鎳基金屬有機(jī)框架衍生的雙功能電催化劑用于析氫和析氧反應(yīng)
閆大強(qiáng),張林,陳祖鵬,肖衛(wèi)平,*,楊小飛,*
1南京林業(yè)大學(xué)理學(xué)院,材料物理與化學(xué)研究所,南京 210037
2南京林業(yè)大學(xué)化學(xué)工程學(xué)院,南京 210037
1 Introduction
The rapid consumption of non-renewable resources has caused serious energy and environmental crisis, hence, the researchers are exploring alternative energy systems1–3.Electrocatalytic water splitting involved a cathode hydrogen evolution reaction (HER) to produce hydrogen gas and an anode oxygen evolution reaction (OER) to produce oxygen gas receives significant attention. The process is clean,environmental-friendly and the generated oxygen/hydrogen gas could be converted into electricity in a fuel cell4,5. The main concern lies in the higher activation energy barrier existed in both HER and OER sides, which require electrocatalysts to achieve high-efficiency HER and OER performance6,7.Nowadays, precious metals as Pt8and RuO29have been reported to present excellent HER and OER performance, respectively,but their practical employment is limited by the scarcity and high price. Hence, the research on substituting catalysts to achieve comparable bifunctional catalytic activity with Pt and RuO2is of great significance10–13.
Nowadays, the 3dtransition metals have the potential to be alternatives for Pt-group electrocatalysts due to their low-cost and high-efficient HER/OER performance14–18. In particular,Ni-based materials which are stable in alkaline solution, have attracted extensive efforts as a substitutive HER catalyst since Ni-based materials show near zero ΔGH*19, as well as the OER catalyst since it can activate oxygen species20. The activities of Ni-based catalysts are closely related to the number of active sites and the structure of the carbon supports21,22. Particularly,metal-organic-frameworks (MOFs) possess modular nature with metal-based units and special organic ligands23,24. After annealed, metal atoms could be converted into unsaturated metal-based active sites and the organic ligands could be carbonized retaining the frame structure of the MOFs precursor,which could provide sufficient active sites and certain pore structure25,26. This could accelerate the charge transfer efficiency and be beneficial to achieve excellent HER and OER performance.
Here, Ni-MOFs precursor was synthesizedviaa liquid phase coordination reaction by using Ni2+and benzene-1,3,5-tricarboxylic acid27. After high-temperature annealing treatment, Ni nanoparticles werein situgrown on the rod-shaped carbon substrate forming Ni/C catalysts. The composite material obtained under the optimal conditions exhibits an overpotential of 120 mV for HER and 350 mV for OER in 1.0 mol·L?1KOH electrolyte at a current density of 10 mA·cm?2. Presumably, good structural, the abundant surface area of carbon substrate elevated HER/OER activity owing to their synergistic advantages of accessible active sites and enhanced electronic conductivity.
2 Experimental
2.1 Materials
Nickel chloride hexahydrate (NiCl2·6H2O, A.R., 99.9%metals basis, China), benzene-1,3,5-tricarboxylic acid(C6H3(CO2H)3, 98%, China) were purchased from Aladdin.Ethanol (A.R., ≥ 99.7%, China), potassium hydroxide (KOH,A.R. China) and sodium hydroxide (NaOH, A.R. China) were acquired from Sinopharm Chemical Reagent Co., Ltd., no additional treatment was required.
2.2 Synthesis
The Ni/C nanomaterials were synthesizedviaa liquid phase coordination reaction followed by an annealing process. The detailed composition steps are as follows: First, 1307.3 mg NiCl2·6H2O was dissolved in the solution mixed with 25 mL deionized water and 50 mL absolute ethanol; 840.56 mg benzene-1,3,5-tricarboxylic acid were dissolved into 50 mL 0.24 mol·L?1NaOH solution. Then, the benzene-1,3,5-tricarboxylic acid solution was slowly added to the nickel solution under magnetic stirring, and the mixture was stirred for 10 min and then stayed at room temperature for 24 h. The green product was washed repeatedly with ethanol and deionized water, and finally dried under vacuum at 60 °C. Finally, the sample obtained under H2thermal annealing of Ni-MOFs at 600 °C, 700 °C, 800 °C for 4 h with a ramping rate of 5 °C·min?1, named Ni/C-H2-600,Ni/C-H2-700, and Ni/C-H2-800, respectively. Ni/C-Ar-700 was obtained from thermal annealing of Ni-MOFs under Ar at 700 °C for 4 h with a ramping rate of 5 °C·min?1.
2.3 Materials characterization
The powder X-ray diffraction data is obtained by irradiating CuKα(λ= 0.15418 nm) using the multifunctional horizontal Xray diffractometer manufactured at 10 (°)·min?1(XRD, Ultima IV, Japan Rigaku, Japan); Laser Raman data was tested on Raman spectrometer (Raman, DXR-532, USA Thermo,Madison, WI, USA); X-ray photoelectron spectroscopy data was obtained by irradiating AlKαmonochromatic ray (XPS, AXIS UltraDLD, Japan Shimadzu Corporation, Japan); Scanning electron microscope images of the samples were obtained with a scanning voltage of 12 kV using a field emission scanning electron microscope model (SEM, JSM-7600F, Japan Electronics Corporation, Japan); Transmission electron microscopy image of the sample was obtained at a transmission voltage of 300 kV (TEM, JEM-1400, Japan Electronics Corporation).
2.4 Electrochemical measurements
The electrochemical measurement was performed in 1.0 mol·L?1KOH solutionviaa three-electrode system on electrochemical workstation (CHI 760E, Shanghai Chenhua,China). The graphite rod and reversible hydrogen electrodes(RHE) were used as counter electrodes and reference electrodes,respectively. 2.0 mg catalyst and 1 mg Vulcan XC-72 were added to 0.20 mL Nafion/isopropyl alcohol (1‰) to prepare the catalyst ink. 30 μL ink was distributed evenly on a glassy carbon rotating electrode with a diameter of 5.0 mm, where the loading amount of the catalyst was 1.53 mg·cm?2. The prepared electrode was activated by cyclic voltammetry (CV) scan in the potential range of 0.05–1.0 V to remove impurities on the surface. The corresponding linear sweep voltammetry (LSV) curve of hydrogen evolution reaction and oxygen evolution reaction were obtained at a scan rate of 5 mV·s?1in the potential range of ?0.5?0.2 V and 1.0–1.7 V (95% iR compensation) under rotation of 1600 rpm. The double-layer capacitance (Cdl) values were derived by linear fitting the curves of (ja?jc)/2 plotted against the scan rate. Electrochemical impedance spectroscopy was tested at 0 V (vs. RHE) potential with a frequency range of 100 kHz–1 Hz. The stability measurement was using a carbon fiber paper loaded catalyst (loading amount, 1.53 mg·cm?2) as the working electrode and tested under the potential at a current density of 10 mA·cm?2for 10 h.
3 Results and disscussion

Fig. 1 Scheme illustration of the synthetic process of Ni/C.
The strategy to synthesize Ni/C is illustrated in Fig. 1. Ni-MOFs was synthesized by coordination of Ni2+ion-containing compound and carbonic acid-containing benzene-1,3,5-tricarboxylic acid. Then, the Ni-MOFs was used as the precursor for annealing treatment from 600 °C to 800 °C. In detail, the MOFs were firstly annealed in argon for 3 h to remove impurities and achieve efficient carbonization of MOFs, generating rich pore structure and potential attachment sites for metal atoms.After that, the as-prepared materials were remained in hydrogen for 1 h to reduce Ni nanoparticles. Consequently, the rod-shaped porous carbon skeleton was obtained, while Ni nanoparticles were located on the carbon support to form the Ni/C catalyst.
The synthesized samples were characterized by scanning electron micrograph (SEM) as shown in Fig. 2. The prepared Ni-MOFs exhibited a rod-shaped structure with a diameter of 1.2–1.5 μm and a length of about 4 μm, which provided sufficient interface during annealing treatment for the attachment of Nibased nanoparticles (Fig. 2a). After annealing under argon at 700 °C, Ni/C-Ar-700 showed a coral-reefs-like morphology where the Ni nanoparticles were accumulated on the carbon rods(Fig. 2b). In contrast, porous rods of Ni/C-H2-700 with a large number of Ni nanoparticles on the surface were obtained after reduction under H2at 700 °C, leading to a good exposure of the active centers (Fig. 2c). The SEM image and corresponding EDS-mappings of Ni/C-H2-700 confirmed the co-existence of Ni, O, and C in the entire sample (Fig. 2d–g). EDX spectra of Ni/C-H2-700 provided further evidence for the presence of Ni,O, and C (Fig. 2h).
Transmission electron microscopy (TEM) was employed to observe the distribution of Ni nanoparticles on the carbon matrix. TEM images of Ni/C-H2-700 (Fig. 3a,b) displayed that the carbon matrix retained the rod-shaped morphology, Ni nanoparticles with a diameter of about 20–80 nm were dispersed on the porous carbon rod. The plane spacing in the HRTEM image (Fig. 3c) was calculated to be 0.201 nm, corresponding to the Ni (111) crystal plane. The HAADF-STEM image and EDSmappings of Ni/C-H2-700 further confirmed that the rod-shaped transparent schema and black nanoparticles corresponded to the carbon support and Ni nanoparticles (Fig. 3d–g).

Fig. 2 Scanning electron microscopy (SEM) images of (a) precursor of Ni-MOFs, (b) Ni/C-Ar-700 and (c) Ni/C-H2-700. (d–g) SEM image and corresponding EDS-mappings of Ni/C-H2-700. (h) EDX spectra of the content of C, O, Ni element in Ni/C-H2-700.
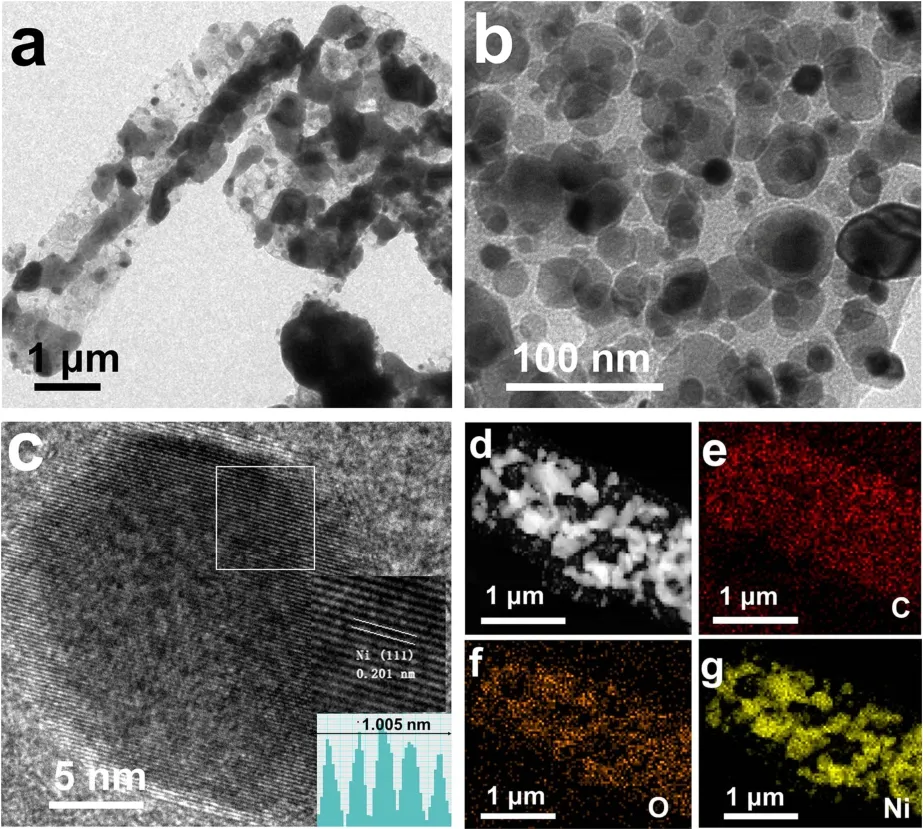
Fig. 3 (a, b) Transmission electron microscopy (TEM) and(c) high-resolution transmission electron microscopy (HRTEM)images of Ni/C-H2-700. (d–g) HAADF-STEM image and mapping analysis of C, O, Ni in Ni/C-H2-700.
Fig. 4a compared the X-ray diffraction (XRD) patterns of Ni/C-H2-600, Ni/C-H2-700, Ni/C-H2-800, Ni/C-Ar-700 samples. The characteristic peaks located at 44.5°, 51.8°, and 76.5° could be considered as the cubic Ni phase (PDF card # 04-0850), while the remaining special peaks corresponded to the NiO phase (PDF card # 44-1159). Hence, the Ni-MOFs annealed under argon atmospheres could acquire the mixture of Ni and NiO, however, only the Ni phase could be seen obviously under hydrogen conditions. Fig. 4b illustrated the Raman spectrum of each sample, there are two distinct characteristic peaks at 1306.7 and 1587.8 cm?1, corresponding to the D-band and G-band of the carbon matrix28, respectively. The X-ray photoelectron spectroscopy (XPS) further verified the coexistence of Ni, O,and C on the surface of Ni/C-H2-700 (Fig. 4c). The XPS spectra of Ni 2p3/2shown in Fig. 4d could be divided into three peaks located at 854.3, 856.4, and 852.9 eV, attributing to Ni2+2p3/2,Nix+2p3/2, and Ni02p3/2, respectively29. Compared with the standard value, the Ni2+2p3/2peak in Ni/C-H2-700 shifted to a higher oxidation state and increased the binding energy, which may be due to synergistic effects between Ni particles and carbon support. Besides, the peak area of Ni0is much higher than that of Ni2+, verifying that the content of Ni is relatively higher than NiO. The fine peaks of the C 1sspectrum were at 284.8,285.6, and 289.3 eV, belonging to C―C, C―O, and C=O (Fig.4e). Interestingly, the spectrum of O in Ni/C-H2-700 is fitted from the component peaks of 529.8 and 531.9 eV. The characteristic peak of 531.9 eV could be attributed to the absorbed oxygen, while the characteristic peak of 529.8 eV belongs to Ni―O, which could be due to the oxidation of Ni exposed to air (Fig. 4f).

Fig. 4 (a) X-ray diffraction (XRD) patterns and (b) Raman spectra of all samples Ni/C-H2-600, Ni/C-H2-700, Ni/C-H2-800, Ni/C-Ar-700,respectively; (c) XPS survey spectrum and high-resolution XPS spectra of (d) Ni 2p, (e) C 1s, (f) O 1s of Ni/C-H2-700 sample.

Fig. 5 (a) CV and (b) LSV curves of Ni/C-H2-600, Ni/C-H2-700, Ni/C-H2-800, Ni/C-Ar-700. (c) The corresponding Tafel plots of samples.(d) The double-layer capacitance (Cdl) calculated by liner fitting of the capacitive currents of different catalysts versus scan rate from 10 mV?s?1 to 160 mV?s?1. (e) EIS plots (0 V vs. RHE). (f) The (i–t) curve of HER durability of Ni/C-H2-700 tested at a constant potential at 10 mA·cm?2.
The electrocatalytic HER performances of Ni/C-H2-600,Ni/C-H2-700, Ni/C-H2-800, and Ni/C-Ar-700 catalysts were tested and compared. Fig. 5a displayed CV curves of different samples at a scan rate of 50 mV·s?1and Ni/C-H2-700 showed the highest capacitance. It could be seen from LSV curves shown in Fig. 5b that the overpotential of Ni/C-H2-700 is 120 mV at the current density of 10 mA·cm?2, which is much lower than that of Ni/C-H2-600 (250 mV) and Ni/C-H2-800 (348 mV), Ni/C-Ar-700 (275 mV). Compared with Ni/C-H2-700 sample, the Ni/CH2-600 might have a low carbonization degree and the Ni/C-H2-800 might lose the good frame structure, which resulted in the much lower HER activity. Tafel curve calculated according to the Tafel equation (η=a+blog|j|) was used to further analyze the catalytic kinetics. The Ni/C-H2-700 exhibited a lower Tafel slope of 121 mV·dec?1and a faster kinetic rate relative to Ni/CH2-600 (131 mV·dec?1), Ni/C-H2-800 (200 mV·dec?1), and Ni/C-Ar-700 (176 mV·dec?1) (Fig. 5c). The double-layer capacitance (Cdl) of the sample was calculated based on the cyclic voltammetry curves in the non-faraday region (0.3–0.5 V)(Fig. 5d). TheCdlof Ni/C-H2700 is 2.85 mF·cm?2, much higher than that of other catalysts, indicating a higher electrochemical active surface area (ECSA) since it is supposed to be proportionable toCdl. Furthermore, the electrode dynamics were further analyzed by electrochemical impedance spectroscopy(EIS) at 0 V (vs. RHE) (Fig. 5e). Ni/C-H2-700 showed the lowest resistance during the catalytic reaction system, which is conducive to promote electron transfer. Based on the above results and analysis, pore structure, large ECSA and high electronic conductivity contribute majorly to the high efficiency of HER catalytic efficiency on Ni/C-H2-700. Furthermore, the HER durability of Ni/C-H2-700 was tested by the potentiostatic method. The current density was almost no attenuation after 10 hours durable test and showed an ideal performance (Fig. 5f).
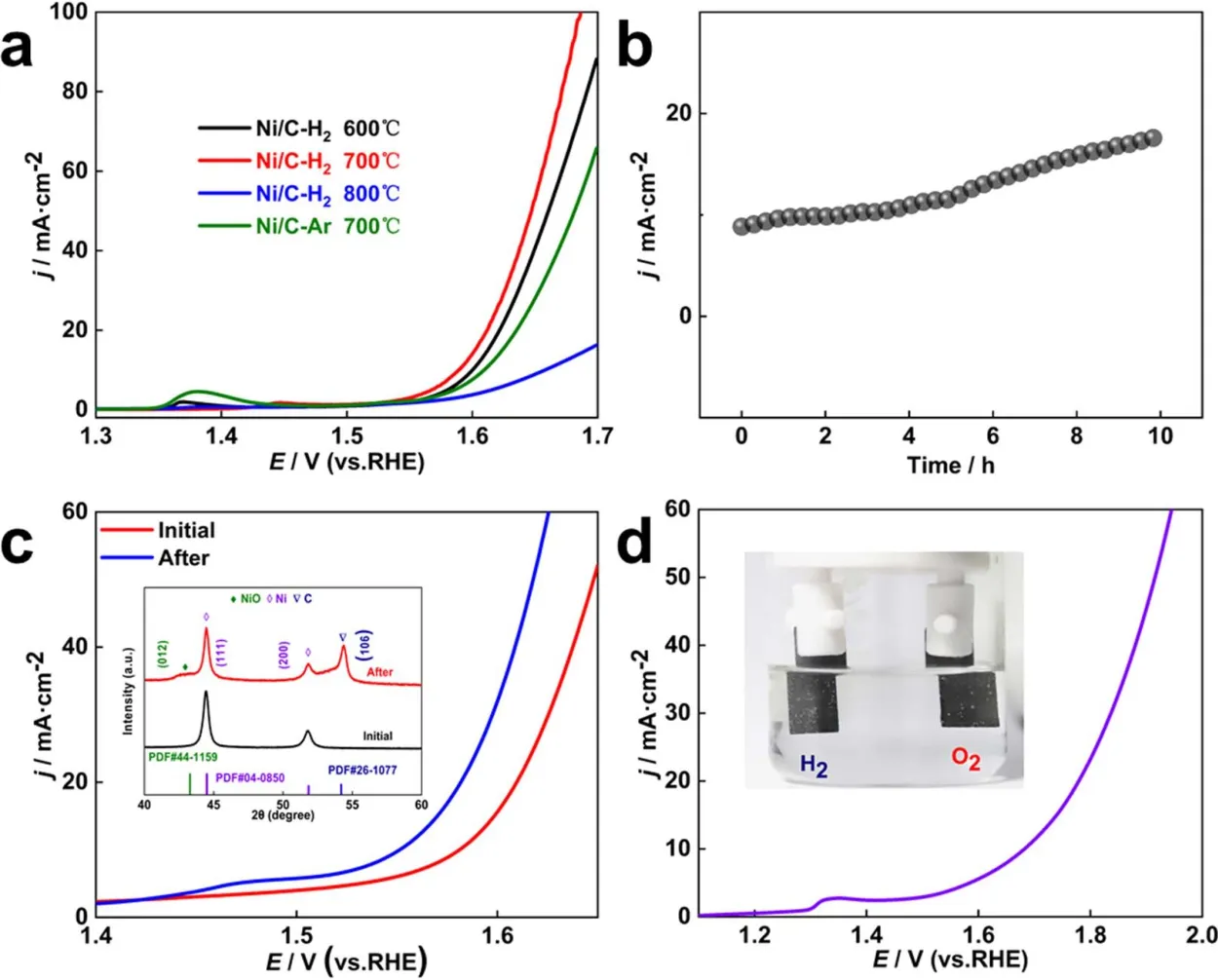
Fig. 6 (a) The OER polarization curves of Ni/C-H2-600, Ni/C-H2-700, Ni/C-H2-800 and Ni/C-Ar-700. (b) Long-term electrochemical OER durability tested at a constant potential at 10 mA?cm?2 of Ni/C-H2-700. (c) Polarization curves and XRD patterns of Ni/C-H2-700 before and after OER durability tests. (d) Polarization curve of Ni/C-H2-700 for overall water-splitting in 1.0 mol·L?1 KOH solution. The inset shows O2 and H2 evolution at the anode and cathode electrode.
Similarly, electrocatalytic OER activity of all samples was measured in 1.0 mol·L?1KOH as shown in Fig. 6a. Ni/C-H2-700 required an overpotential of 350 mV at a current density of 10 mA·cm?2, which was lower than Ni/C-H2-600 (370 mV), Ni/CH2-800 (430 mV), and Ni/C-Ar-700 (380 mV). The long-term electrochemical OER performance of Ni/C-H2-700 was obtained by maintaining overpotential at 350 mV. Compared with the initiative value, the current density was enhanced and the overpotential was reduced after long-term operation for 10 h(Fig. 6b). Fig. 6c showed the polarization curves and XRD patterns of Ni/C-H2-700 before and after OER durability test. It can be seen that the peak of NiO (PDF card # 44-1159) appeared after durable tests. The oxygen evolution performance has been improved, which could be owing to the partially oxidation of Ni during the process of oxygen evolution reaction. As displayed in Fig. 6d, Ni/C-H2-700 required a low cell voltage of 1.71 V to obtain the current density of 10 mA·cm?2when used as both the anode and cathode electrodes to driving overall water-splitting in 1.0 mol·L?1KOH solution. Based on the analysis, Ni/C-H2-700 exhibited excellent HER and OER activity and durability and could be used as a bifunctional catalyst for water splitting.
4 Conclusion
In summary, an effective bifunctional nickel-based electrocatalyst on porous carbon rods for HER and OER was synthesizedviaa liquid phase coordination reaction followed by an annealing process. Ni/C-H2-700 exhibited the optimized catalytic performance in 1.0 mol·L?1KOH solution. The overpotentials for HER and OER at a current density of 10 mA·cm?2were as low as 120 and 350 mV, respectively.Moreover, the Ni/C-H2-700 has also shown a much stable performance under the long-term durability test. This work verifies that it is practical to obtain bifunctional catalysts of HER and OER for water splitting by adjusting the annealing atmosphere and temperature from MOF precursors.

