Carrier and magnetism engineering for monolayer SnS2 by high throughput first-principles calculations*
Qing Zhan(詹慶) Xiaoguang Luo(羅小光) Hao Zhang(張皓) Zhenxiao Zhang(張振霄)Dongdong Liu(劉冬冬) and Yingchun Cheng(程迎春)
1Key Laboratory of Flexible Electronics&Institute of Advanced Materials,Jiangsu National Synergetic Innovation Center for Advanced Materials,Nanjing Tech University,Nanjing 211816,China
2Frontiers Science Center for Flexible Electronics(FSCFE),Shaanxi Institute of Flexible Electronics(SIFE)&Shaanxi Institute of Biomedical Materials and Engineering(SIBME),Northwestern Polytechnical University(NPU),Xi’an 710129,China
Keywords: SnS2,high throughput first-principles calculations,carrier,magnetism
1. Introduction
Tin disulfide(SnS2)is van der Waals semiconductor with an indirect band gap of 1.58 eV.[1]Free-standing SnS2monolayer can be prepared by mechanical exfoliation,[2]liquid exfoliation,[3]as well as chemical vapor deposition. SnS2has been demonstrated for various applications such as solar cells,[4]field-effect transistors,[5]lithium-ion batteries,[6]photothermal therapeutics,[7]catalysis and sensors.[8,9]To extend its application,chemical doping has been proposed experimentally and theoretically as an effective strategy.[10-14]For example,SnS2monolayer doped with Fe has been verified as a two-dimensional(2D)dilute magnetic semiconductor.[15-17]Till now various chemical doping, such as Li, B, C, N,O, F,Na, Mg, Al, P, Cl, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn,Ga, As, Se , Br, Sr, Y, Zr, Nb, Mo, Tc, Ru ,Rh, Pd, Ag, Cd,In, Sn, Te, I, W, Re, Os, Ir, Pt, Au and Hg have been investigated by first-principles calculations.[1,18-23]However,there are some other elements doping absent,such as Be,Si,S,Sc,Ge, Rb, Sb, Ba, Lu, Hf, Ta, Tl, Pb, Bi, Po, At, Fr and Ra. In addition,debates remain. For example,Li and Na doped SnS2is predicted to be magnetic,[24]which is different from nonmagnetic results.[18]Therefore,a comprehensive investigation of chemical doping in SnS2is necessary.[25]
In this paper, we systematically investigate the stability,electronic structure and magnetic properties of monolayers SnS2doped with foreign elements,based on high-throughput first-principles calculations. We firstly screen out the elements which can be effectively doped in SnS2with high stability.Then the effect of dopants on carrier in SnS2is discussed.More importantly,we find that monolayer SnS2systems doped with elements in VIB,VIIB,VIIIB8,and VIIIB9 groups show large binding energies with non-zero magnetic moment,indicating high possibility of fabrication experimentally and application in spintronics.Our present work provides a comprehensive view regarding chemical doping in SnS2and that predicts chemical doping is an effective strategy to functionalize SnS2for various applications, such as field effect transistor, optoelectronic device and spintronic technology.
2. Computational details
First-principles calculations are carried out by using the projector-augmented wave method implemented in the Viennaab initiosimulation package (VASP) based on density functional theory.[26]The Perdew-Burke-Ernzerhof(PBE) functional of the generalized gradient approximation(GGA) is adopted for electron exchange and correlation interactions.[27,28]The van der Waals interactions between layers are corrected using the DFT-D3 functional.The kinetic energy cut-off of the wave function is set to 500 eV.To mimic the defects in monolayer SnS2, a 4×4×1 supercell is used and a 4×4×1k-mesh sampling is applied. The ions relaxation is achieved until the force for per atom is less than 0.01 eV/?A and the total energy converges to 10?5eV.A vacuum spacing of 20 ?A is used to prevent interaction between adjacent slabs.
3. Results and discussion
3.1. Structural properties and binding energies
Pristine monolayer SnS2is hexagonal with space groupD3d(Pˉ3m1). The optimized lattice constants of the 4×4×1 supercell area=b=14.80 ?A.When removing one Sn/S atom from the monolayer SnS2supercell,a VSn/VSwill be created with corresponding vacancy concentration of 6.25%/3.125%.Figure 1 shows the structure of a 4×4×1 SnS2supercell doped byXSnandXS.

Fig. 1. Top view of the relaxed structures for (a) pristine, (b) Sn-vacant monolayer SnS2, (c) single XSn doped monolayer SnS2, (d) S-vacant monolayer SnS2, (e) single XS doped monolayer SnS2 (XSn and XS indicate Sn and S substitutions). The purple, yellow, green and orange balls denote the Sn,S,XSn and XS atoms,respectively.
3.2. Binding energy
To check the stability of different dopants,we calculated the binding energies for various doping systems, as summarized in Fig. 2. The binding energyEbis defined asEb=Ev+μ?Ed,whereEvis the energy of a relaxed SnS2monolayer with one Sn/S vacancy,μis the energy of the doped atom,andEdis the energy of a relaxed SnS2monolayer with one Sn/S atom replaced by aXSn/XSatom.
Figure 2(a)shows the binding energy of main group metal doped in SnS2. Only the binding energy of Al and Si is higher than that of Sn, indicating Al and Si are energetically favorable doped in SnS2. The binding energies of Be, Ca, Sr, Ba,Ga, Ge, Pb, In, and Sb are close to that of Sn, suggesting the high possibility of doping those different elements in SnS2. It has been reported that Al and Sr can be doped in SnS2by a simple soft chemical route.[29,30]Therefore,it is expected that Si, Be, Ca, Ba, Ga, Ge Pb, In and Sb can be verified experimentally in future.
Figure 2(b) shows the binding energies for non-metal doped SnS2. Taking the binding energy of S doping as a reference,we can claim that B,C,O and F are easy to be doped in SnS2. The C doped SnS2has been fabricated by using hydrothermal method and demonstrated as a high-efficient solar fuel catalyst under visible light.[31]Because Se and Te can also replace S in SnS2,[23]we can estimate that N, Se, and Cl are possible to be doped in SnS2from the binding energy shown in Fig.2(c). However,there are few reports regarding B,O,N,and Cl doping in SnS2.
Figure 2(c)shows the binding energy of transition metal doped in SnS2. Take the binding energy of Sn as a reference, we can find that Sc, Y, Lu, Ti, Zr, Hf, V, Nb, Ta,Cr, Mo, W, Mn, Tc, Re, Fe, Ru, Os, Co, Rh, Ir, Ni and Pt can be doped in SnS2. Ni, Co and Mn doped SnS2have been synthesized by solvothermal method and can be applied for supercapacitors.[32]Fe, Cr and Y doped SnS2have also been successfully prepared by thermolysis method.[31,33]V and W doped SnS2have been demonstrated by spray pyrolysis technique.[34]Therefore,our prediction is consistent with previous experimental work.
Figure 3(a) shows the spin polarized DOS of pristine monolayer SnS2. The band gap is 1.487 eV according to DFT calculation. Si has same valence electrons as Sn, and therefore, SiSnis isovalent doping, which will induce band gap change without defect states.The DOS shown in Fig.3(b)confirms this assumption and SiSnwill induce the change of band gap(1.367 eV).Because Al atom has one less electron than Sn atom,it is expected a p-type doping for Al substitution. From the DOS shown in Fig.3(b),we can clearly observe the Fermi level cross the band edge states of the valence band,which is the signature of p-type doping. We expect SbSndefect will induce n-type doping because Sb has one more valence electron than Sn. However,the defect states locate in the middle of the band gap and the Fermi level is far from the conduction band or valence band edge as shown in Fig. 3(d). Therefore, SbSndefect will introduce trap states in SnS2, which is consistent with previous experimental and theoretical results.[35,36]
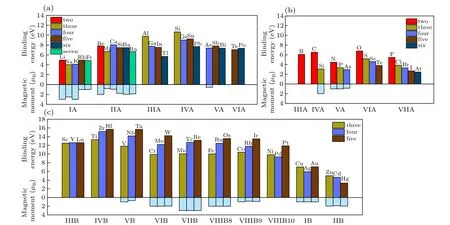
Fig.2. Binding energies and magnetic moments of monolayer SnS2 doped by(a)group A metal atoms from the period IA to VIA,(b)nonmetal from IIIA to VIIA,(c)group B metal atoms from IB to VIIIB.
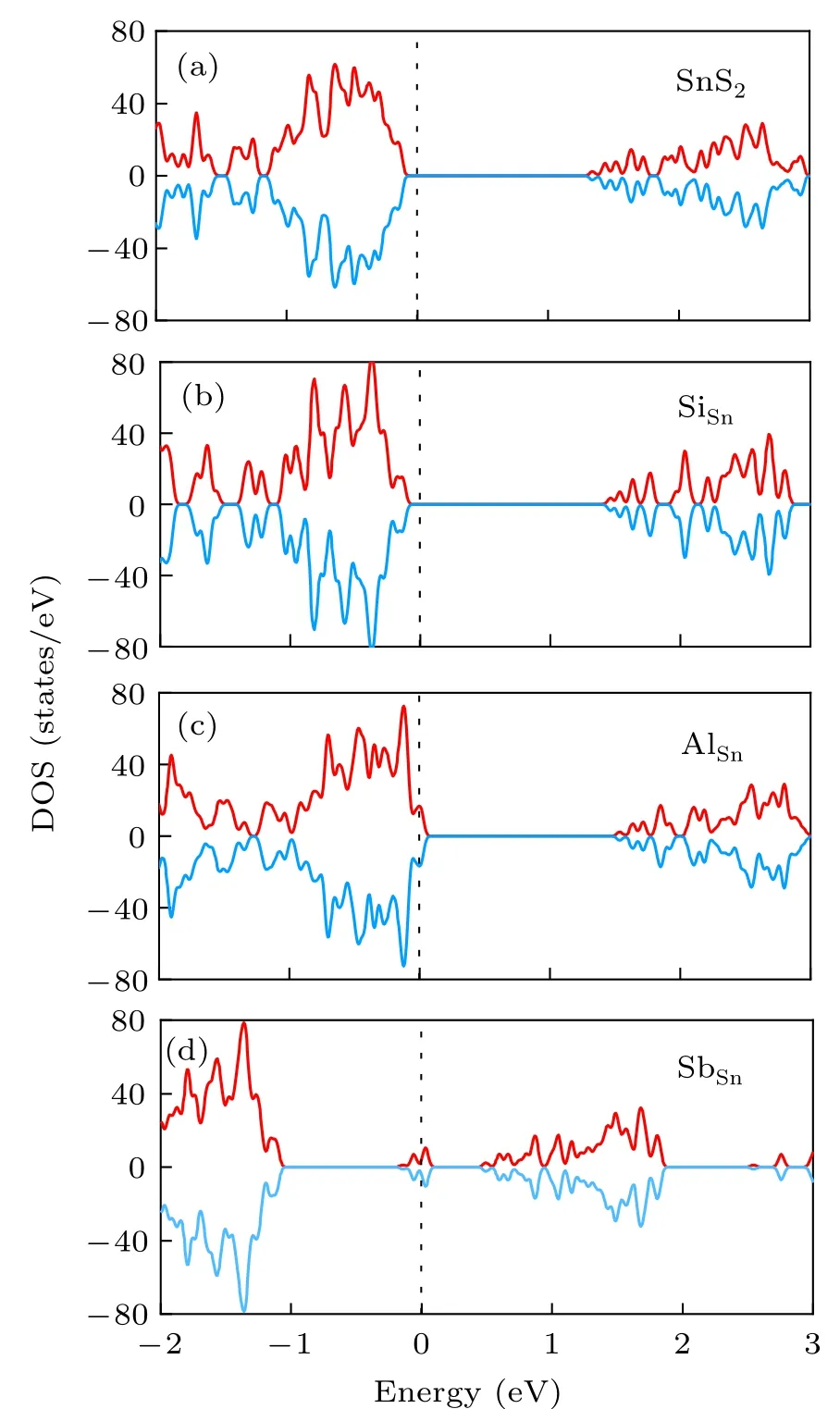
Fig.3. Spin polarized density of states of(a)pristine,(b)SiSn,(c)AlSn,and(d)SbSn doped monolayer SnS2.

Fig.4. Spin polarized density of states of(a)CS,(b)FS,and(c)OS doped monolayer SnS2.
Figure 4(a) shows the DOS for SnS2with CSdefect.There is an occupied defect state above the valence band edge,indicating the p-type doping of CS.In contrast,FSdefect leads to a defect state close to the conduction band edge,indicating a n-type doping scenario,as shown in Fig.4(b).As a typical isovalent doping,OSleads to the change of band gap(1.454 eV)without defect states in the band gap as shown in Fig. 4(c).Similarly, SeSand TeSalso induce change of band gap without defect states.
The valence state of transition metal ranges from +2 to+5. Therefore, it is also expecting p-type and n-type doping by transition metals substitution. Figure 5(a) shows the band structure of Hf doped SnS2,which evidently shows there is no defect state in the band gap. The absence of defect state can be attributed to the same valent state of Hf as that of Sn in SnS2. In addition, both HfS2and monolayer SnS2are in the same space group and the in-plane lattice parameters for each compound are similar. Because of the band gap of monolayer HfS2is smaller than that of SnS2, the doping of Hf in SnS2will reduce the band gap of SnS2. The band gap of Hf doped SnS2with doping concentration of 6.25%is 1.228 eV,which is smaller than that of pristine SnS2. We also can find that Zr,Pt,Pd,and Ni have the similar effect to Hf. The DOS for ScSnin Fig.5(b)shows that the Fermi level is on the valence band edge, indicating a p-type doping nature. YSnand LuSnhave the same scenario.

Fig. 5. Spin polarized density of states of (a) HfSn, and (b) ScSn, doped monolayer SnS2.
For non-metal doping, the dopant with high binding energy is absent of magnetic moment. There are some dopants with non-zero magnetic moment, such as NS, PSand AsS.However, the binding energies for those dopants are low, indicating difficulty of formation. For dopant from IA group,the binding energy is low though magnetism is introduced by Li,Na,K doping. The introduction of magnetism by elements in IA group is consistent with previous calculation.[24]For dopant in IIA group,the formation energies are larger than that in IA group. The magnetic moments for those dopants range from 1.0μBto 2.0μB. Figure 6(a)shows the DOS of monolayer SnS2with CaSndefect. The spin up and spin down DOS are unsymmetrical, indicating that Ca doping possibly leads to magnetism in SnS2. Moreover, the Fermi level crosses the valence band edge,suggesting p-type doping.
For transition metal doping,the dopant from VIB,VIIB,VIIIB8 or VIIIB9 groups is with local magnetic moment and has high binding energies. The magnetic moments are around 2.0μB,3.0μB,2.0μBand 1.0μBfor single dopant from VIB,VIIB,VIIIB8 and VIIIB9 groups,respectively.Magnetism has been predicted/observed in 2D transition metal doped monolayer 2D semiconductors, such as Mn doped MoS2,[37]Fe doped SnS2.[16]Figure 6(b)shows the spin polarized DOS of Mn doped SnS2. Though Re and Mn are in the same group and magnetic moments both are~3.0μB, the spin polarized DOS of Re doped SnS2shown in Fig. 6(c) is quite different from that of Mn doped SnS2. There are two more gap states in the spin up channel for Re doped SnS2. V and Nb in VB group also introduce magnetism in SnS2,while the magnetism is absent in Ta doped SnS2. Therefore, the doping scenarios are different from elements in the same group.
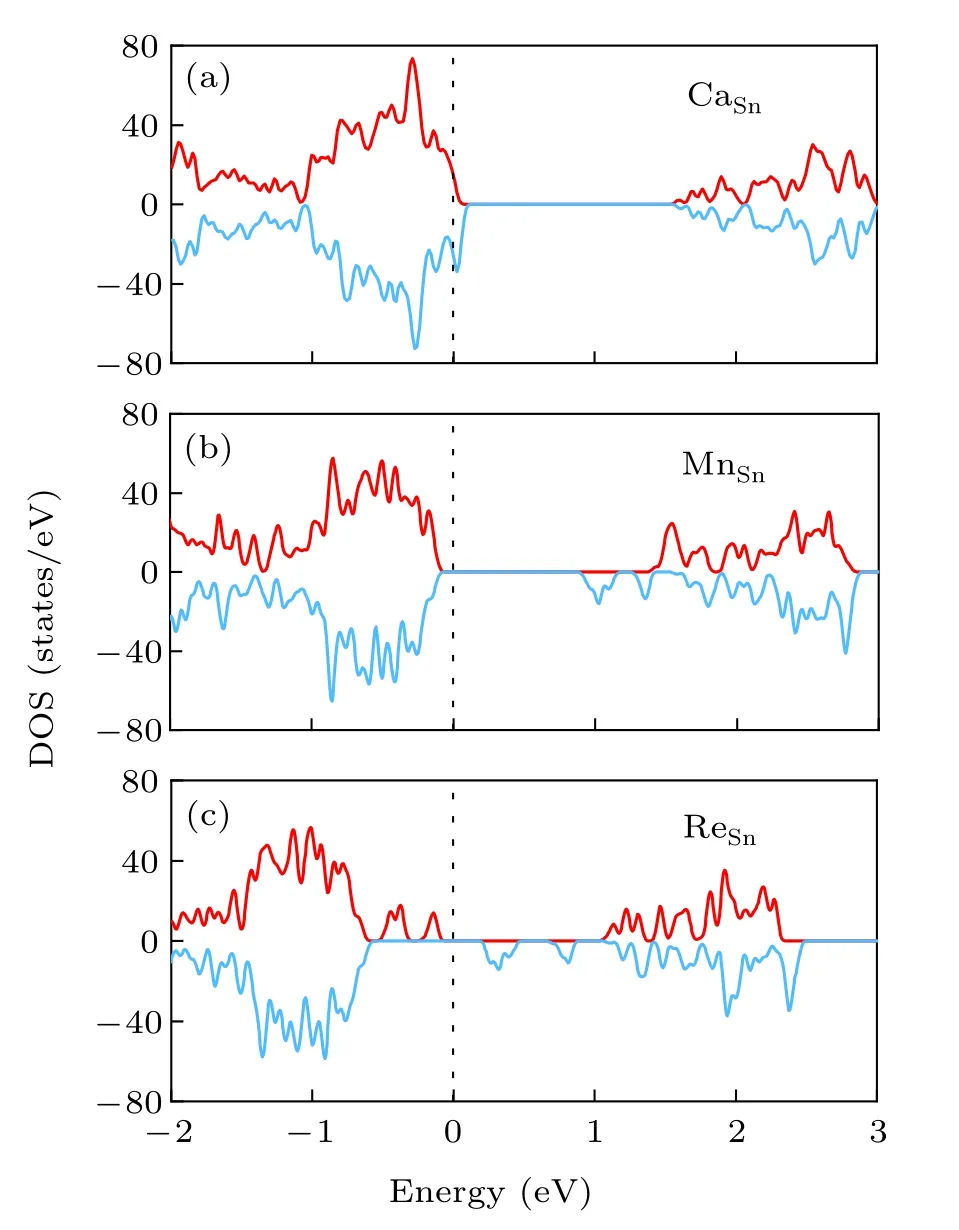
Fig.6. Spin polarized density of states of(a)CaSn,(b)MnSn,and(c)ReSn doped monolayer SnS2.
Due to valence state of each element is different from each other, the doping scenarios, including binding energy,electronic and magnetic properties, are different. Doping a specific element in SnS2depends on various factors, such as growth temperature, type of reactant, and abundance of reactant. In our present work, the calculated binding energy is applied to check the possibility of doping foreign element. To further verify the possibility of doping, the formation energy for doping on chemical potential should be investigated.However, it has been proven efficient and validity of evaluating the doping possibility by using the binding energy.[18,22,24,37]Some predictions by using such method have been experimentally verified,such as Fe,Co,and Mn doping in MoS2.[38-40]By evaluating the binding energy shown in Fig.2 and the carrier type for different element doping shown in Figs. 3 and 4, and Figs. S1-S6 in supporting information, we conclude that CS, AlSn, ScSn, LuSn, and YSnare promising experimentally realized for p-type doping, as well as FS, SbSnand TaSnfor n-type doping without magnetism. In addition, we screen out some foreign elements doping that may introduce magnetism in SnS2.Under doping concentration of 6.25%,the ferromagnetic state is 0.60 meV,2598.95 meV,637.75 meV,and 906.58 meV lower than non-magentic or antimagnetic state for Ca,Mn,Re,and Fe substitutional doping,respectively,see Table S1 in supporting information. The Curie temperature(TC)is an important parameter of a ferromagnetic semiconductor.Based on the mean-field theory and Heisenberg model we estimateTCusing the relationkBTC=(2/3)?E,[41,42]wherekBand ?Eare the Boltzmann constant and the energy difference of ferromagnetic and non-magnetic/antiferromagnetic states,respectively. The estimated values ofTCare listed in Table S1,together with magnetic moments. TheTCof ReSnis around 30 K,while others are lower than 10 K.It is well known that doping concentration also affect magnetic coupling and the value ofTC. We take Re and Fe doped SnS2as two representatives. The two Re ions are antiferromagnetic coupled,while Fe ions remains ferromagnetic coupled for doping concentration of 12.5%. The detailed magnetic ground state and further exploration of highTCof a certain doping should be investigated in future.
4. Conclusion
By using high throughput first-principles calculation,we comprehensively investigate the substitutional doping in monolayer SnS2. Metal dopants from group A can induce ptype doping(AlSn),deep states in the gap(SbSn),or band gap change without defect states (SiSn). However, metal dopants from group A can not introduce n-type doping from our calculations. Nonmetal dopants from group A not only can introduce n-type doping(FS),p-type doping(CS),and band gap variation (OS). Transition metal dopants from VB to VIIIB9 can introduce magnetism in SnS2and high binding energy for each dopant indicates high possibility of growth transition metal doped SnS2. Our present work provides a comprehensive view of chemical doping effect on carrier and magnetism of SnS2, which is important for field effect transistor, optoelectronic device and spintronics.
- Chinese Physics B的其它文章
- Erratum to“Floquet bands and photon-induced topological edge states of graphene nanoribbons”
- Viewing the noise propagation mechanism in a unidirectional transition cascade from the perspective of stability*
- Nonlinear signal transduction network with multistate*
- Optical strong coupling in hybrid metal-graphene metamaterial for terahertz sensing*
- Any-polar resistive switching behavior in Ti-intercalated Pt/Ti/HfO2/Ti/Pt device*
- Magnetic two-dimensional van der Waals materials for spintronic devices*

