過敏反應是其最常見的不良反應之一,是束縛中藥注射劑進步的瓶頸。雙黃連注射劑是由黃芩、金銀花和連翹三味草藥制備而成,具有清熱解毒和抗菌消炎等作用,是臨床常用藥物[1-2]。然而隨著其臨床應用日益廣泛,ADR報道亦日趨增多[3-4]。近年來雙黃連注射劑多次被國家藥品不良反應監測中心通報其引起的包括過敏反應[4-6],因此建立針對過敏反應的靈敏可靠的非臨床評價方法是目前亟須解決的問題。
急性過敏反應主要分為2個類別,一類是I型過敏反應,一類是類過敏反應[7]。與典型的過敏反應(I型過敏反應)不同,藥物的類過敏反應直接引起肥大細胞或嗜堿性粒細胞脫顆粒,或激活補體系統間接引起該細胞釋放生物活性遞質,從而誘發一系列類過敏癥狀。有研究顯示3/4的急性過敏反應為類過敏反應,發生率遠高于典型的過敏反應[8],并且由于類過敏反應在患者首次用藥即可產生,嚴重的可為過敏性休克,直接威脅生命。但是目前對其發生機制卻并不明確,也無標準的安全性評價模型,嚴重制約了中藥注射劑的安全性及其質量控制水平。

