Probing thermal properties of vanadium dioxide thin films by time-domain thermoreflectance without metal film?
Qing-Jian Lu(陸青鑑),Min Gao(高敏),?,Chang Lu(路暢),Fei Long(龍飛),Tai-Song Pan(潘泰松),and Yuan Lin(林媛),4,?
1School of Materials and Energy,University of Electronic Science and Technology of China,Chengdu 610054,China
2State Key Laboratory of Electronic Thin Films and Integrated Devices,University of Electronic Science and Technology of China,Chengdu 610054,China
3Guangxi Key Laboratory of Optical and Electronic Materials and Devices,School of Materials Science and Engineering,Guilin University of Technology,Guilin 541004,China
4Medico-Engineering Cooperation on Applied Medicine Research Center,University of Electronic Science and Technology of China,Chengdu 610054,China
Keywords:vanadium dioxide thin film,thermal conductivity,time-domain thermoreflectance
1.Introduction
Vanadium dioxide(VO2),by virtue of its sharp metal–insulator transition(MIT)near room temperature and the associated dramatic changes in electrical and optical properties,presents promising applications in electrical and optical switches.[1–6]While the predominant research thrust has been oriented to the intensive exploration of its phase transition mechanism and applications in memory devices and other smart devices,the mechanistic exploration of its thermal transport mode has attracted much less attention.[7–12]Only very recently,Sangwook et al.[10]and Jin et al.[11]have done some researches on the thermal transport mode of VO2,including the abnormal low electronic thermal conductivity and the non-quasiparticle transport mechanism in the metallic phase,which provides a different perspective to further understand the electron–electron interaction in this strongly correlated material.
Sangwook et al.’s work focused on single-crystal VO2nanowire,but VO2thin films were technically more useful in the predicted devices.In this study,we use the timedomain thermoreflectance(TDTR)method[13–17]to investigate the cross-plane thermal properties of VO2thin films.The TDTR has been demonstrated to be a powerful method to investigate the thermal properties of thin films and multilayered films.[18–21]Unlike the most widely used 3ωmethod in measuring the thermal properties of films,TDTR allows detecting the thermal properties of electrically conducting or semiconducting materials.In practical,most TDTR experiments require a thin metal layer(Pt,Au,Mo or Al)as a thermoreflectance layer in probing the surface temperature changes because the reflectivity of metal layer changes linearly with temperature.Some previous work utilized the TDTR to investigate the cross-plane thermal properties of VO2films by depositing a metal layer on the top of the film.[7,8]However,the interfacial thermal resistance between the metal layer and the specimen was difficult to eliminate,which might cause additional measurement uncertainties.On the other hand,the reflectivity and transmittance of VO2film change with temperature,which is similar to those of the metallic thermoreflectance layer,suggesting the possibility of measuring the thermal transport properties of VO2film by using TDTR without depositing any metal layer on the film.
In this study,we fabricate VO2thin films on the Al2O3(10-10)substrate and fluorine mica substrate,respectively.The VO2thin film epitaxially grown on the Al2O3substrate exhibits high crystalline quality and bonds well with the substrate.The fluorine mica substrate is a van de Waals(vdW)substrate,which means a weak effect on the in-plane growth direction of the as-grown film.By measuring the TDTR spectra of the VO2films on these two different substrates below and above the phase transition temperature through using the TDTR method without depositing any metal layer on the films,we observe the reasonable thermal conductivities of VO2thin films when the thickness values of the films are greater than 100 nm.Meanwhile,by comparing the thermal conductivity changes of VO2films on different substrates across the MIT,we find that it is correlated with the electrical conductivity change rate.Our results show that the TDTR method can be feasible in investigating the thermal transport properties of VO2films without depositing any metal thermoreflectance layer.Meanwhile,the limitation of this method will also be discussed.
2.Experiment
The VO2thin films were grown on Al2O3(10-10)substrate and fluorine mica substrate by using a polymer-assisted deposition(PAD)technique.[22–24]First,the polymer solution was prepared by dissolving 2.0-g polyethyleneimine(PEI,Mw≈10000),2.0-g ethylenediamine tetra acetic acid(EDTA)and 0.8-g ammonium metavanadate(NH4VO3)in 60-mL deionized(DI)water.Then the solution was filtered in an ultrafiltration device(Amicon 8050)to obtain a precursor solution containing 20.5-mg V ions per mL as measured by inductively coupled plasma optical emission spectrometer(ICPOES).The as-prepared precursor solution was spin-coated on the Al2O3(10-10)and fluorine mica substrates at a rate of 6500 rpm for 40 s,respectively.Finally,the precursor films were annealed at 505°C in forming gas(H2=1.5%and N2=98.5%)for 2 h.The spin-coating and annealing process was repeated to obtain VO2thin films of various thickness values.A detailed description of the synthesis process can be found in previous literature.[23,24]The VO2thin films of four different thickness values were grown on the Al2O3substrates and the fluorine mica substrates.Using scanning electron microscopy,the thickness values were determined to be about 30 nm,60 nm,110 nm,and 150 nm,respectively.
The thermal conductivity of the VO2thin film was measured by a nanosecond pulse laser thermal reflector system(Nano TR,PicoTherm,Japan).The experimental set-up is schematically shown in Fig.1.A ceramic heating plate,controlled by a direct current(DC)power,was used to heat the samples and the temperature of the ceramic heating plate was detected by a thermocouple thermometer.A 1550-nm pulsed laser with pulse width of 1 ns and energy of 100 mW was used to heat the sample and a 785-nm continuous wave laser was used to probe the variation of temperature of the sample with time.A lock-in amplifier in this system was used to measure a small change of temperature with time.
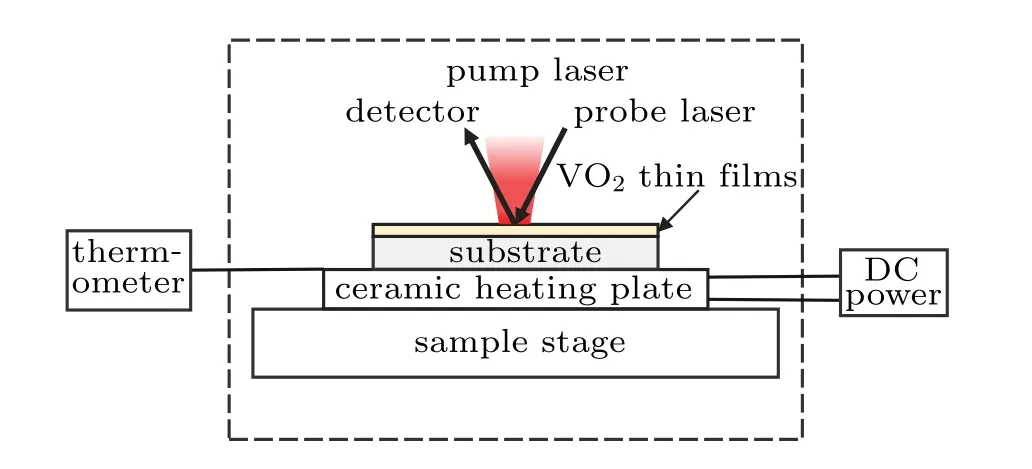
Fig.1.Schematic set-up of nanosecond pulse laser thermal reflection system.
The crystal quality of the VO2thin films was examined by a high-resolution x-ray diffractometer(HR-XRD,Bruker D8 Advance).The variation of the resistance of the thin films with temperature was measured by B2902A(Keysight),while the temperatures of the samples were controlled by LakeSore 325 during the measurements.
3.Results and discussion
The crystal structures of the VO2thin films on the fluorine mica and Al2O3(10-10)substrates are investigated by XRDθ–2θscans as shown in Fig.2.For the VO2thin films on the Al2O3substrates,only the x-ray diffraction peak of VO2(?402)is observed besides the peaks from the substrates.Additionally,our previousΦ-scan confirmed the epitaxial growth of VO2on Al2O3(10-10)substrate.[24]For the VO2thin films on the fluorine mica substrates,the x-ray diffraction peaks of VO2(011)and VO2(022)are detected besides the peaks from the substrates as shown in Fig.2,suggesting the growth mode with a highly preferred crystalline orientation.However,theΦ-scan does not detect the diffraction peak of VO2,which is possibly due to the disordered in-plane crystalline alignment.
The TDTR is employed to measure the thermal conductivity of the VO2thin film.First,we measure the thermal reflectance curves of 60-nm-thick VO2thin films coated with two kinds of 100-nm-thick metal films(Pt film and Mo film)as shown in Fig.S1 in the supplementary materials.The large difference between the observed two thermal reflectance curves indicates that the interface thermal resistance between the metal film and the sample should be carefully controlled to reduce the interface effect.To explore the methods of measuring TDTR without depositing the metallic thermoreflectance layer,we first check the change of the reflectivity of VO2film with temperature.As demonstrated by the measurements with a 785-nm laser,the reflectivity of 150-nm-thick VO2film exhibits an approximately linear relationship with temperature in both the insulating state and the metallic state as shown in Fig.S2 in the supplementary materials.This implies that the thermal reflectance curve of VO2film can be obtained through the TDTR method without depositing an extra metal thermoreflectance layer on the top.However,in the phase transition process,the reflectivity of vanadium dioxide film displays sharp changes,which in turn can present abnormal thermal reflectance curves in TDTR.It should be noted that by measuring the temperature dependent reflectivity of the VO2film coated with 100-nm Pt film(shown in Fig.S3 in the supplementary materials),we find that it also exhibits a sharp change in the phase transition process,thereby confirming that the abnormal reflected signals of the VO2film cannot be completely screened even by the 100-nm-thick Pt film.Therefore,we can analyze only the thermal reflectance curves of VO2films beyond the phase transition region by using this method.
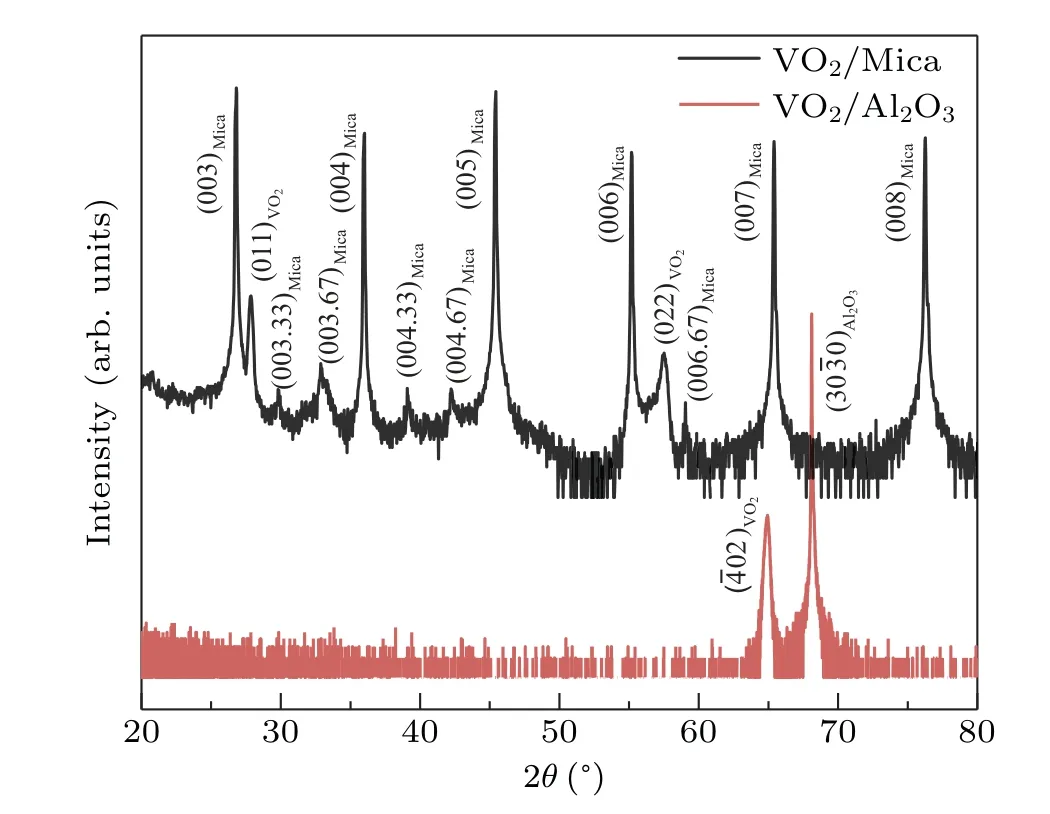
Fig.2.X-ray diffraction of VO2 thin films grown on Al2O3(10-10)substrate and fluorine mica substrate,respectively.
In order to obtain the thermal conductivity of the VO2thin film through the thermal reflection curve of the film,the Thermal Analysis for Thin Films software supplied in the nanoTR system is used to analyze the curve.This simulation method uses a mirror image method.After heating the surface with a pulse,the temperature of the specimen surface changes according to the following equation:
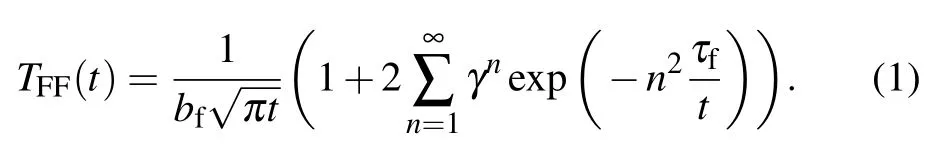
Here,τfrepresents the characteristic time(thermal diffusion time)that describes the thermal diffusion from the surface of the thin film to the interface between the film and its substrate,
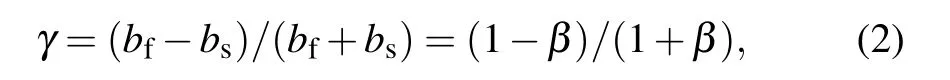
with bfand bsbeing the thermal effusivity of the thin film and the substrate,respectively.andβ=bs/bfrepresenting the thermal effusivity ratio.
By using the analysis software,the thermal diffusion time τfand a dimensionless parameterγfor the thin film are determined through fitting this equation over an optimum time span with the top film used as the first layer and the substrate as the second layer.Then the thermal conductivity of the film is calculated from the thermal diffusion time according to

whereλfis the thermal conductivity of the film,dfis the thickness of the film,ρfis the density of the film,and cfis the specific heat capacity of the film.
To verify the above argument,we first measure and simulate the thermal conductivities of VO2thin films with thickness values of 30 nm,60 nm,and 110 nm on the Al2O3(10-10)and fluorine mica substrates,which are shown in Fig.S4 and Table S1(supplementary materials).Even though the results show that the simulated thermal conductivities of all the VO2thin films at the metallic phase(353 K)are larger than those at the insulating phase(297 K),in consistence with that expected from the properties of VO2,the data of 30-nm-thick and 60-nm-thick VO2thin films are much lower than the values of VO2reported in Refs.[8,10].Furthermore,it should be noted that the thermal conductivity increases as the thickness of the VO2film increases.This is caused by the short heat flow diffusion time when the thickness of the VO2thin film is too small.Based on the calculation above,for the film with thickness below 100 nm,a picosecond laser should be utilized to improve the temperature response time.
On the other hand,because of the fast temperature response of VO2to infrared light,reasonable thermal conductivity value of 150-nm VO2thin film should be able to be measured with a nanosecond laser.We measure the thermal reflectance curves of 150-nm-thick VO2thin films on the Al2O3(10-10)substrate and the fluorine mica substrate,without an extra metal layer on their top,at 297 K and 353 K,respectively,and the curves are shown in Figs.3(a)–3(d).The fitting results are also shown in Fig.3.At 297 K,the simulated thermal diffusion time of the 150-nm-thick VO2thin films on Al2O3(10-10)substrate and the fluorine mica substrate are 13.19 ns and 9.97 ns,respectively.Utilizing Eq.(3)and the heat capacity per unit volume of VO2in Ref.[6],the simulated thermal conductivity of the 150-nm-thick VO2thin films on the Al2O3(10-10)substrate and the fluorine mica substrate are 4.86 W/mK and 6.43 W/mK,respectively.At 353 K,the thermal conductivity of the 150-nm-thick VO2thin films on Al2O3(10-10)substrate and fluorine mica substrate are 7.62 W/mK and 7.06 W/mK,respectively.While the temperature increases to 373 K,the thermal reflectance curve of the 150-nm-thick VO2thin film on Al2O3(10-10)is the same as that at 353 K as shown in Fig.S5 in the supplementary materials.In this case,the thermal conductivity of VO2thin film at 353 K is a stable value of metallic VO2.It is obvious that the thermal conductivity of the VO2thin film on the Al2O3(10-10)substrate has a larger rate of change across the phase transition than that on the fluorine mica substrate.The calculated phonon thermal conductivity of metallic phase of VO2has a reduction resulting from the calculated phonon thermal conductivity of insulating phase of VO2.[10]Therefore,the increase of thermal conductivity of the VO2thin film across MIT is due to the increase of electronic thermal conductivity.In order to explore the difference,the electrical conductivity of VO2thin film should be considered.
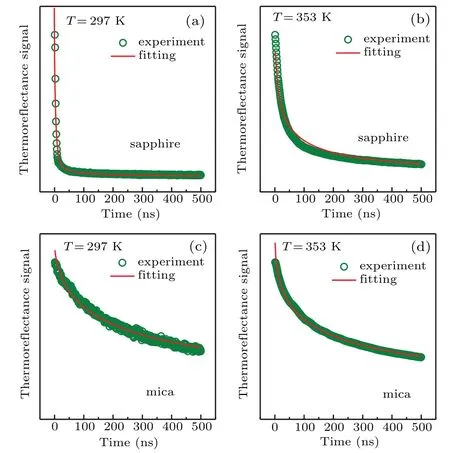
Fig.3.Thermal reflectance curves of 150-nm-thick VO2 thin films grown on Al2O3(10-10)at(a)297 K and(b)353 K,respectively.Thermal reflectance curves of 150-nm-thick VO2 thin films grown on fluorine mica at(c)297 K and(d)353 K,respectively.Olive circles represent experimental data,and red solid lines denote fitting data.
The temperature-dependent electrical conductivities of the 150-nm-thick VO2thin films on different substrates are measured by four-point-probes,and the results are shown in Fig.4.For the 150-nm-thick VO2film on Al2O3(10-10),the electrical conductivity changes from 37.6 S/m(at 297 K)to 1.44×106S/m(at 353 K),while the electrical conductivity varies from 46.2 S/m(at 297 K)to 1.09×105S/m(at 353 K)for the VO2thin film on the mica substrate.In the metallic state,the electrical conductivity of the VO2thin film on the sapphire substrate is an order of magnitude higher than that on the mica substrate.This phenomenon should be attributed to the crystalline quality of the films.The VO2thin films epitaxially grown on Al2O3(10-10)have better crystallinity and less defects.However,the interaction force between the fluorine mica substrate and the VO2film is the van der Waals force,and the XRD pattern in Fig.2 indicates that the VO2thin film has only a preferred growth orientation along〈011〉.Thus,the VO2thin film has more defects,which increases the elastic electron scattering weight.
It should be noted that the metallic phase of VO2is not regarded as a conventional Fermi liquid,[25,26]where the transport mode of charge and the transport mode of heat current are separated.[10]In this case,the electronic thermal conductivity of metallic VO2is not proportional to its electrical conductivity,which means that it does not obey the Wiedemann–Franz(WF)law.However,it has been found that disorders can recover the WF law.[11]Consequently,because VO2thin film has more defects than the single-crystalline VO2nanowires,the electronic thermal conductivity in the VO2thin film is related to its electrical conductivity.

Fig.4.Temperature-dependent electrical conductivities of 150-nmthick VO2 thin films grown on(a)Al2O3(10-10)and(b)fluorine mica,respectively.
4.Conclusions
In this work,the thermal conductivities of the VO2thin films on different substrates are successfully measured by TDTR without depositing any metal thermoreflectance layer.When the thickness is greater than 100 nm,the thermal conductivity value is consistent with those measured by other methods.A comparison of thermal conductivity among the VO2thin films on different substrates shows that the thermal conductivity change rate across MIT is related to the electrical conductivity change rate,implying that the increasing elastic electron scattering weight caused by defects in the VO2thin film can change the electronic thermal transport mode in the metallic phase.
- Chinese Physics B的其它文章
- Origin of anomalous enhancement of the absorption coefficient in a PN junction?
- Protection of isolated and active regions in AlGaN/GaN HEMTs using selective laser annealing?
- First-principles study of plasmons in doped graphene nanostructures?
- An improved model of damage depth of shock-melted metal in microspall under triangular wave loading?
- Signal-to-noise ratio of Raman signal measured by multichannel detectors?
- Molecular dynamics study of coupled layer thickness and strain rate effect on tensile behaviors of Ti/Ni multilayered nanowires?

