Induction chemotherapy with albumin-bound paclitaxel plus lobaplatin followed by concurrent radiochemotherapy for locally advanced esophageal cancer
Mao-Hui Yan,Fang Liu,Bao-Lin Qu,Bo-Ning Cai,Wei Yu,Xiang-Kun Dai
Mao-Hui Yan,Fang Liu,Bao-Lin Qu,Bo-Ning Cai,Wei Yu,Xiang-Kun Dai,Department of Radiotherapy,The First Medical Center of the Chinese People's Liberation Army(PLA)General Hospital,Beijing 100853,China
Abstract BACKGROUNDAlbumin-bound paclitaxel(ABP)has been used as second- and higher-line treatments for advanced esophageal cancer,and its efficacy and safety have been well demonstrated.Lobaplatin(LBP)is a third-generation platinum antitumor agent;compared with the first two generations of platinum agents,it has lower toxicity and has been approved for the treatment of breast cancer,small cell lung cancer,and chronic granulocytic leukemia.However,its role in the treatment of esophageal cancer warrants further investigations.AIMTo investigate the efficacy and safety of induction chemotherapy with ABP plus LBP followed by concurrent radiochemotherapy(RCT)for locally advanced esophageal cancer.METHODSPatients with pathologically confirmed advanced esophageal squamous cell carcinoma(ESCC)at our hospital were enrolled in this study.All patients were treated with two cycles of induction chemotherapy with ABP plus LBP followed by concurrent RCT:ABP 250 mg/m2,ivgtt,30 min,d1,every 3 wk;and LBP,30 mg/m2,ivgtt,2 h,d1,every 3 wk.A total of four cycles were scheduled.The dose of the concurrent radiotherapy was 56-60 Gy/28-30 fractions,1.8-2.0 Gy/fraction,and 5 fractions/wk.RESULTSA total of 29 patients were included,and 26 of them completed the treatment protocol.After the induction chemotherapy,the objective response rate(ORR)was 61.54%,the disease control rate(DCR)was 88.46%,and the progressive disease(PD)rate was 11.54%;after the concurrent RCT,the ORR was 76.92%,the DCR was 88.46%,and the PD rate was 11.54%.The median progression-free survival was 11.1 mo and the median overall survival was 15.83 mo.Cox multivariate analysis revealed that two cycles of induction chemotherapy followed by concurrent RCT significantly reduced the risk of PD compared with two cycles of chemotherapy alone(P=0.0024).Non-hematologic toxicities were tolerable,and the only grade 3 non-hematologic toxicity was radiation-induced esophagitis(13.79%).The main hematologic toxicity was neutropenia,and no grade 4 adverse event occurred.CONCLUSIONInduction chemotherapy with ABP plus LBP followed by concurrent RCT is effective in patients with locally advanced ESCC,with mild adverse effects.Thus,this protocol is worthy of clinical promotion and application.
Key Words:Esophageal squamous cell carcinoma;Esophagus cancer;Induction chemotherapy;Concurrent radiochemotherapy;Radiotherapy;Chemotherapy;Albuminbound paclitaxel;Lobaplatin
INTRODUCTION
Esophageal cancer is one of the most common forms of cancer worldwide,with an estimated 572000 new cases and 509000 deaths in 2018[1,2].The most common type of esophageal cancer in China is esophageal squamous cell carcinoma(ESCC),which accounts for 89% of all esophageal cancer cases[3].The 5-year survival rate of Chinese ESCC patients is 20%-30% overall[4].The preferred treatment modality for esophageal cancer is surgery,but 80% of patients are no longer eligible for radical surgery upon diagnosis[5,6].Concurrent chemoradiotherapy has been found to yield better overall survival than radiotherapy[7-9].While definitive radiochemotherapy(RCT)remains the mainstay of treatment for locally advanced esophageal cancer[10],the treatment modalities have long been controversial.Several clinical trials have explored and evaluated the multidisciplinary treatments for advanced unresectable esophageal cancer,but there is still no standardized treatment protocol.In the COSMOS trial[11],the 1-year survival rate of patients with esophageal cancer treated by surgery after induction chemotherapy with docetaxel plus cisplatin and 5-fluorouracil(DCF)regimen was 67.9%,which confirmed the efficacy of induction chemotherapy followed by definitive RCT in treating esophageal cancer.Another multicenter randomized controlled trial(JCOG1510)[12]further confirmed that induction chemotherapy + surgery or induction chemotherapy + concurrent definitive RCT was superior to concurrent definitive RCT in terms of overall survival(OS)in patients with locally advanced unresectable ESCC.Paclitaxel combined with carboplatin is one of the standard chemotherapy regimens recommended in guidelines,but there is limited evidence for other taxanes and platinum agents.Nanoparticle albumin-bound(nab)-paclitaxel has been shown to provide significant efficacy and safety benefits over paclitaxel and is currently approved for the treatment of breast cancer,lung cancer,pancreatic cancer,and melanoma.In recent years,albumin-bound paclitaxel(ABP)has been used as second- and higher-line treatments for advanced esophageal cancer,and its efficacy and safety have been well demonstrated.Lobaplatin(LBP)is a thirdgeneration platinum antitumor agent;compared with the first two generations of platinum agents,it has lower toxicity and has been approved for the treatment of breast cancer,small cell lung cancer,and chronic granulocytic leukemia.However,its role in the treatment of esophageal cancer warrants further investigations.
Thus,we conducted the present prospective study to investigate the efficacy and safety of induction chemotherapy with ABP plus LBP followed by concurrent RCT in the treatment of locally advanced esophageal cancer.
MATERIALS AND METHODS
Subjects
ESCC patients attending our hospital were included according to the following inclusion criteria:(1)Patients voluntarily participated in this study,had good compliance,could cooperate with the trial requirements during the observation and follow-up periods,and signed an informed consent form;(2)Patients with histopathologically confirmed stage III or IVA unresectable advanced ESCC,which had not been treated with anti-tumor drugs other than the study drug within the past 4 wk;and the patient could receive the specialized anti-tumor treatment;(3)Patients with at least one measurable lesion[≥ 1 cm on computed tomography(CT)or ≥ 2 cm on other imaging modes];(4)Patients with an Eastern Cooperative Oncology Group score of ≤ 2 and having indications for chemotherapy;(5)Patients who lost ≤ 10% of the body weight in the last 6 mo and could tolerate radiotherapy;(6)Males or females aged 18-76 years;(7)Patients with the following laboratory-confirmed bone marrow,liver,kidney,and heart functions within 7 d before the first dose:White blood cell count ≥ 3000/μL,absolute neutrophil count ≥ 1500/μL,platelet count ≥ 100000/μL,and hemoglobin ≥ 9.0 g/dL;aspartate aminotransferase and alanine aminotransferase ≤ 2.5 times upper limit of normal(ULN),and alkaline phosphatase ≤ 4 times ULN,and total bilirubin ≤ 1.5 times ULN;serum creatinine ≤ 1.5 times ULN and blood urea nitrogen ≤ 2.5 times ULN;prothrombin time and/or international normalized ratio or partial thromboplastin time ≤ 1.5 times ULN;and left ventricular ejection fraction ≥ 60% as assessed by Doppler ultrasound,and electrocardiographic findings were basically normal;and(8)Females must use contraception during the treatment and within 6 mo upon the completion of the treatment,and they should not be pregnant or lactating;and males must take birth control measures during the treatment and within 6 mo upon the completion of the treatment.
The exclusion criteria were:(1)Patients with severe acute infection,purulent/ chronic infection,or protracted wound healing;(2)Patients with esophageal perforation(e.g.,with existing or possible tracheoesophageal fistula),which had shown obvious symptoms and multiple distant metastases;(3)Patients who had abnormal coagulation and/or bleeding tendency(e.g.,active peptic ulcers)or were receiving a thrombolytic or anticoagulant therapy;(4)Patients with pre-existing severe cardiac disease,including:Congestive heart failure,uncontrollable high-risk arrhythmia,unstable angina pectoris,myocardial infarction within 6 mo,severe heart valve disease,and resistant hypertension;(5)Patients with uncontrollable neurological or psychiatric diseases or mental disorders;the patients had poor compliance and were unable to follow the treatment protocol or describe their treatment responses;and(6)Patients with severe cirrhosis and/or severe renal insufficiency.
Treatments
All patients were treated with two cycles of induction chemotherapy with ABP(Keaili,produced by CSPC Ouyi Pharmaceutical Co.,Ltd)combined with LBP:ABP 250 mg/m2,ivgtt,30 min,d1,every 3 wk;and LBP,30 mg/m2,ivgtt,2 h,d1,every 3 wk.Concurrent RCT was given after the induction chemotherapy.Intensity-modulated radiation therapy(56-60 Gy/28-30 fractions,1.8-2.0 Gy/fraction,5 fractions/wk)was applied as the radiotherapy.Two cycles of chemotherapy was applied on days 1 and 21 of radiotherapy,and the chemotherapy regimen was the same as that in the induction chemotherapy.
Evaluation of efficacy and adverse events
Chest CT,magnetic resonance imaging,and positron emission tomography-CT(if necessary)were performed after induction chemotherapy,1 mo after concurrent RCT,and at each follow-up visit.The efficacy was evaluated using the benchmarks of Response Evaluation Criteria in Solid Tumors version 1.1,which included complete response(CR),partial response(PR),stable disease(SD),and progressive disease(PD);the objective remission rate(ORR)was calculated using the following formula:ORR=(CR + PR)/total cases × 100%.
The adverse events were graded according to the US NCI Common Terminology Criteria for Adverse Events version 3.0.Adverse events occurring during the study period were recorded continuously.
After the completion of treatment,all patients were followed by telephone or outpatient visits.
Primary and secondary outcome measures
The primary outcome measure was progression-free survival(PFS).The secondary outcome measures included OS,ORR after induction chemotherapy,ORR after concurrent RCT,correlations with prognostic factors,and adverse events.
Statistical analysis
Examination indicators at baseline were described.Count data are presented with the number and percentage of cases;for measurement data,the mean,standard deviation,median,and maximum and minimum values were calculated.For the efficacy indicators,ORR is presented using the number and percentage of cases and PFS and OS are described using Kaplan-Meier curves;the median and 95% confidence interval(CI)were calculated,and factors affecting disease progression were explored using COX multivariate regression,in which hazard ratio(HR)and 95%CI are listed.The types and severity of adverse events during the trial were described,and the incidence of adverse events was calculated(presented as number and percentage of cases).All statistical analyses were performed using SPSS 19.0 software package.
RESULTS
Treatment completion
A total of 29 patients with locally advanced ESCC were included in this study between April 2019 and October 2020,and the baseline data of these patients are shown in Table 1.Three patients withdrew from the study because of surgery(n=1),PD after enrollment without treatment(n=1),and loss to follow-up(n=1).All patients(100%)completed the induction chemotherapy.The completion rate of the entire study protocol(2 cycles of induction chemotherapy + 2 cycles of concurrent RCT)was 65.38%(n=17).Five patients(19.23%)underwent two cycles of induction chemotherapy + one cycle of concurrent RCT,and the reasons for not completing the entire protocol were grade III radiation-induced esophagitis in three cases and grade III myelosuppression in two.Four(13.79%)patients underwent two cycles of induction chemotherapy only,among whom three experienced disease progression and one refused to receive concurrent radiotherapy.
Short-term efficacy
After two cycles of induction chemotherapy,ORR was 61.54%(n=16);the disease control rate(DCR)was 88.46%(n=23),among which PR was achieved in 16(61.54%)cases and SD in 7(26.92%);PD was noted in three(11.54%)cases,includingin situprogression(n=1),mediastinal lymph node progression(n=1),and esophagotracheal fistula(n=1).Among patients who completed induction chemotherapy + concurrent RCT,the evaluated ORR was 76.92%(n=20)and the DCR was 88.46%(n=23),among which PR was achieved in 20(76.92%)cases and SD in 3(11.54%);PD was noted in three(11.54%)cases(Table 2).
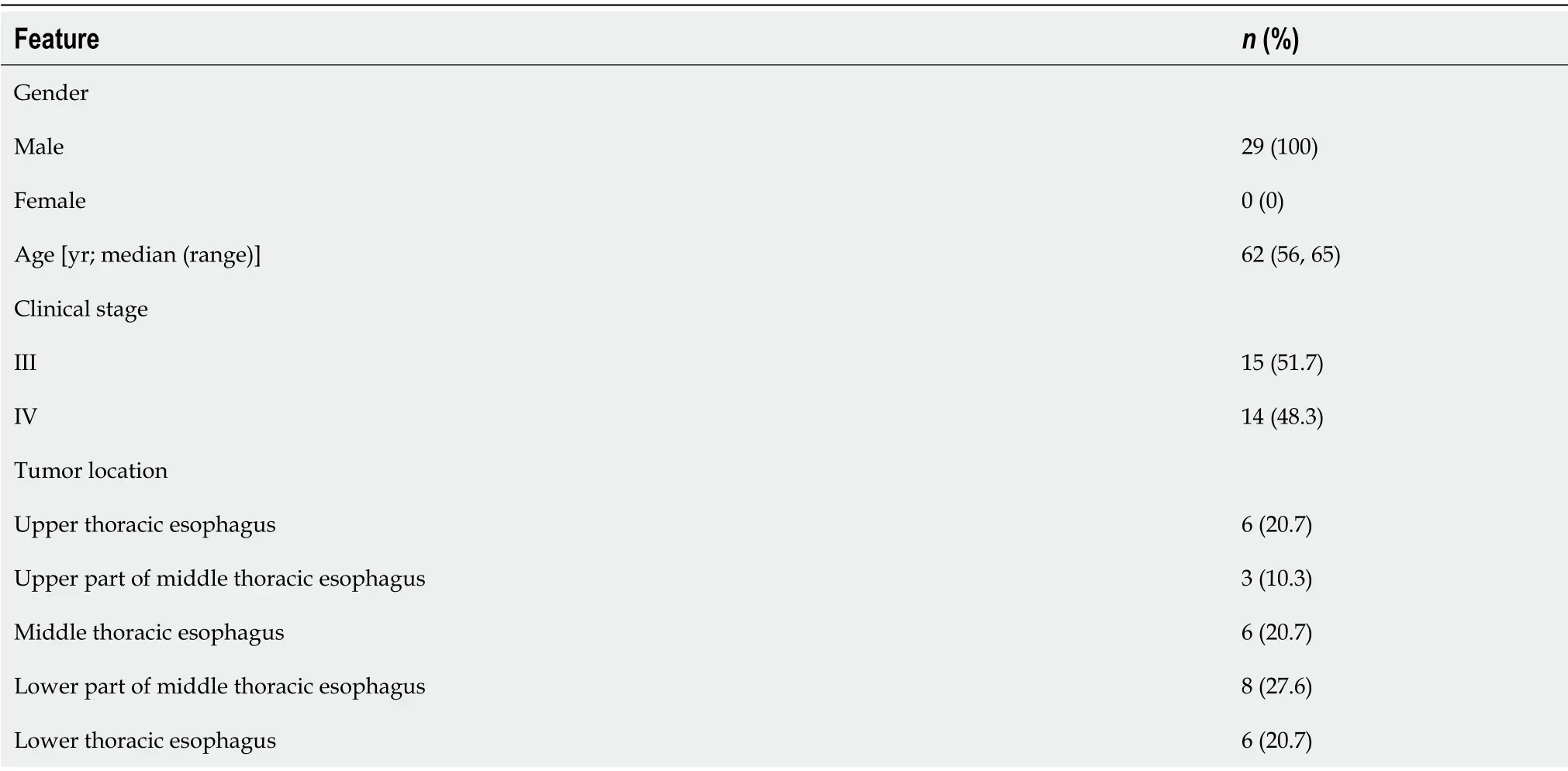
Table 1 Baseline characteristics of patients
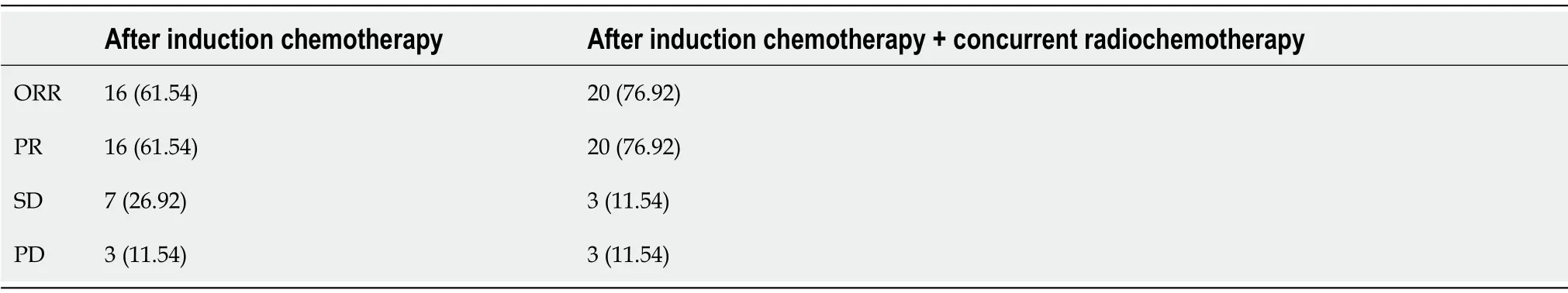
Table 2 Short-term efficacy,n(%)
Follow-up and long-term outcomes
By October 2020,29 patients had been followed for a median of 15.28(5-28)mo.Disease progression occurred in 14(48.28%)patients,including four(17.24%)cases ofin situprogression,two(6.90%)cases of lymph node metastasis,and seven(24.14%)cases of distant metastasis[including two(6.90%)cases of liver metastasis,one(3.45%)case of brain metastasis,three(10.34%)cases of lung metastasis,and one(3.45%)caseof spleen and kidney metastases].Thirteen patients died.The median PFS was 11.1 mo,the median OS was 15.83 mo,and the 1-year OS was 42%(Figure 1).
Adverse events and safety
The incidence of post-treatment hematologic toxicities is as follows.After the treatment,the incidence of grade 3 anemia,leukopenia,neutropenia,and thrombocytopenia was 0%,10.35%,6.9%,and 0%,respectively.The incidence of non-hematologic toxicities including decreased appetite,fatigue,radiation-induced esophagitis,decreased body weight,and abnormal liver function was 13.79%,13.79%,34.48%,3.45%,and 3.45%,respectively.These non-hematologic toxicities were generally grade 1 or 2;the only grade 3 non-hematologic toxicity was radiation-induced esophagitis,and no grade 4 toxicity was noted(Table 3).
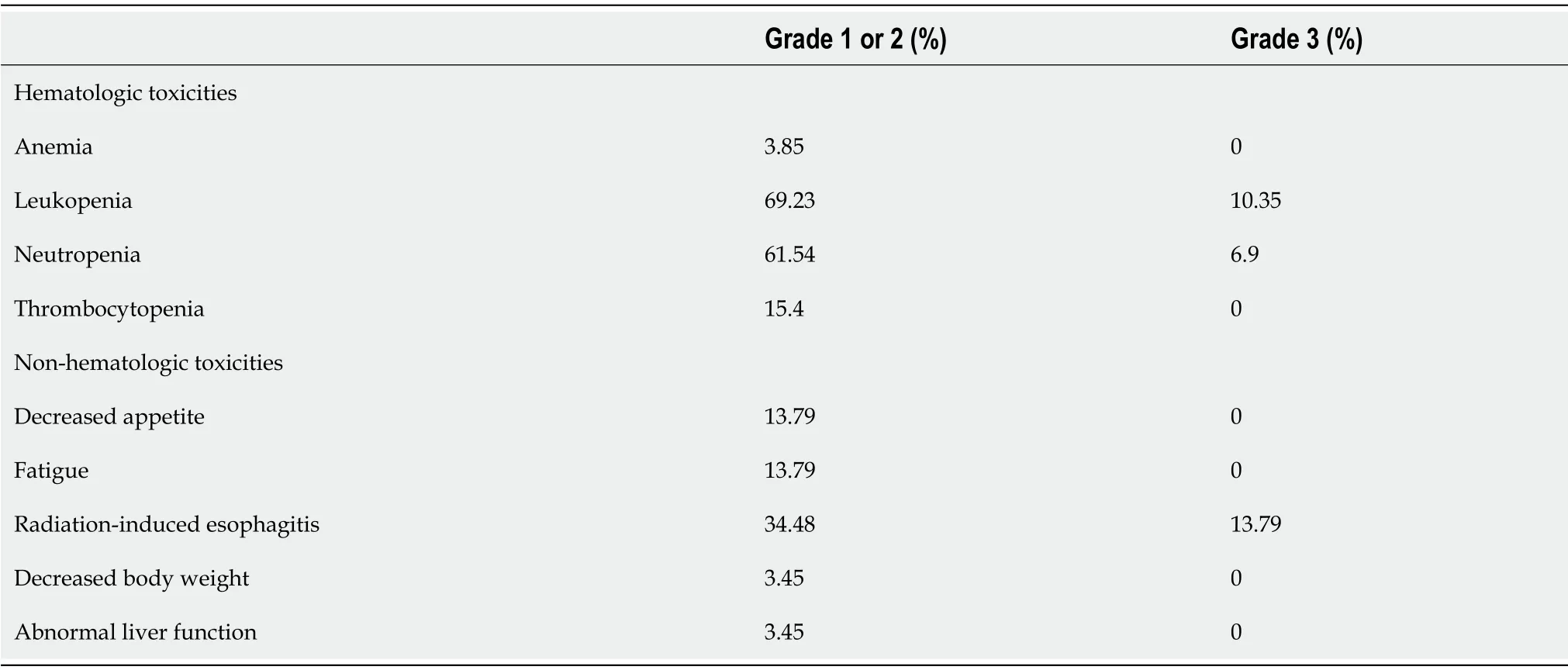
Table 3 Adverse events(n=29)
Results of multivariate Cox analysis
The number of chemotherapy cycles was a statistically significant factor affecting the prognosis(P=0.0024).Patients with esophageal cancer who received three(HR=0.0555;95%CI:0.0066-0.4668)or four cycles of chemotherapy(HR=0.0043;95%CI:0.0002-0.0992)had a significantly lower risk of disease progression compared to those who received only two cycles of induction chemotherapy.
The prognostic impact of the overall nutritional score was not statistically significant(P=0.0826);however,compared to patients with an initial score of 5,patients with an initial score of 1(HR=0.0037;95%CI:0.0001-0.2715),3(HR=0.0077;95%CI:0.0001-0.6176),and 4(HR=0.0131;95%CI:0.0002-0.7049)had better prognoses(Figure 2).
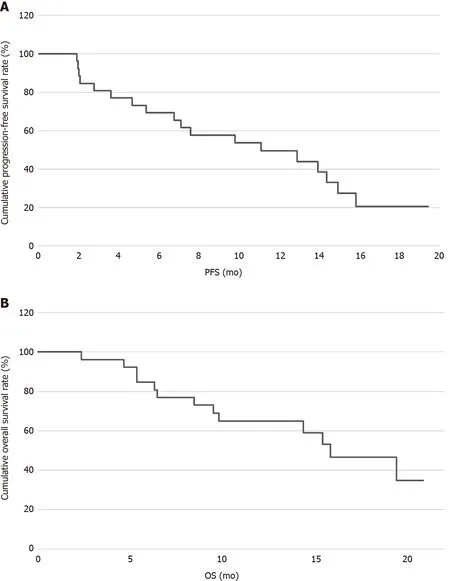
Figure 1 Cumulative progression-free survival and overall survival.A:1-year progression-free survival;B:1-year overall survival.PFS:Progression-free survival;OS:Overall survival.
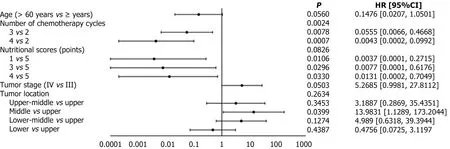
Figure 2 Multivariate Cox analysis.
DISCUSSION
The efficacy of induction chemotherapy followed by definitive RCT in treating esophageal cancer has been proved in recent years.In the COSMOS trial[11],the 1-year survival rate of patients with esophageal cancer treated by surgery after induction chemotherapy with DCF regimen reached 67.9%.Another multicenter randomized controlled trial(JCOG1510)[12]further confirmed that induction chemotherapy + surgery or induction chemotherapy + concurrent definitive RCT was superior to concurrent definitive RCT in terms of OS in patients with locally advanced unresectable ESCC.In the current study,the ORR after two cycles of induction chemotherapy with ABP combined with LBP was 61.54%,with a DCR of 88.46%;100% of our subjects completed the induction therapy without severe hematologic toxicities or chemotherapy-induced nausea and vomiting.Twenty-two patients further received concurrent RCT;65.38% of them completed induction chemotherapy plus concurrent RCT,while 19.23% received one cycle of concurrent RCT due to radiation-induced esophagitis or myelosuppression.The ORR was 76.92% and disease control rate was 88.46% after the completion of induction chemotherapy followed by concurrent RCT,which were comparable to those of previous studies;the median PFS was 11.1 mo and the median OS was 15.83 mo;notably,the 1-year PFS rate was 49.45% and the 1-year OS rate was 64.96%.Cox multivariate analysis of the efficacy and prognosis concluded that two cycles of induction chemotherapy followed by concurrent RCT significantly reduced the risk of disease progression compared with two cycles of chemotherapy only(P=0.0024),suggesting that induction chemotherapy combined with definitive RCT is an efficacious and well-tolerated treatment modality in patients with esophageal cancer.With fewer and milder toxicities,it enhances chemotherapy tolerability and prolongs survival.
Wanget al[13]demonstrated that weekly nab-paclitaxel plus cisplatin with concurrent definitive radiotherapy is an effective and well-tolerated treatment option for ESCC.In addition,Wanget al[14]compared the values of nanoparticle albuminbound paclitaxel plus cisplatin(nab-TP)vssolvent-based paclitaxel plus cisplatin(sb-TP)and found that nab-TP demonstrated a higher ORR(50%vs30%,P=0.082)and disease control rate(81%vs65%,P=0.124)than sb-TP,as well as a longer median PFS(6.1 mo,95%CI:5.3-6.9)(P=0.029).In contrast,LBP used in our current study is a newgeneration platinum drug.Many studies have shown that LBP has therapeutic efficacy in the treatment of advanced esophageal cancer,with tolerable side effects[15-17].Among patients who received induction therapy plus concurrent RCT,the incidence of grade 3 leukopenia and neutropenia was 10.35% and 6.9%,respectively.The nonhematologic toxicities including decreased appetite,fatigue,weight loss,and abnormal liver function were generally grade 1 or 2;the only grade 3 non-hematologic toxicity was radiation-induced esophagitis,and no grade 4 toxicity was noted.Thus,ABP combined with LBP has acceptable adverse effects and can achieve good long-term survival in patients with locally advanced ESCC when used either as an induction chemotherapy regimen or as a concurrent chemotherapy regimen.
However,although RCT is the main treatment modality for patients with stage II-III esophageal cancer who refuse surgery or has a contraindication for surgery and in patients with locally advanced unresectable(stage IVa)esophageal cancer[18,19],different patterns of recurrence and metastasis still occur.Sudoet al[20]reported the types of recurrence after definitive chemoradiotherapy:The incidence of luminal relapse,regional relapse,distant metastasis,new cancer diagnosed by esophagogastroduodenoscopy(NC-E),and new cancer other than NC-E(NC-O)was 14%,6%,19%,17%,and 8%,respectively.In the present study,disease progression occurred in 14(48.28%)patients,including five(17.24%)cases ofin situprogression,two(6.90%)cases of lymph node metastasis,and eight(27.59%)cases of distant metastasis[including two(6.90%)cases of liver metastasis,one(3.45%)case of brain metastasis,three(10.34%)cases of lung metastasis,and one(3.45%)case of spleen and kidney metastases].The results of this study also suggested that nutritional scores before and during treatment had an impact on prognosis,which needs to be further verified in studies with larger sample sizes.
CONCLUSION
In conclusion,induction chemotherapy with ABP plus LBP followed by concurrent RCT is effective in patients with locally advanced ESCC,with mild adverse effects.Thus,this protocol is worthy of clinical promotion and application.However,as an interim report,this study was limited by its short follow-up intervals,and patients’ survival and tumor recurrence/metastasis need to be further investigated.In addition,the induction chemotherapy regimen as well as the optimal dose and fractionation schedule of the definitive RCT for ESCC deserves further clinical research.
ARTICLE HIGHLIGHTS
Research background
The most common type of esophageal cancer in China is esophageal squamous cell carcinoma(ESCC),which accounts for 89% of all esophageal cancer cases.The 5-year survival rate of Chinese ESCC patients is 20%-30% overall.The preferred treatment modality for esophageal cancer is surgery,but 80% of patients are no longer eligible for radical surgery upon diagnosis.
Research motivation
We conducted the present prospective study to investigate the efficacy and safety of induction chemotherapy with albumin-bound paclitaxel(ABP)plus lobaplatin(LBP)followed by concurrent radiochemotherapy(RCT)in the treatment of locally advanced esophageal cancer.
Research objectives
This study aimed to investigate the efficacy and safety of induction chemotherapy with ABP plus LBP followed by concurrent RCT for locally advanced esophageal cancer.
Research methods
Patients with pathologically confirmed advanced ESCC were enrolled in this study.All patients were treated with two cycles of induction chemotherapy with ABP plus LBP followed by concurrent RCT.A total of four cycles were scheduled.
Research results
Cox multivariate analysis revealed that two cycles of induction chemotherapy followed by concurrent RCT significantly reduced the risk of progressive disease compared with two cycles of chemotherapy alone.Non-hematologic toxicities were tolerable,and the only grade 3 non-hematologic toxicity was radiation-induced esophagitis.The main hematologic toxicity was neutropenia,and no grade 4 adverse event occurred.
Research conclusions
Induction chemotherapy with ABP plus LBP followed by concurrent RCT is effective in patients with locally advanced ESCC,with mild adverse effects.Thus,this protocol is worthy of clinical promotion and application.
Research perspectives
As an interim report,this study was limited by its short follow-up intervals,and patients’ survival and tumor recurrence/metastasis need to be further investigated.
 World Journal of Gastrointestinal Oncology2021年11期
World Journal of Gastrointestinal Oncology2021年11期
- World Journal of Gastrointestinal Oncology的其它文章
- Hepatocellular carcinoma biomarkers,an imminent need
- Anatomical vs nonanatomical liver resection for solitary hepatocellular carcinoma:A systematic review and meta-analysis
- Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma:A systematic review
- Cell-free DNA liquid biopsy for early detection of gastrointestinal cancers:A systematic review
- Colorectal cancer in Arab world:A systematic review
- Genetic variation of TGF-ΒR2 as a protective genotype for the development of colorectal cancer in men
