Influence of Fe2O3 on Release Mechanism of NH3 and Other Nitrogen-Containing Compounds from Pyrolysis of Three Typical Amino Acids in Urban Sludge
XU Lianlian(徐蓮蓮), YANG Zaifu(楊再福)
1 College of Energy and Environment, Anhui University of Technology, Ma’anshan 243002, China 2 College of Environmental Science and Engineering, Donghua University, Shanghai 200051, China
Abstract: To elucidate the effects of Fe2O3 on nitrogen transformation during sludge pyrolysis, thermogravimetry coupled with mass spectrometry (TG-MS) was used to investigate the influences of Fe2O3 on the pyrolysis characteristics and the release of important gaseous NOx precursors such as HCN and NH3 during pyrolysis of three typical amino acids in urban sludge. The results show that after Fe2O3 addition, the total weight loss rate of the three amino acids and the initial decomposition temperature of proline are reduced. The release amounts of NH3, HCN, CH3CN, and HNCO from these three representative amino acids—glumatic, arginine, and proline, decrease in the order of arginine, glutamic, proline. The generation of Fe-N complexes, reduces the generation of NH3, HCN, CH3CN, and HNCO while the catalysis effects of Fe2O3 on the formation of H and H2 play a promoting role in the generation of NH3, HCN, CH3CN, and HNCO. The results would provide an experimental and theoretical basis for subsequent research on the NOx precursor formation mechanisms during pyrolysis or combustion of Fe-containing sludge or sludge with additives containing Fe.
Key words: sludge; thermogravimetry coupled with mass spectrometry (TG-MS); Fe2O3; amino acid; nitrogen-containing compound
Introduction
Large amount of urban sludge is generated annually, especially in developing countries,e.g., China. In 2019, the sludge production was more than 39 million tons based on wet weight[1]. At present, sludge is mostly processed through landfilling, composting and incineration. Among these methods, incineration exhibits many advantages,e.g., fast speed, partial recovery of energy. Therefore, sludge is often incinerated industrially after being dried[2-3]. However, because sludge is rich in nitrogen, a large quantity of nitrogen oxide is generated in the incineration process, resulting in serious environmental pollution[3-5]. The mechanism by which HCN and NH3are generated from the pyrolysis of sludge as precursors of NOxhas attracted much attention[6-10].
Nitrogen in urban sludge comprises protein nitrogen, inorganic ammonium nitrogen, heterocyclic nitrogen,etc., with protein nitrogen being the most abundant component. Previous studies have used protein and amino acids, the building blocks of protein, as model compounds for pyrolysis to illustrate the nitrogen-releasing mechanism of biomass and sludge[7, 9-11].
Fe-containing minerals have a great impact on the pyrolysis process of solid fuels, such as coal and biomass, and the migratory behavior of nitrogen in thermal processes[12-13]. Wangetal.[14]found that Fe2O3could catalyze and decompose the heavy components of the primary pyrolysis products of coal. Xuetal.[15]used low-rank coal, coke generated from the pyrolysis of lignite by adding Fe and Ca, and commercial activated carbon as catalysts in the reaction that NH3decomposes into N2and H2. It was found that Fe plays a very important role in this reaction, and that low-rank coal rich in iron can be used as a kind of prospective catalytic material in the removal and purification of low-concentration NH3. Xuetal.[16]found that Fe reduced the production rate of NH3by decomposing it to N2and H2during the evaporation of biomass. Chengetal.[17]revealed the impact of minerals on the release of nitrogen components during the burning process of anthracite by showing that Fe2O3could promote the generation of NH3. At present, the content of Fe-containing components in the sludge used in the burning process are very high due to the flocculants containing Fe used in the primary treatment process of sewage sludge[18]. Therefore, it is necessary to study the impact of minerals containing Fe on the nitrogen migratory characteristics of sludge or corresponding model compounds containing nitrogen. Zhangetal.[19-20]studied the impact of minerals on the evolution behaviors of NH3and HCN through microwave pyrolysis of sludge, and they found that most minerals, among which the effect of Fe2O3on the generation of NH3was the most powerful, substantially suppressed the generation of NH3and HCN. Yietal.[21]investigated the influence of mixed Fe/Ca additives on the generation of HCN and NH3during the pyrolysis of some N-containing model compounds such as protein, proline, and phenylalanine with a fixed bed reactor at 873, 1 073, and 1 273 K. The results showed that the intermediates (FeNxand CaCxNy) played important roles in the reduction of HCN and NH3. Weietal.[22]used differential scanning calorimetry-mass spectrometer (DSC-MS) and fixed bed experiment to investigate the influences of Fe2O3on glycine pyrolysis characteristics. The results showed Fe2O3can reduce the release of NH3and HCN.
Although many kinds of amino acids are contained in sludge, there is less information about the generation mechanism of gaseous NOxprecursors such as HCN and NH3when Fe2O3is added to other typical amino acids in sludge. In this paper, we chose three typical amino acids with different structures whose content in sludge is high,i.e., glutamic acid, arginine, and proline, as research subjects and use thermogravimetry coupled with mass spectrometry (TG-MS) to study the impact of Fe2O3on the generation of NH3and other nitrogen-containing (N-containing) compounds in a slow pyrolysis process, which can provide both an experimental and theoretical basis for subsequent research on the NOxprecursor formation mechanisms during pyrolysis or combustion of Fe-containing sludge or sludge with additives containing Fe. Our study is helpful for controlling N conversion pathways during sludge pyrolysis and predicting N migratory behavior during sludge combustion.
1 Experiments
1.1 Materials
Amino acids used in experiments included glumatic acid, arginine, and proline (mass purity > 98.5%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), which were indicated by letters E, R, P, respectively according to the literatures[23-27]. All samples were dried for 10 h at 45 ℃ prior to each test. Fe2O3(analytical reagent (AR), mass purity > 99%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and dried amino acids were mixed at mass ratios (Fe2O3/amino acid) of 0∶10, 1∶10, 2∶10 (1∶5), 3∶10, 4∶10 (2∶5) and 5∶10 (1∶2), and these samples were respectively marked as (E,R,P)-(0,1,2,3,4,5). Samples E-(0,1,2,3,4,5) were glutamic acid mixed with Fe2O3at different mass ratios (Fe2O3/glutamic acid). Samples R-(0,1,2,3,4,5) were arginine mixed with Fe2O3at different mass ratios (Fe2O3/arginine). Samples P-(0,1,2,3,4,5) were proline mixed with Fe2O3at different mass ratios (Fe2O3/proline).
1.2 Methods
For TG-MS experiments, a Netzsch STA 449C thermogravimetric analyzer (TGA, Selb, Germany) and a Netzsch QMS 403 mass spectrometer (MS, Selb, Germany) were adopted. During the test process, sample volume of amino acids was fixed at about 10 mg, and samples were placed into alumina crucibles of the TGA. Air inside the system was purged three times with argon (volume purity > 99.999%). The flow rate of argon was 50 mL/min. The heating rate was 20 ℃/min. The temperature was between 50 ℃ and 900 ℃.
2 Results and Discussion
2.1 Thermogravimetric experiment
Figure 1 shows the TG and derivative thermogravimetric analysis (DTG) curves obtained from the pyrolysis of amino acids with and without the addition of Fe2O3. Normalization was done with the original weight of amino acids as the benchmark. As depicted in Fig. 1, comparatively intense loss of weight occurs with the amino acids respectively at 204, 240, and 223.5 ℃. After Fe2O3addition, the initial temperature of the pyrolysis of proline decreases to 204 ℃. This is because that Fe2O3can reduce the activation energy of the initial decomposition. It is found from Figs. 1(e)-1(f) that an obvious second weight loss step occurs in the main pyrolysis step of proline with Fe2O3addition, as Fe2O3can substantially change some of the reaction paths during the proline pyrolysis process.
After Fe2O3addition, the total weight loss rate of the three amino acids is reduced in the major weight loss stage, which results from the fact that Fe2O3can generate Fe-N complexes in the pyrolysis process[22, 28-34]. The chemical formula is shown in
Fe2O3+Amino-N→FeCxNy.
As the content of Fe2O3increases, weight loss substantially occurs, respectively at 548 ℃, 548 ℃, and 528 ℃ in the pyrolysis of glutamic acid, arginine, and proline. This occurs because of the decomposition of Fe-N complexes[29-30], while the difference in the initial temperature of weight loss occurs because the Fe-N complexes generated from different amino acids are different.

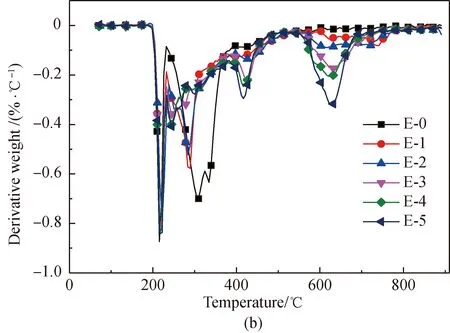
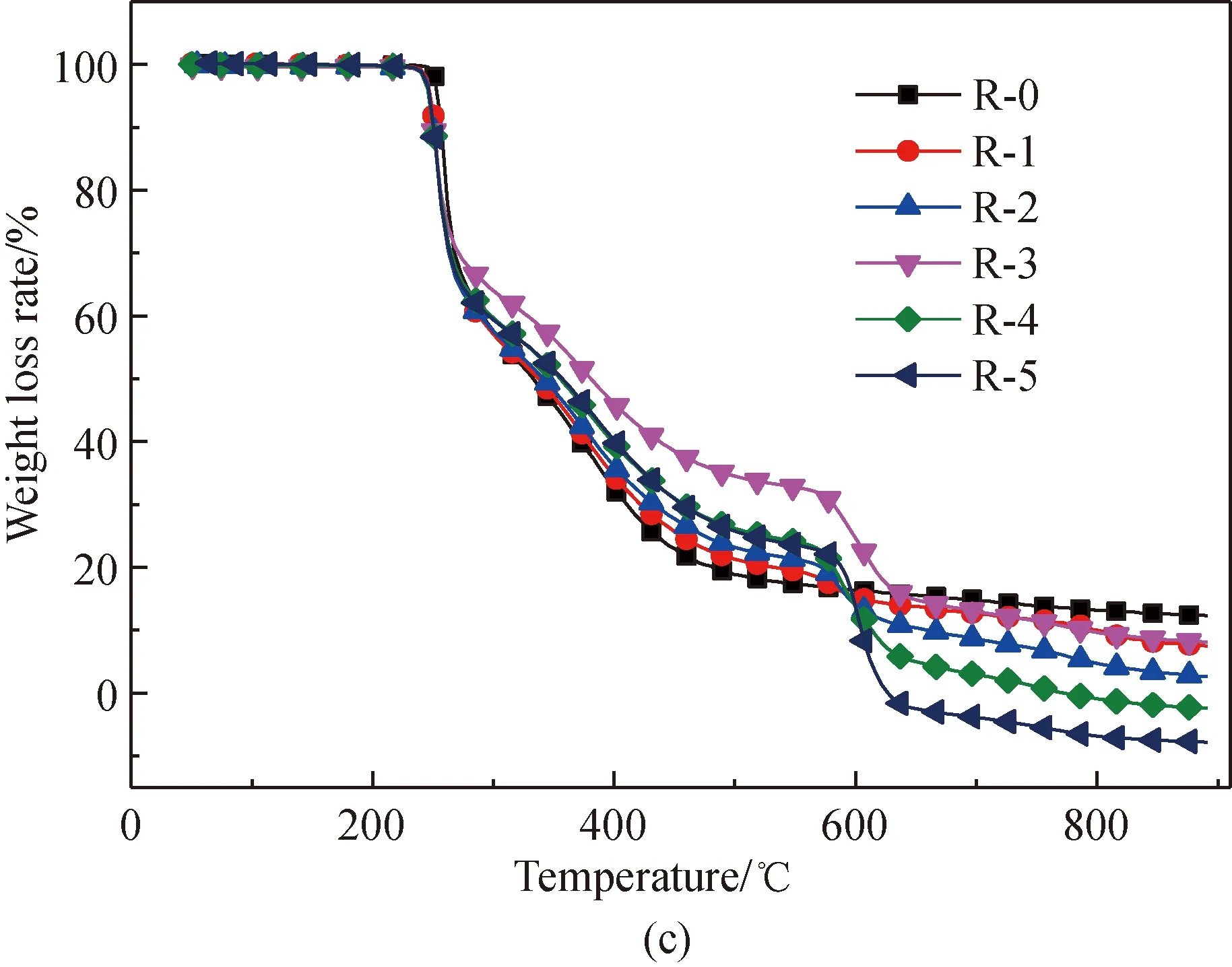
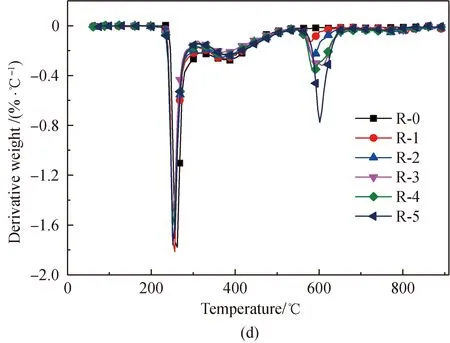
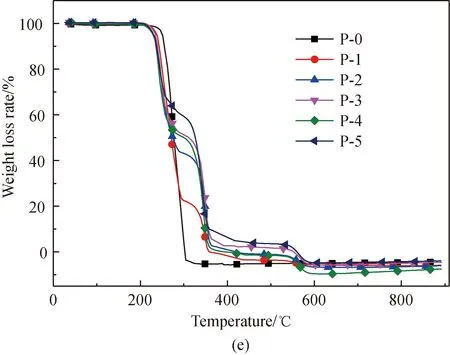
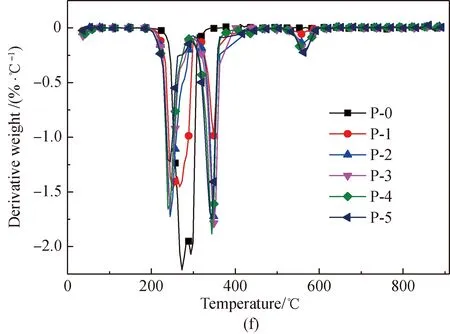
Fig. 1 Pyrolysis TG and DTG curves of glutamic acid, arginine, and proline with added Fe2O3: (a),(c) and (e) TG;(b), (d) and (f) DTG
2.2 Release characteristics of NH3 and other gases
A comparison of the release characteristics of NH3and other gases for different amino acids in the pyrolysis process is shown in Fig. 2. It is found that the evolution amounts of gases (NH3, HCN, CH3CN, and HNCO from the three kinds of amino acids are in the descending order of arginine, glutamic acid, proline. The reason is that arginine has a single carboxyl and multiple aminos, glutamic acid has two carboxyls and a single amino, and proline is a cyclic amide-type substance with nitrogen fixed on the ring. The nitrogen evolution capacity of these three different structures is in descending order[35-36]. Meanwhile, the evolution amounts of HCN, CH3CN, and HNCO from these amino acids are far less than those of NH3; the amount of NH3evolved from glutamic acid and proline is almost the same; and the ratio of the evolution amount of NH3and HCN from glutamic acid is lower than those of arginine and proline. These results are as follows.
(1) Structurally, the evolution of NH3from arginine with multiple amino acids is easier[35-37].
(2) The pyridine pyrolysis of proline with its five-membered ring, causes nitrogen to escape in the forms of —NH and —NH2, which are more likely to generate NH3and accompanied by a small amount of HCN, CH3CN, and HNCO[38-39].
(3) Glutamic acid is more likely to deaminize and generate NH3in the slow pyrolysis process, but its dual-carboxyl structure makes it also easier for intra-molecular or multiple-molecule reactions to generate a cyclic amide structure, which decomposes and then generates HCN[35, 37].
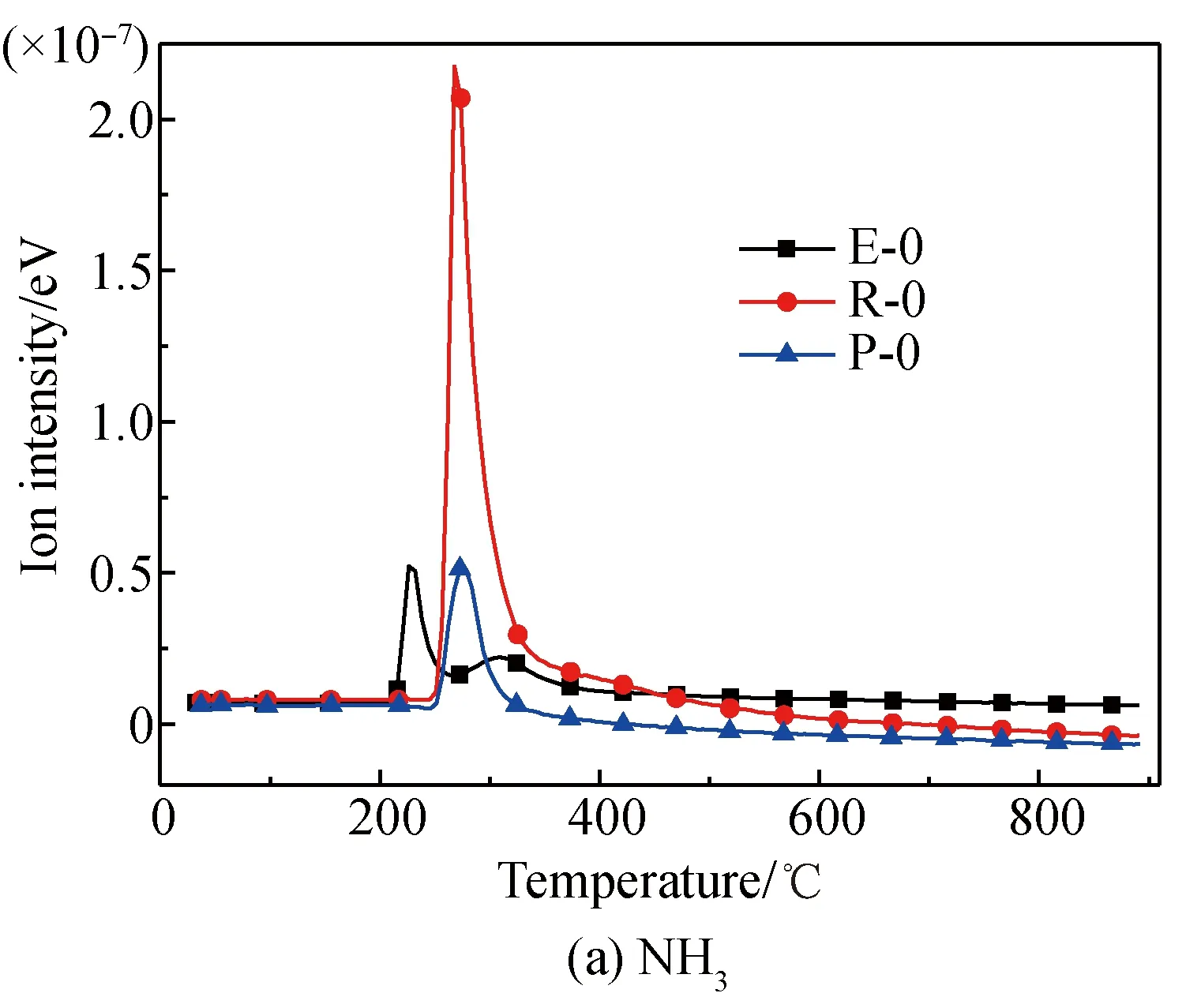
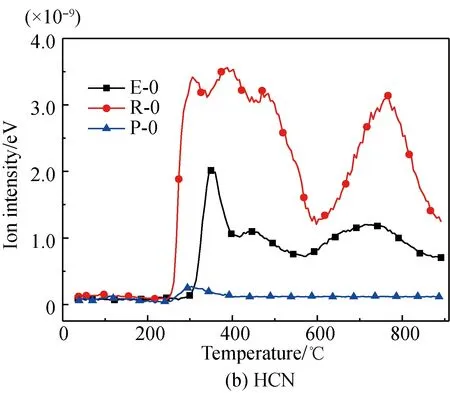
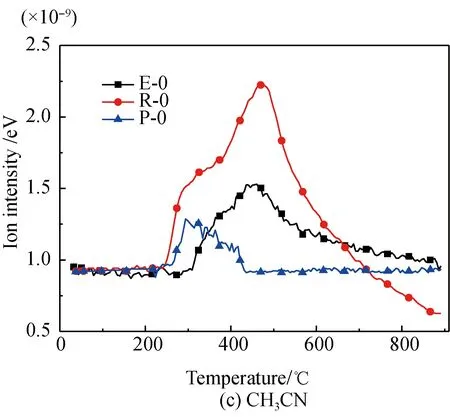
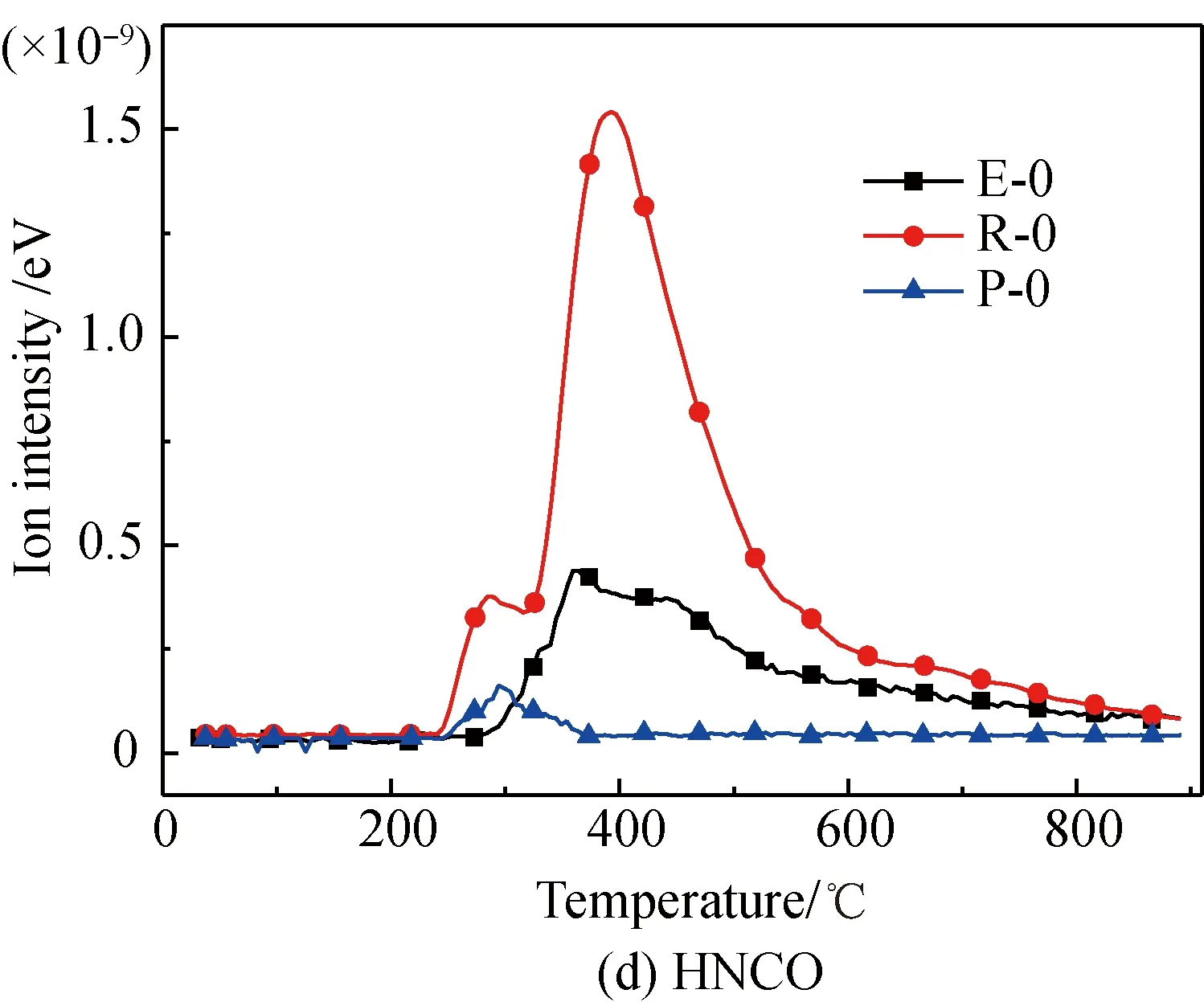
Fig. 2 Comparison of N-containing gas releasecharacteristics for different amino acids
The NH3evolution curves from glutamic acid pyrolysis before and after Fe2O3addition are shown in Fig. 3(a). It is found that before Fe2O3addition, the evolution temperature of NH3lays within the sections of 204-267.5 ℃ and 267.5-397 ℃, with the peak temperatures respectively being 226.3 ℃ and 309.3 ℃. The first temperature section 204-267.5 ℃ corresponds to the initial deamination process. The subsequent peak in the second temperature section 267.5-397 ℃ corresponds to the deamination process of such amide-type materials as pyroglutamic acid generated via pyrolysis, or further deamination of 4-aminobutyric acid generated by decarboxylation[37, 39-41]. After Fe2O3addition, the two evolution sections gradually merge into one with no obvious change in the initial and terminal temperatures, while the peak temperature changes to approximately 232 ℃. The reason for the change in these sections is that Fe-N complexes[22, 28-34]are generated from the reactions of Fe2O3and aminos of glutamic acid, which suppresses or prevents the deamination process at the temperature section of 267.5-397 ℃. Meanwhile, the phenomenon of alternatively decreasing and slight increasing the evolution amount of NH3within this section occurs with increasing Fe2O3, which is due to the fact that while suppressing the evolution of NH3, Fe2O3also has a slight promoting effect. Therefore, the overall evolution performance is the combined result of suppression and promotion effects. The promotion effect occurs because Fe2O3has a catalyzing effect on the dehydration and dehydrogenation reaction, serving as a hydrogen source for the generation of NH3[22, 30, 42-43]. In addition, after Fe2O3addition, a slight increase in NH3occurs near 397 ℃, which is caused by the decomposition or deamination of Fe-N complexes[22, 29-30].
The HCN evolution curves of glutamic acid pyrolysis before and after Fe2O3addition are shown in Fig. 3(b). It was found that the evolution temperature of HCN lays within the sections of 267.5-416.8 ℃, 416.8-567.9 ℃, and 567.9-876.1 ℃, with the peak temperatures respectively being 349.5, 440.9, and 711.6 ℃. The first temperature section 267.5-416.8 ℃ corresponds to the generation of such amide-type substances as pyroglutamic acid via pyrolysis or the denitrification and dehydrogenation of 4-aminobutyric acid. The peak value in the second temperature section 416.8-567.9 ℃ is due to breakage and dehydrogenation of some other amides, while the subsequent peak in the third temperature section 567.9-876.1 ℃ corresponds to the further breakage and degeneration of some difficultly decomposed N-containing heterocycles or cyanides[37, 39-41].
After Fe2O3addition, the first two evolution sections of HCN merge into a large section with three small peaks that first increases, then decreases, and then increases again with increasing Fe2O3/glutamic acid ratios. This is because Fe2O3presents two different influence mechanisms on the evolution of N-containing gases of different amino acids. The rising under promotion is due to the catalytic effects of Fe2O3on the formation of H and H2that are necessary for the generation of HCN[22, 30, 42-43]. The decrease under suppression was also due to the fact that Fe-N complexes are generated from the reactions of Fe2O3and aminos of glutamic acid[22, 28-34], which reduced the formation of the generation path of HCN. The multiple peaks within the large section are due to multiple generation paths of HCN. Furthermore, the third peak at the temperature section 567.9-876.1 ℃ before Fe2O3addition disappears after Fe2O3addition. This is most likely because that N-containing substances release at an earlier stage or are fixed within the carbon. As the Fe2O3/glutamic acid mass ratio arrives at 50%, a smaller evolution peak occurs near 548 ℃, which is on account of the decomposition of Fe-N complexes.
The CH3CN and HNCO evolution curves of glutamic acid pyrolysis before and after Fe2O3addition are shown in Fig. 3(c). The initial evolution temperature of CH3CN and HNCO is 267.5 ℃, which is the same as the initial temperature of HCN evolution. The evolution of CH3CN peaks at 445.8 ℃, which is close to the second peak evolution value of HCN. Also, a peak value and a shoulder peak of HNCO occur at 359.2 ℃ and 440.9 ℃, respectively, which are also close to that of HCN. The results show that the generation of CH3CN and HNCO is also due to the breakage and hydrogenation of amides, including pyroglutamic acid or 4-aminobutyric acid. As for CH3CN, the evolution peak becomes higher and sharper after Fe2O3addition. This is because Fe2O3promotes the generation of products like the initial cyclic amide, which generates more CH3CN through further reactions. As for HNCO, the initial evolution temperature increases to 204 ℃ after Fe2O3addition. This is the same as the initial evolution temperature of NH3, indicating that Fe2O3promotes deamination reactions of glutamic acid at the initial stage of pyrolysis. Meanwhile, the evolution amount of HNCO is reduced because Fe-N complexes are generated in the pyrolysis process and Fe2O3inhibits the reactions by which amides break and then generate HNCO[22, 28-34]. With increasing Fe2O3, the evolution amount of CH3CN first increases, then decreases, and then increases again, while that of HNCO first decreases, then increases, and then decreases again. This is because, similar to HCN, the formation of Fe-N complexes[22,28-34], suppresses the generation of CH3CN and HNCO, while the catalysis effects of Fe2O3on the generation of H and H2[22,30,42-43]play a promoting role in the generation of CH3CN and HNCO. It is seen that the promotion effect plays a dominant role in CH3CN, while suppression does in HNCO.
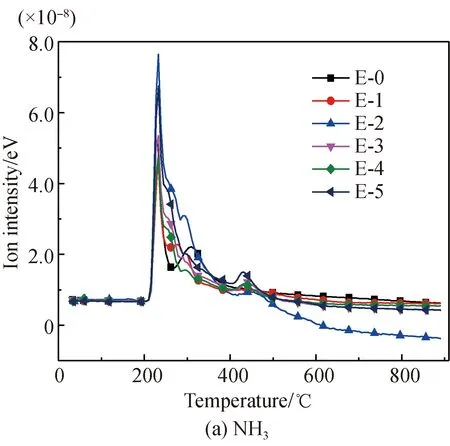
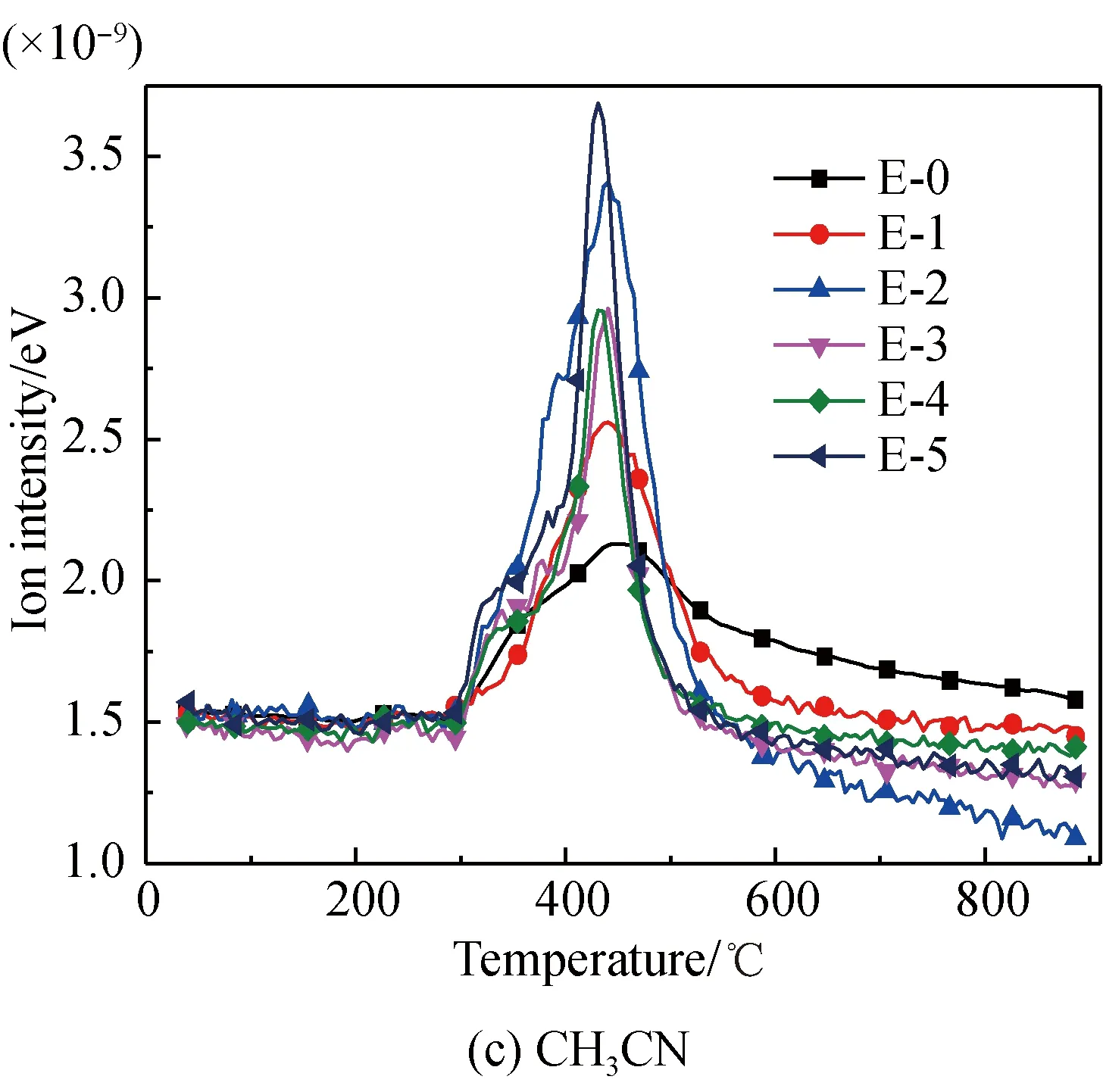
Fig. 3 N-containing gas release characteristics of glutamic acid pyrolysis before and after Fe2O3 addition
The NH3evolution curves of arginine pyrolysis before and after Fe2O3addition are shown in Fig. 4(a). It is found that NH3only evolves in the temperature range of 240-359.2 ℃ for arginine without Fe2O3, with the only peak value at 267.8 ℃, which is different from glutamic acid. This occurs because arginine has a totally different structure than glutamic acid. Arginine has many amino groups, so there is only the reaction of deamination to generate NH3. After Fe2O3addition, the evolution amount of NH3is reduced. This is because Fe-N complexes are generated, or the occurrence of such reaction paths as cyclization of amino are promoted, which suppresses the deamination reaction[22, 28-34]. There is a slight increase when the added amount increases from 10% to 20%, which occurs because Fe2O3catalyzes the generation of H and H2[22, 30, 42-43]in the pyrolysis process, which shows a promotion effect.
The HCN evolution curves of arginine pyrolysis before and after Fe2O3addition are shown in Fig. 4(b). It is found that before Fe2O3addition, HCN releases within the ranges of 240-340.1 ℃, 340.1-460 ℃, 460-597.5 ℃, and 597.5-900 ℃, with peak temperatures respectively being 305.9, 387.7, 469.8, and 766.2 ℃. Figure 4(c) shows the CH3CN evolution curves of arginine pyrolysis before and after Fe2O3addition. It is found that CH3CN starts to release at 240 ℃, and the peak temperature is 474.6 ℃. Figure 4(d) shows the HNCO evolution curves of arginine pyrolysis before and after Fe2O3addition. It is found that, before Fe2O3addition, the evolution temperatures of HNCO are in the temperature ranges of 240-315.8 ℃ and 315.8-650 ℃, and its peak temperatures are 284.9 ℃ and 392.5 ℃ respectively. The initial evolution temperature of these three gases is the same as that of NH3, which indicates that while deamination is underway, there are small amounts of side reactions generating imide and nitriles, and then generating HCN, CH3CN, and HNCO. Three consecutive evolution ranges occur for HCN at the initial pyrolysis process, an overlapping peak occurs for CH3CN, and a double overlapping peak occurs for HNCO. This is all because that the generation side reactions of the three gases originate from the dehydrogenation and decomposition of different amides or cyclic amides formed from amino groups at different parts of arginine. The fourth evolution range of HCN is due to the decomposition and dehydrogenation of amide, pyridine, and other N-containing heterocycles at high temperatures, which are difficult to decompose under low temperatures. There is no generation of CH3CN and HNCO during the high temperature interval, which is respectively because no methyl or easily decomposable amide complexes exist in the product at high temperatures.
After Fe2O3addition, the first small HCN release range is suppressed, while the other two ranges tend to merge into one, accompanied by the trend of an initial increase followed by a later decrease. This is because Fe2O3suppresses the initial deamination reactions and side reactions of HCN generation at the same time. And some Fe-N complexes easy to decompose are decomposed into HCN[29-30]. Besides, Fe2O3catalyzes the initial cyclization reactions mentioned previously, and then HCN is generated through decomposition of the cycles at the later stage. Also, Fe2O3reduces the activation energy for the HCN generation reactions or catalyzes the generation of H and H2that are necessary for the generation of HCN at the later stage. And then, overdose Fe2O3can slightly suppress the generation of HCN at the later stage. In addition, the last evolution range of HCN is suppressed. This is because nitrogen is fixed to form Fe-N complexes or heterocyclic nitrogen products that are hard to decompose and enter into solid products[29-30].
After Fe2O3addition, the evolution of CH3CN increases firstly and decreases later, which is due to the fact that similar to glutamic acid mentioned previously. The impact of Fe2O3on the evolution of CH3CN from arginine is the result of all sorts of promotion and suppression effects. And the amount of HNCO is reduced. This is because the suppression effects due to the fact that the generation of Fe-N complexes[22, 28-34],etc. play the dominant role after Fe2O3addition.
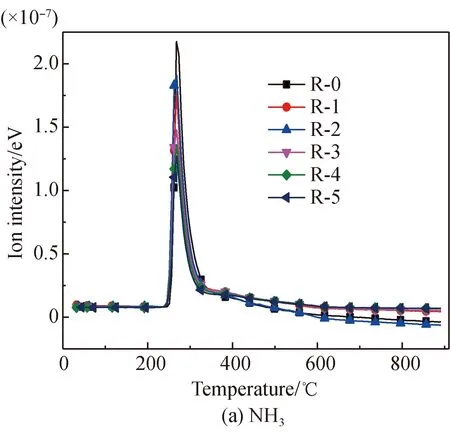
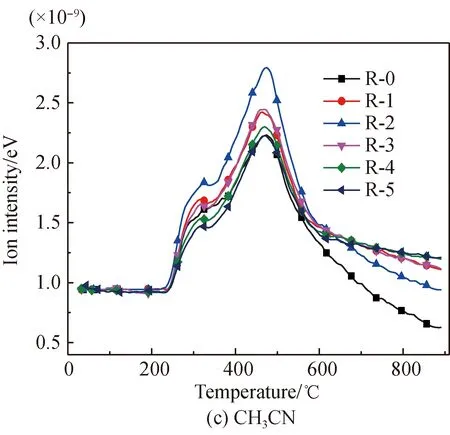
Fig. 4 N-containing gas release characteristics of arginine pyrolysis before and after Fe2O3 addition
The NH3evolution curves of proline pyrolysis before and after Fe2O3addition are shown in Fig. 5. It is found that, before Fe2O3addition, NH3only evolves in the temperature range of 246.2-397.2 ℃, with the peak temperature being at 273 ℃. NH3is generated from the deamination reaction of the cyclic amino group of proline during this range. HCN, CH3CN, and HNCO all start to evolve in trace amounts near 246.2 ℃, with the peak temperature being at 294.1 ℃, and the evolution amounts are far less than that of NH3.
After Fe2O3addition, the evolution of NH3occurs in the temperature ranges of 204-324.3 ℃ and 324.3-435.7 ℃, with peak temperatures respectively being approximately 262 ℃ and 368 ℃. In the first range, the evolution temperature and the evolution amount of NH3are reduced. This is because Fe2O3reduces the initial activation energy of the cyclic amino reactions, while the generation of Fe-N complexes reduces the evolution amount of NH3[22,28-34]. In the second range, NH3is generated through the decomposition of Fe-N complexes.
After Fe2O3addition, the evolution amounts of HCN, CH3CN, and HNCO greatly increase, with two temperature ranges and two peak temperatures almost equal to that of NH3. This is mostly because Fe2O3reduces the activation energy of the reactions of breakage and dehydrogenation of the five-membered ring of proline highly, and such products as amides decompose to HCN, CH3CN, and HNCO. Meanwhile, Fe-N complexes that are formed at the first stage decompose into HCN, CH3CN, and HNCO at the second stage. When Fe2O3is added at amounts up to 40% or 50%, there is a small amount of HCN evolved in the temperature range of 528 -612 ℃. This is because, with the existence of large amounts of Fe2O3, small amounts of heterocyclic complexes, which are difficult to decompose under low temperatures, decomposes into a small amount of HCN under higher temperatures. No CH3CN or HNCO presents in this high temperature range. This is because no methyl and easily decomposable amide complexes exist at high temperatures.
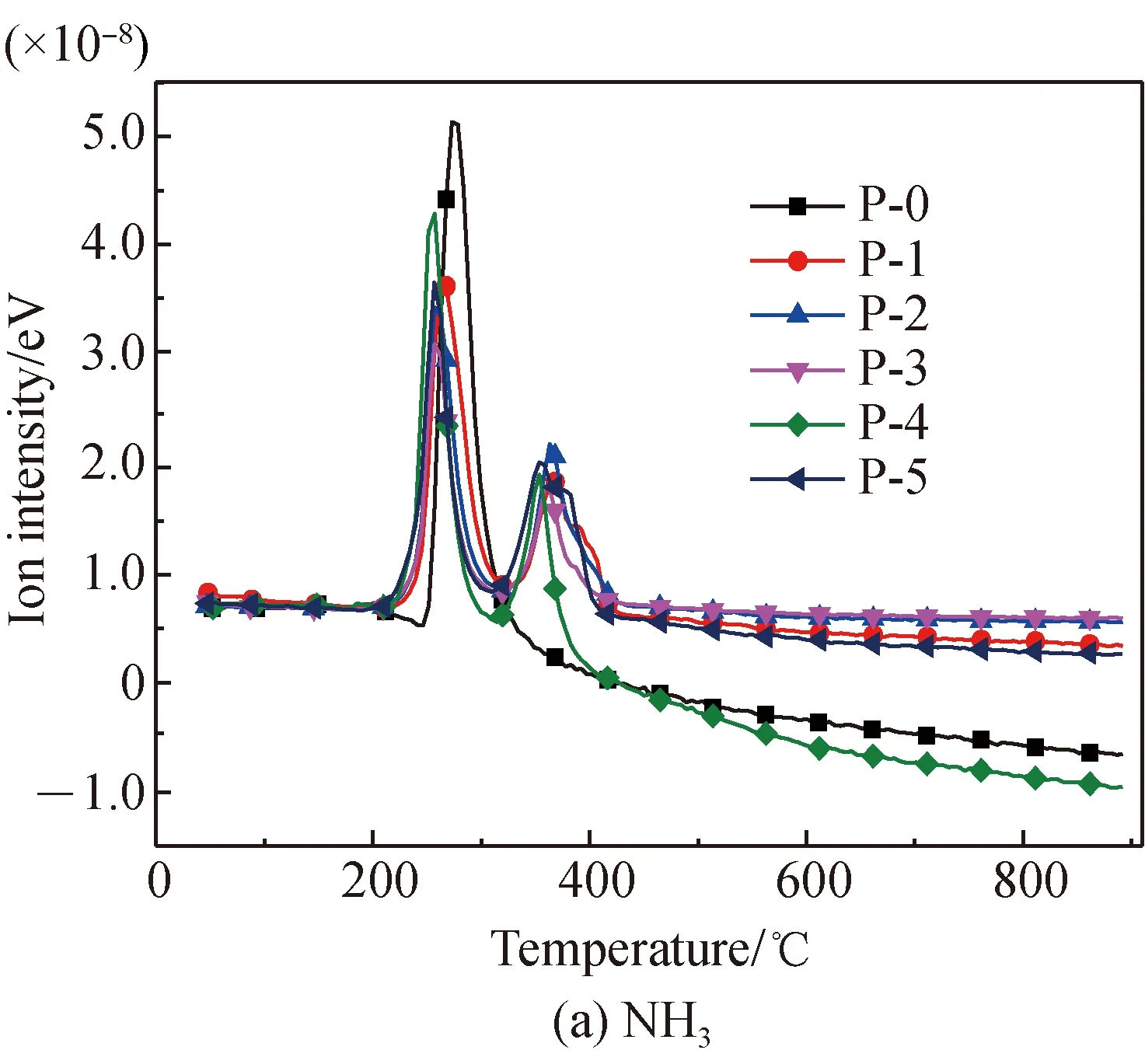
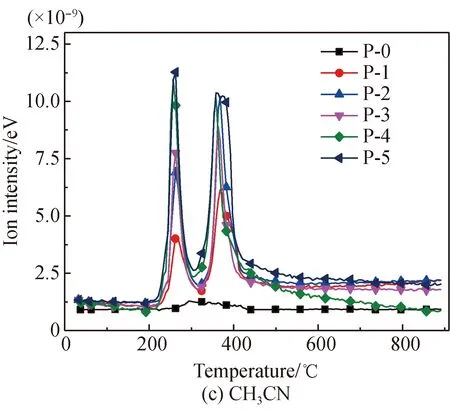
Fig. 5 N-containing gas release characteristics of proline pyrolysis before and after Fe2O3 addition
From all the discussions, Fe2O3presents two different influence mechanisms on the evolution of N-containing gases of different amino acids. The generation of Fe-N complexes[22,28-34]from the reactions of Fe2O3and amino acids or NH3, HCN, CH3CN and HNCO reduces the generation of NH3, HCN, CH3CN, and HNCO, while the catalysis effects of Fe2O3on the generation of H and H2play a promoting role in the generation of NH3, HCN, CH3CN, and HNCO[22, 30, 42-43]. The mechanism is shown in Fig. 6.
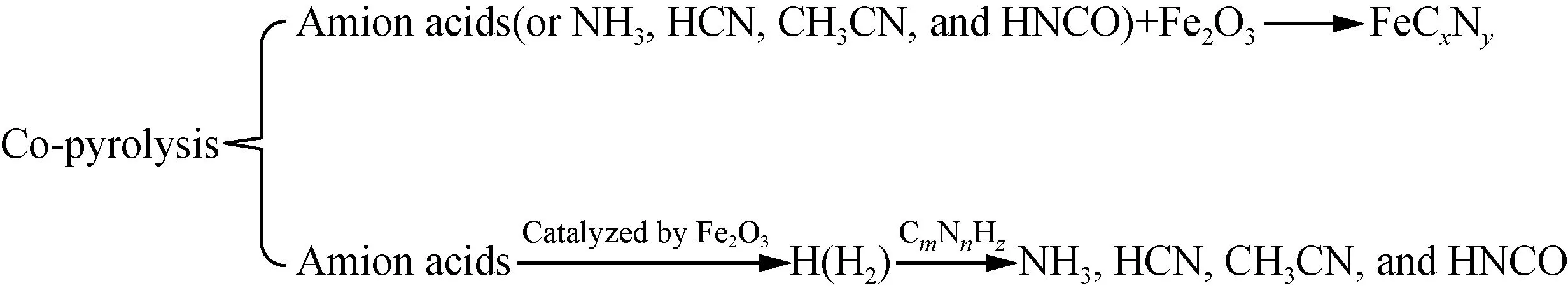
Fig. 6 Effects of Fe2O3 on the pyrolysis of amino acids
3 Conclusions
(1) Fe2O3can reduce the initial decomposition activation energy of proline and substantially change the pathway of its pyrolysis reaction. After Fe2O3addition, Fe-N complexes are generated, and continue to decompose under higher temperatures.
(2) The structures of arginine, glutamic acid, and proline determine their evolution capacities for N-containing gases such as NH3in descending order, and the evolution amounts of HCN, CH3CN, and HNCO are far less than that of NH3. In addition, the evolution amount of NH3is almost the same between glutamic acid and proline, while the NH3and HCN evolution amounts of glutamic acid are far less than that of argine and proline.
(3) Fe2O3presents two different influence mechanisms on the evolution of N-containing gases of different amino acids, and the dominant influence varies with different categories of amino acids and gases, evolution temperature ranges,etc.The generation of Fe-N complexes from the reactions of Fe2O3and amino acids or NH3,etc.reduces the generation of NH3, HCN, CH3CN, and HNCO, while the catalysis effects of Fe2O3on the generation of H and H2play a promoting role in the generation of NH3, HCN, CH3CN, and HNCO.
 Journal of Donghua University(English Edition)2021年4期
Journal of Donghua University(English Edition)2021年4期
- Journal of Donghua University(English Edition)的其它文章
- Influence Mechanism of Clothing Anchor Features on Consumers’ Purchase Intention
- Performance Evaluation of a Molten Carbonate Fuel Cell-Graphene Thermionic Converter-Thermally Regenerative Electrochemical Cycles Hybrid System
- Long Text Classification Algorithm Using a Hybrid Model of Bidirectional Encoder Representation from Transformers-Hierarchical Attention Networks-Dilated Convolutions Network
- Estimating Mechanical Vibration Period Using Smartphones
- Meta-Path-Based Deep Representation Learning for Personalized Point of Interest Recommendation
- Design and Characterization of Electrical Connections for Conductive Yarns
