Role of nutritional ketosis in the improvement of metabolic parameters following bariatric surgery
INTRODUCTION
Ketogenesis primarily occurs in the liver at rates proportional to total fat oxidation under conditions of reduced glucose availability such as fasting or very low-calorie ketogenic diets (VLCKDs). In brief, under these conditions, lipolysis-derived free fatty acids (FFAs) undergo beta-oxidation and are broken down into acetyl CoA, which is then converted to ketone bodies (KB), namely acetone, acetoacetate (AcAc), and betahydroxy butyrate (BHB), in the mitochondrial matrix of hepatocytes. KBs, namely BHB, AcAc and acetone, transfer lipid-derived energy from the liver, which cannot use them as a fuel, to extrahepatic organs (
, central nervous system, heart, skeletal muscle, kidney), serving as an energy substrate alternative to glucose[1]. Over the past few years, the interest in KBs and nutritional ketosis has progressively increased, largely due to the discovery that, besides serving as energy substrates, KBs may also exert favourable metabolic effects[2,3], serving as metabolic regulators and signalling molecules. In particular, BHB exerts antioxidant and anti-inflammatory effects, may affect epigenetics by inhibiting histone deacetylation, suppresses the activity of the sympathetic nervous system and reduces lipolysis and, through unknown mechanisms, to play a role in appetite suppression[4,5]. In healthy individuals, even small increases in KB levels were shown to lower glucose and circulating FFA independent of insulin and glucagon[6], and to attenuate the glycaemic response to an oral glucose tolerance test by increasing insulin sensitivity[7], suggesting a direct metabolic effect of KBs. Bariatric metabolic surgery (BMS) offers a unique opportunity to study nutritional ketosis, avoiding the complexity of a nutritional intervention such as VLCKD that would need greater effort from patients and also greater costs[8]. Similar to VLCKDs, BMS involves a marked energy deficit that results in massive mobilization of FFAs from adipose tissue and therefore ketogenesis[9,10]. The role of BMS in achieving sustained weight loss (WL), improving obesity-related comorbidities and reducing mortality is well established[11]. However, not all subjects respond to a similar extent[12], those with cardiometabolic abnormalities such as diabetes (especially when long-standing or poorly controlled) and arterial hypertension exhibiting poorer WL after surgery[13,14]. To the best of our knowledge, no studies have assessed the relationship between ketogenic capacity, as reflected by KB production in response to marked calorie restriction, and WL after BMS. We hypothesized that subjects with reduced ketogenic capacity are poorer responders to BMS in terms of WL 6 mo surgery.
What a feast! he exclaimed; will anyone come and share it? Moo-oo, sounded close behind him, and looking round he saw a great red ox, which said, I have much pleasure in accepting your kind invitation
MATERIALS AND METHODS
Study design
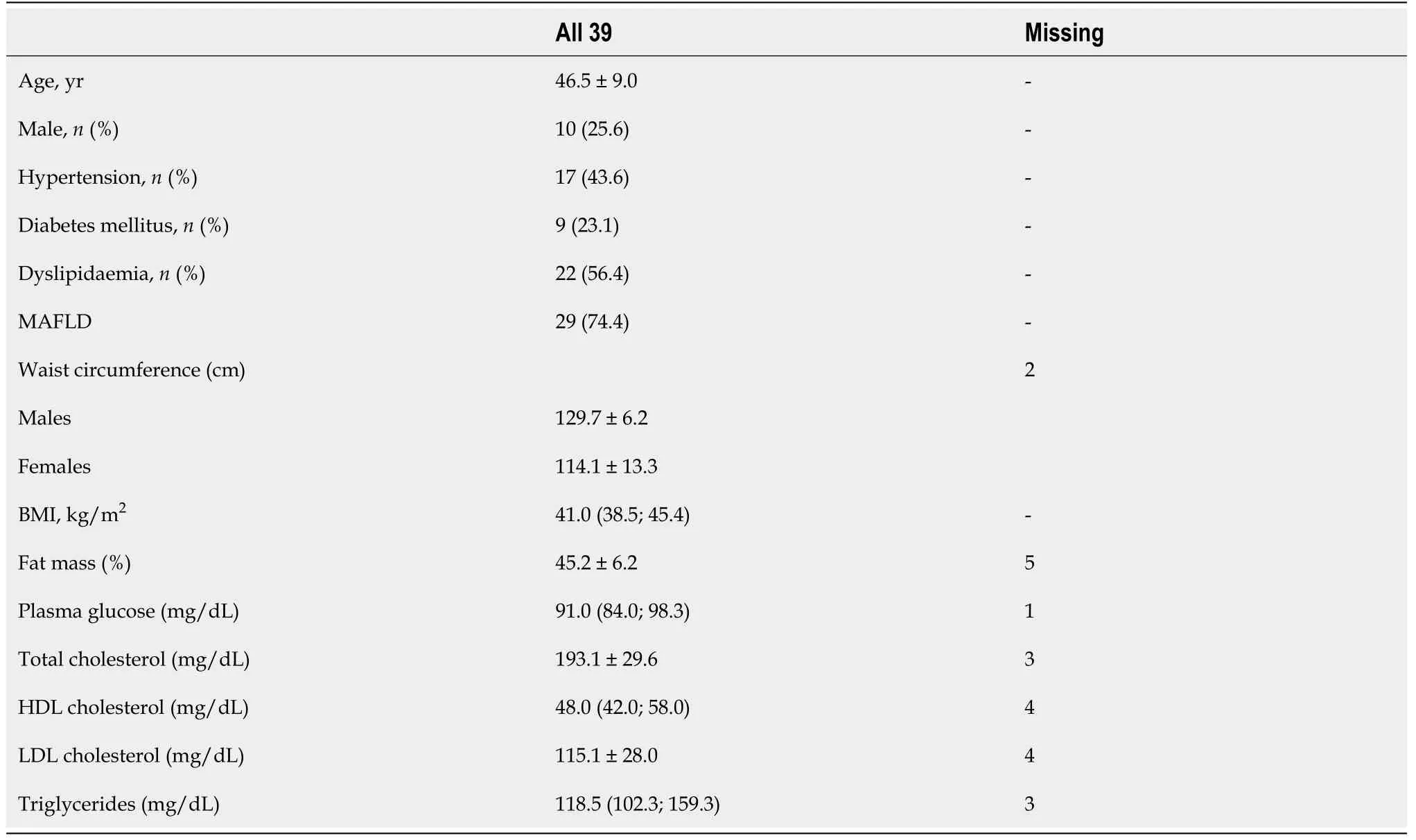
This was an observational, retrospective, single-centre study part of the KETO-BMS study. Male and female subjects aged 18-65 years who underwent laparoscopic sleeve gastrectomy at San Raffaele Scientific Institute from May 2016 to November 2018 and had urinary KB measured within two months of surgery and a follow-up of at least 6 mo were included. The protocol was approved by the Institutional Ethics Committee, and all patients provided informed consent. All patients underwent routine assessments prior to BMS, including medical history, physical examination, measurement of anthropometrics [height (cm), weight (kg) and body mass index (BMI), calculated as the ratio between the weight and the height squared, waist circumference (WC)], body composition (measured by electric bio-impedance in the fasting state using a BIA AKERN device and the software Bodygram PLUS software, Akern, Montachiello, Italy). Metabolic parameters including fasting plasma glucose (FPG), total, highdensity lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, and triglycerides were collected. During the first 8 wk after surgery, patients meet with a registered dietitian and subsequently with a staff physician for nutritional assessment and guidance. As per institutional protocols, during this time frame patients move from clear liquids to pureed foods, progressively increasing to approximately 750-900 kcal daily, depending on protein requirements (up to 1.5g/kg IBW). After the first 8 wk, patients move to solid foods and gradually increase the daily energy intake. Assessments are scheduled every 3-6 mo for the first 12 mo, and annually thereafter. Follow-up outpatient visits include medical history review, physical examination, measurement of anthropometrics, and laboratory assessments as per current recommendations[15].
KB production
KB production was assessed by the presence of acetoacetic acid in urine using an automated dipstick urinalysis (Aution MAX and Aution Sticks, Menarini Diagnostics, Florence, Italy). This is a semiquantitative method that detects urinary acetoacetic acid at concentrations ranging from 5 mg/dL to 150 mg/dL.
ONCE upon a time there lived in a certain village a little country girl, the prettiest creature who was ever seen. Her mother was excessively fond of her; and her grandmother doted on her still more. This good woman had a little red1 riding hood1 made for her. It suited the girl so extremely well that everybody called her Little Red Riding Hood2.
Statistical analysis
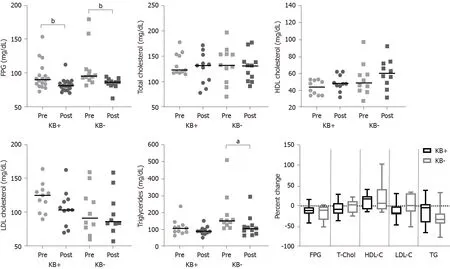
Laboratory variables at 6 mo were available for a subgroup of patients. No statistically significant differences in percent change from pre-surgery to 6 mo were detected between groups (Figure 2), although patients who had developed nutritional ketosis tended to have a greater percent increase in HDL cholesterol and greater percent reductions in total and LDL-cholesterol, whereas those who did not develop ketosis tended to have a greater reduction in triglycerides.
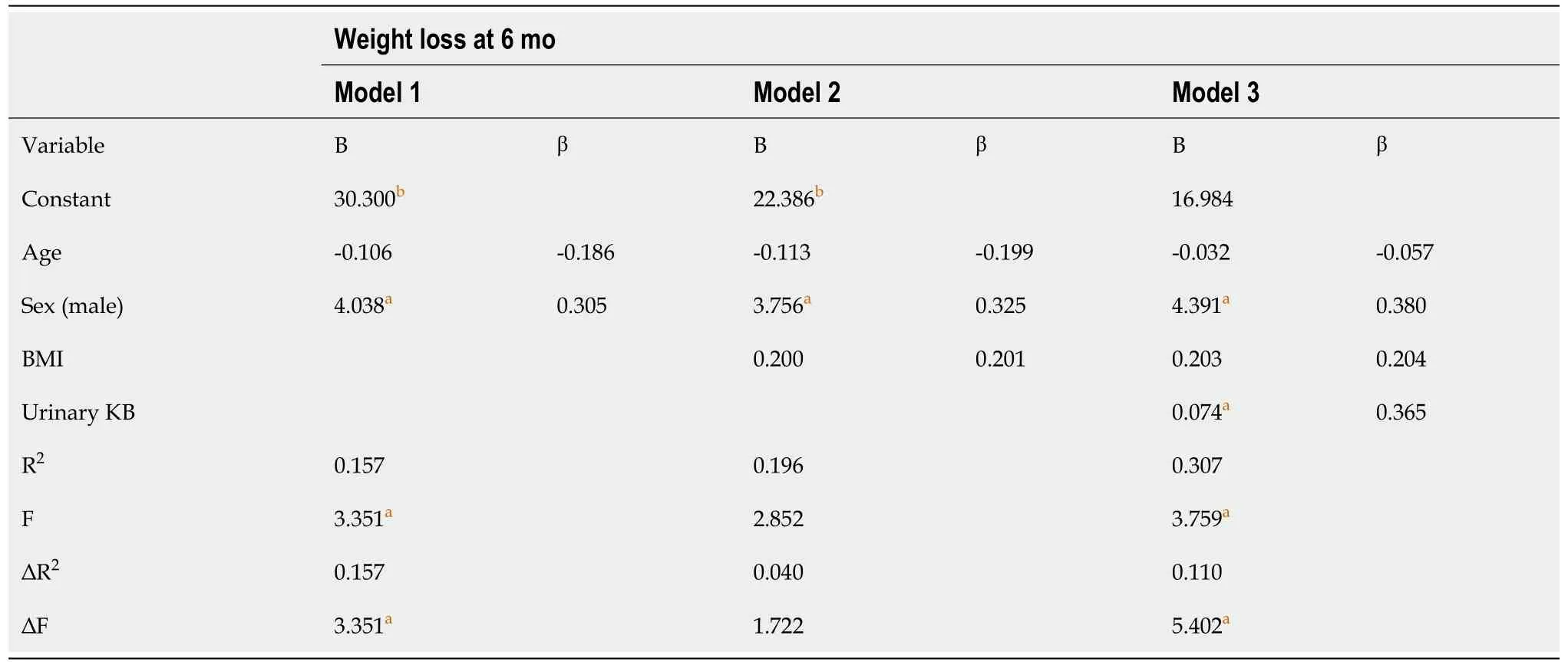
Our primary objective was to examine the relationship between KB production and WL at 6 mo after BMS. One-way analyses of covariance were conducted to examine the effect of sex and pre-operative cardiometabolic conditions [diabetes mellitus (DM), hypertension, dyslipidaemia] on WL at 6 mo, with age included as a covariate. Bivariate correlation analyses were performed to examine the relationship of WL at 6 mo with pre-operative BMI, fat mass, FPG, total cholesterol, triglycerides and postoperative urinary KBs. Relevant variables that were significantly correlated were included in a hierarchical multiple-regression analysis, while controlling for sex, age and BMI. All variables were screened for violations of the assumptions relevant to each of the statistical analysis performed. Statistical significance was set at
< 0.05. Statistical analysis was conducted using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, United States: IBM Corp.).
RESULTS
Patient population
A total of 39 patients were included in the analysis. Patient characteristics are depicted in Table 1. Patients were middled-aged, mostly females. Metabolic-associated fatty liver disease was the most prevalent obesity complication, followed by dyslipidaemia, hypertension and DM.
KB production
Dejected, the pastor and his wife looked at the defaced wall. There was obviously no chance to repair the damage before Christmas. Nearly three months of hard work had been washed way. Yet the young couple accepted the damage as God’s will and set about cleaning up the damp debris5.
WL after surgery
In this analysis of patients who underwent BMS, we found that KB production during marked calorie restriction after surgery predicted WL at 6 mo. Patients who developed nutritional ketosis had greater WL at 6 mo and tended to have a greater WL at 12 mo after surgery, as compared with those who did not develop nutritional ketosis.
Urinary KB (Spearman’s rho 0.398,
= 0.012) significantly correlated with WL at 6 mo, whereas age (
= 0.290), BMI (
= 0.056), fat mass (
= 0.735), FPG (
= 0.680), total cholesterol (
= 0.508) and triglycerides (
= 0.976) did not. After adjustment for age, there was a statistically significant difference in WL at 6 mo between males and females, F(1, 36) = 5.221,
= 0.028, partial η
= 0.127. There was no statistically significant difference between patients with or without DM, hypertension, or dyslipidaemia, therefore these variables were not included in the regression model. At hierarchical multiple regression, urinary KBs and male sex emerged as significant predictors of WL at six months. The full model statistically significantly predicted WL at 6 mo, R
= 0.31, F(4, 34) = 3.76,
= 0.012 (Table 3). Urinary KBs also correlated with WL at 12 mo (Spearman’s rho 0.356,
= 0.036).
Descriptive statistics were obtained for all study variables. Normality was assessed with the Shapiro-Wilk test. Continuous variables were expressed as mean ± SD or median (25
-75
percentile), depending on data distribution. Categorical variables were summarised as counts and percentages. Missing data were not imputed. The
test, Welch
-test, or Mann-Whitney
-test were used for between-group comparisons, depending on variable distribution. The Fisher’s exact test was used to assess the association between categorical variables and KB production.
“When you have reached your fifteenth year,”9 said the grand-mother, “you will have permission to rise up out of the sea, to sit on the rocks in the moonlight, while the great ships are sailing by; and then you will see both forests and towns
DISCUSSION
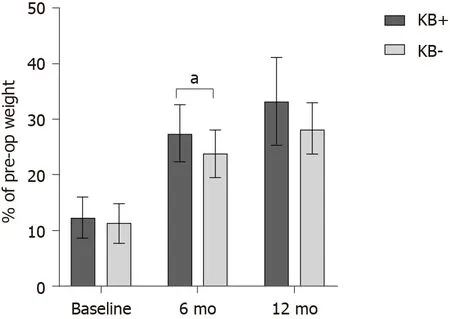
Mean WL at 6 mo was 26.4% ± 5.1% of pre-operative weight in the whole group. WL at 6 mo was significantly greater in patients who had developed post-operative ketosis (
= 0.035; Figure 1). Time of assessment was similar between those who did or did not develop ketosis (6.1 ± 0.9 mo
6.1 ± 0.5 mo, respectively;
= 0.931). In 35 patients who had available data at 12 mo (89.7% of the total, 24 and 11 in the group with and without ketosis, respectively), WL also tended to be greater in those with postoperative ketosis (
= 0.067, Figure 1). Time of assessment was similar between those who did or did not develop ketosis (11.9 ± 0.9 mo
12.1 ± 1.2 mo, respectively;
= 0.590).
I now see that praying is such a powerful act. Prayer is the most powerful tool a Christian has. When I pray for those around me, it also blesses my life, and it changes my perception of others. I realized I needed God s blessings4 to see the world through loving eyes. The prayers I said for others turned out to help me the most.
Little information is available on KB production after BMS. Crujeiras
[9] reported that patients who underwent BMS developed mild ketosis at one month after surgery. Thereafter, KBs decreased and returned to pre-operative levels at 3 mo. The association between nutritional ketosis and WL was not explored in that study, which had a different aim. There may be different explanations for the association between post-operative nutritional ketosis and the greater WL at 6 mo observed in our study. It has been reported that conditions of altered glucose metabolism such as type 2 DM negatively impact WL after BMS[16,17]. Ketogenic capacity might be a proxy of glucometabolic health. Previous studies suggested that ketogenic capacity is impaired in women with obesity as compared to normal-weight controls[18], in the pathogenesis of non-alcoholic liver disease and progression to non-alcoholic steatohepatitis, and even hepatocellular carcinoma[19-21]. Furthermore, studies in mice indicate that impaired ketogenesis may play a role in fatty liver injury and dysregulated glucose homeostasis[22-24]. Patients who did not develop nutritional ketosis in our cohort had significantly higher FPG and triglycerides, indicating worse glucometabolic status. Impaired ketogenesis may be responsible for a diminished extraction of available fat, altered acetyl-CoA balance in mitochondria, and diversion of non-disposed FFAs to other metabolic pathways, possibly including lipogenesis[23]. Conversely, better WL and metabolic responses to BMS in patients with adequate ketogenic capacity might be due to efficient clearance of excess FFAs released from adipose tissue. It has been known for more than 40 years that KBs may have roles beyond serving as energy substrates[25]. Specifically, BHB appears to exert antioxidant and anti-inflammatory effects, to inhibit histone deacetylation and to play a role in appetite suppression[4,5]. In healthy individuals, even small increases in circulating KBs were shown to reduce glucose and triglyceride levels, and to hamper the glycaemic response to an oral glucose load by increasing insulin sensitivity[6,7]. At the time of KB assessment, WL was similar between patients with or without ketosis. However, it is tempting to speculate that exposure to mild ketosis led to an improvement of mitochondrial bioenergetics and metabolic health[3,26,27], which in turn resulted in improved subsequent WL. Despite having significantly higher LDL cholesterol prior to surgery, patients who developed nutritional ketosis exhibited a numerically greater reduction in LDL at 6 mo as compared with patients who did not develop nutritional ketosis (Figure 2). During ketogenesis, acetyl-CoA is converted to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) by mitochondrial HMG-CoA synthase, an enzyme that is also involved in cholesterol synthesis[28]. It is possible that, in conditions of low glucose and high FFA availability, an increase in ketogenesis results in lower rates of
cholesterol synthesis.
Differences in KB production might also be due to differences in diet macronutrient composition. A limitation of our study is that we did not record food intake in the first weeks following BMS. However, all patients received standard dietary recommendations, and compliance was reviewed by dieticians at follow-up assessments. Ketosis develops in conditions of reduced glucose availability and marked calorie restriction[29], such as in the first weeks after BMS. Following BMS, protein-rich foods are prioritized over other foods in order to prevent excess loss of fat-free mass[30]. It is unlikely that some patients ingested relatively high amounts of carbohydrates in the first postoperative weeks. On the other hand, it is possible that some greatly restricted carbohydrates to allow adequate protein intake. Deriving energy from proteins is an expensive process for the body, which may lead to calorie consumption and greater WL as compared with diets that rely on carbohydrates as the main energy source[31-33]. In fact, during carbohydrate restriction most of the body’s glucose requirements are satisfied by gluconeogenesis from amino acids, a process that requires approximately 400-600 kcal/d[32]. In other settings, several studies have demonstrated that very-low carbohydrate ketogenic diets are associated with greater WL as compared to other dietary regimens[34-36]. Diet composition in the first postoperative weeks might influence subsequent WL even in patients undergoing BMS. Other potential limitations are the relatively small sample size and the availability of data on WL at 12 mo only for a subgroup of patients, which might explain the lack of a statistically significant between-group difference in WL at this timepoint. Finally, we did not formally assess the level of physical activity throughout the 12-month follow-up to detect differences that might influence WL. In general, changes in physical activity during the first 6 mo after BMS (
, the timepoint for the assessment of the primary outcome in this study) are small and unlikely to affect WL[37]. We cannot exclude that changes in physical activity during the following months influenced WL at 12 mo.
CONCLUSION
In conclusion, it is possible that both metabolic status and diet composition influenced KB production in our cohort. Urinary KBs are easy to measure, and could be an early predictor of WL after BMS. Increasing evidence indicates that nutritional ketosis may have several health benefits[2,22,38-49]. Our findings add to this knowledge, suggesting that patients who develop nutritional ketosis following BMS might have greater WL and better metabolic responses to BMS.
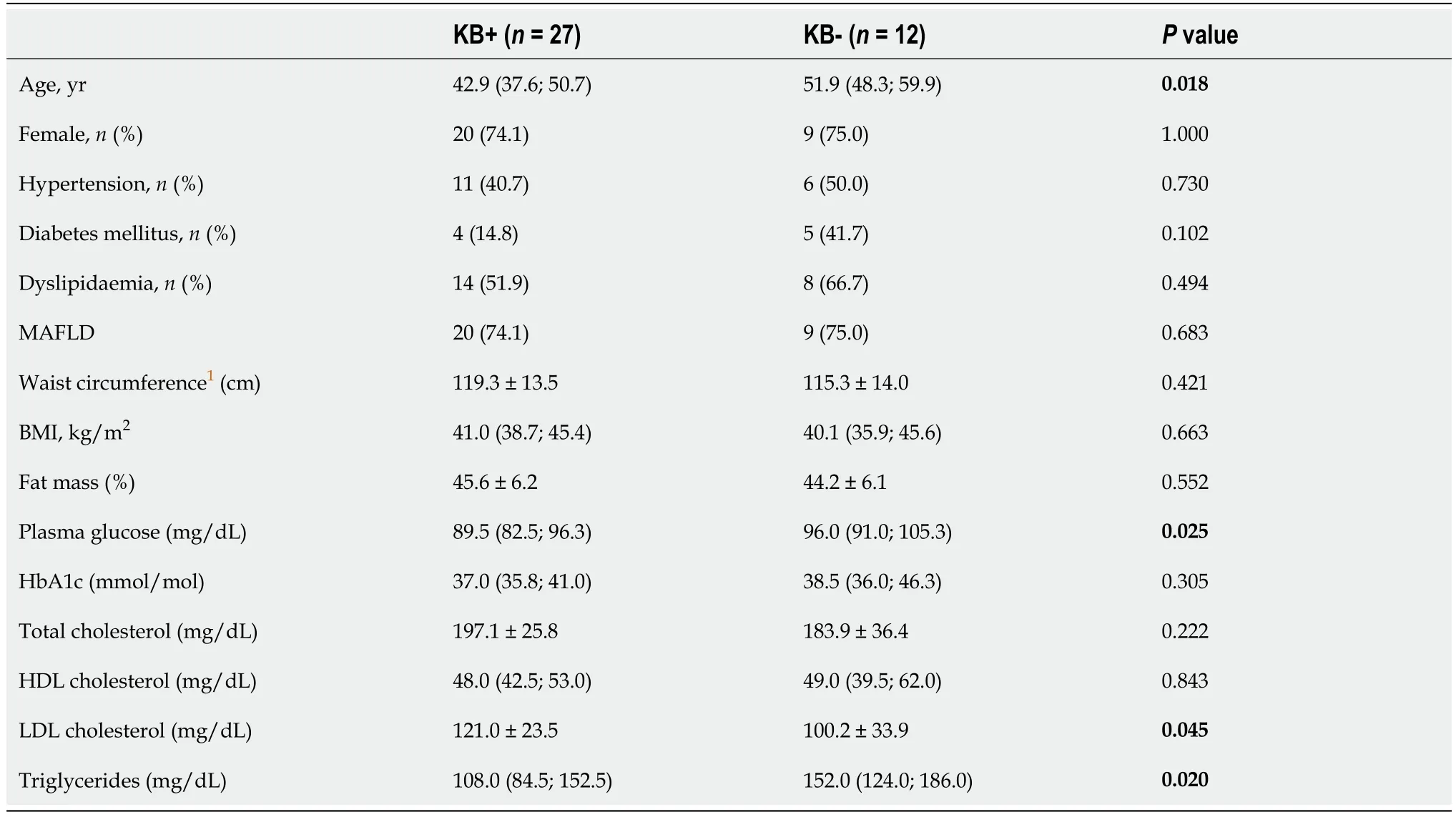
Most patients (69.2%) developed ketosis after a mean of 46.0 ± 13.6 d from surgery. Time from surgery was similar between those who did or did not develop ketosis (44.6 ± 15.0 d
49.0 ± 9.5 d, respectively;
= 0.351). Patients with ketosis were significantly younger and had significantly lower pre-operative FPG and triglyceride levels, but greater LDL cholesterol (Table 2). Urinary KBs were inversely correlated with age (Spearman’s rho -0.519,
= 0.001), FPG (Spearman’s rho -0.366,
= 0.024), and positively correlated with LDL cholesterol (Spearman’s rho 0.426,
= 0.011). There was no correlation between urinary KBs and BMI (
= 0.936), WC (
= 0.619), percent fat mass (
= 0.768), total cholesterol (
= 0.368), HDL cholesterol (
= 0.618) or triglycerides (
= 0.095).
ARTICLE HIGHLIGHTS
Research background
We hypothesized that subjects with reduced ketogenic capacity are poorer responders to BMS in terms of WL. Characterization of the relationship between ketogenic capacity and WL following BMS will help understand the metabolic actions of KB and find out whether KB could be used as a predictor of BMS-induced WL.
Research motivation
Ketone bodies (KB) derived from free fatty acid (FFA) metabolism serve as energy substrates in conditions of reduced glucose availability, but also as metabolic regulators and signalling molecules. Bariatric metabolic surgery (BMS) involves a marked energy deficit that results in massive mobilization of FFAs from adipose tissue, resulting in the activation of ketogenesis. It is not known whether all subjects undergoing BMS become ketotic, and whether there is a relationship between ketogenic capacity and weight loss (WL) following BMS.
Research objectives
We assessed the relationship between KB production in the first weeks after BMS and WL at 6 mo. We also assessed the relationship of KB with metabolic parameters and WL at 12 mo.
Research methods
For this retrospective study, we analyzed data from 39 patients who underwent laparoscopic sleeve gastrectomy, had urinary KB measured within two months of surgery and a follow-up of at least 6 mo. KB production was assessed by the presence of acetoacetic acid in urine using an automated dipstick urinalysis. We compared patients who developed post-operative ketosis with those who did not. The relationship of WL at 6 mo with pre-operative anthropometrics, body composition and metabolic parameters, and with post-operative urinary KBs was studied using bivariate correlation analyses. Variables that were significantly correlated were included in a hierarchical multiple-regression analysis, while controlling for sex, age and BMI.
Jack Robinson: Jacobs has fun by referencing another Jack in folklore, this time the phrase, Faster than you can say Jack Robinson! The phrase dates to at least 1778 since it appears that year in Fanny Burney s novel, Evelina
Research results
This was the first study to specifically assess the relationship of ketogenic capacity with weight and metabolic outcomes. Most, but not all patients (69.2%), developed ketosis after a mean of 46.0 ± 13.6 d from surgery. Patients with ketosis were significantly younger [42.9 (37.6; 50.7) years vs 51.9 (48.3; 59.9) years, P = 0.018] and had significantly lower pre-operative fasting plasma glucose [89.5 (82.5; 96.3) mg/dL vs 96.0 (91.0; 105.3) mg/dL, P = 0.025] and triglyceride levels [108.0 (84.5; 152.5)mg/dL vs 152.0 (124.0; 186.0) mg/dL, P = 0.020], but greater LDL cholesterol (121.0 ±23.5 mg/dL vs 100.2 ± 33.9 mg/dL, P = 0.045). WL at 6 mo was significantly greater in patients who had developed post-operative ketosis (27.5% ± 5.1% vs 23.8% ± 4.3% in the groups with and without ketosis, respectively; P = 0.035). At hierarchical multiple regression, urinary KBs and male sex emerged as significant predictors of WL at 6 mo.
Research conclusions
In keeping with the growing body of evidence indicating that nutritional ketosis has several health benefits, our findings suggest that patients who develop nutritional ketosis following BMS might have greater WL and better metabolic responses to BMS.
Research perspectives
Our findings should be considered hypothesis-generating. Further research is needed to confirm these data in larger populations, and to assess the relationship between ketogenic capacity and metabolic responses to BMS with more sophisticated techniques.
1 Balasse EO, Féry F. Ketone body production and disposal: effects of fasting, diabetes, and exercise.
1989; 5: 247-270 [PMID: 2656155 DOI: 10.1002/dmr.5610050304]
2 Branco AF, Ferreira A, Sim?es RF, Magalh?es-Novais S, Zehowski C, Cope E, Silva AM, Pereira D, Sard?o VA, Cunha-Oliveira T. Ketogenic diets: from cancer to mitochondrial diseases and beyond.
2016; 46: 285-298 [PMID: 26782788 DOI: 10.1111/eci.12591]
3 Miller VJ, Villamena FA, Volek JS. Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health.
2018; 2018: 5157645 [PMID: 29607218 DOI: 10.1155/2018/5157645]
4 Newman JC, Verdin E. Ketone bodies as signaling metabolites.
2014; 25: 42-52 [PMID: 24140022 DOI: 10.1016/j.tem.2013.09.002]
5 Puchalska P, Crawford PA. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics.
2017; 25: 262-284 [PMID: 28178565 DOI: 10.1016/j.cmet.2016.12.022]
6 Mikkelsen KH, Seifert T, Secher NH, Gr?ndal T, van Hall G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males.
2015; 100: 636-643 [PMID: 25415176 DOI: 10.1210/jc.2014-2608]
7 Myette-C?té é, Neudorf H, Rafiei H, Clarke K, Little JP. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals.
2018; 596: 1385-1395 [PMID: 29446830 DOI: 10.1113/JP275709]
8 Boyers D, Retat L, Jacobsen E, Avenell A, Aveyard P, Corbould E, Jaccard A, Cooper D, Robertson C, Aceves-Martins M, Xu B, Skea Z, de Bruin M; REBALANCE team. Cost-effectiveness of bariatric surgery and non-surgical weight management programmes for adults with severe obesity: a decision analysis model.
2021; 45: 2179-2190 [PMID: 34088970 DOI: 10.1038/s41366-021-00849-8]
9 Crujeiras AB, Gomez-Arbelaez D, Zulet MA, Carreira MC, Sajoux I, de Luis D, Castro AI, Baltar J, Baamonde I, Sueiro A, Macias-Gonzalez M, Bellido D, Tinahones FJ, Martinez JA, Casanueva FF. Plasma FGF21 Levels in obese patients undergoing energy-restricted diets or bariatric surgery: a marker of metabolic stress?
2017; 41: 1570-1578 [PMID: 28588304 DOI: 10.1038/ijo.2017.138]
10 Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell Function in type 2 diabetic patients.
2013; 62: 3027-3032 [PMID: 23610060 DOI: 10.2337/db12-1762]
11 Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012.
2014; 149: 275-287 [PMID: 24352617 DOI: 10.1001/jamasurg.2013.3654]
12 Batterham RL, Cummings DE. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery.
2016; 39: 893-901 [PMID: 27222547 DOI: 10.2337/dc16-0145]
13 Cottam S, Cottam D, Cottam A, Zaveri H, Surve A, Richards C. The Use of Predictive Markers for the Development of a Model to Predict Weight Loss Following Vertical Sleeve Gastrectomy.
2018; 28: 3769-3774 [PMID: 30039237 DOI: 10.1007/s11695-018-3417-3]
14 Muraca E, Oltolini A, Binda A, Pizzi M, Ciardullo S, Manzoni G, Zerbini F, Bianconi E, Cannistraci R, Perra S, Pizzi P, Lattuada G, Perseghin G, Villa M. Metabolic and Psychological Features are Associated with Weight Loss 12 Months After Sleeve Gastrectomy.
2021; 106: e3087-e3097 [PMID: 33705552 DOI: 10.1210/clinem/dgab161]
15 Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, Hjelmes?th J, Kinzl J, Leitner DR, Makaronidis JM, Schindler K, Toplak H, Yumuk V. Practical Recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the Post-Bariatric Surgery Medical Management.
2017; 10: 597-632 [PMID: 29207379 DOI: 10.1159/000481825]
16 Cottam S, Cottam D, Cottam A. Sleeve Gastrectomy Weight Loss and the Preoperative and Postoperative Predictors: a Systematic Review.
2019; 29: 1388-1396 [PMID: 30661210 DOI: 10.1007/s11695-018-03666-7]
17 Kitamura R, Chen R, Trickey A, Eisenberg D. Positive and Negative Independent Predictive Factors of Weight Loss After Bariatric Surgery in a Veteran Population.
2020; 30: 2124-2130 [PMID: 32009214 DOI: 10.1007/s11695-020-04428-0]
18 Vice E, Privette JD, Hickner RC, Barakat HA. Ketone body metabolism in lean and obese women.
2005; 54: 1542-1545 [PMID: 16253646 DOI: 10.1016/j.metabol.2005.05.023]
19 Fletcher JA, Deja S, Satapati S, Fu X, Burgess SC, Browning JD. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver.
2019; 5 [PMID: 31012869 DOI: 10.1172/jci.insight.127737]
20 Léveillé M, Estall JL. Mitochondrial Dysfunction in the Transition from NASH to HCC.
2019; 9 [PMID: 31623280 DOI: 10.3390/metabo9100233]
21 M?nnist? VT, Simonen M, Hyysalo J, Soininen P, Kangas AJ, Kaminska D, Matte AK, Venesmaa S, K?kel? P, K?rj? V, Arola J, Gylling H, Cederberg H, Kuusisto J, Laakso M, Yki-J?rvinen H, Ala-Korpela M, Pihlajam?ki J. Ketone body production is differentially altered in steatosis and nonalcoholic steatohepatitis in obese humans.
2015; 35: 1853-1861 [PMID: 25533197 DOI: 10.1111/liv.12769]
22 Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease.
2013; 304: H1060-H1076 [PMID: 23396451 DOI: 10.1152/ajpheart.00646.2012]
23 d'Avignon DA, Puchalska P, Ercal B, Chang Y, Martin SE, Graham MJ, Patti GJ, Han X, Crawford PA. Hepatic ketogenic insufficiency reprograms hepatic glycogen metabolism and the lipidome.
2018; 3 [PMID: 29925686 DOI: 10.1172/jci.insight.99762]
24 Patman G. NAFLD: Ketogenesis could be a determinant of steatohepatitis.
2014; 11: 702 [PMID: 25385228 DOI: 10.1038/nrgastro.2014.189]
25 Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues.
1980; 60: 143-187 [PMID: 6986618 DOI: 10.1152/physrev.1980.60.1.143]
26 Miller VJ, LaFountain RA, Barnhart E, Sapper TS, Short J, Arnold WD, Hyde PN, Crabtree CD, Kackley ML, Kraemer WJ, Villamena FA, Volek JS. A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle while improving metabolic health.
2020; 319: E995-E1007 [PMID: 32985255 DOI: 10.1152/ajpendo.00305.2020]
27 Walton CM, Jacobsen SM, Dallon BW, Saito ER, Bennett SLH, Davidson LE, Thomson DM, Hyldahl RD, Bikman BT. Ketones Elicit Distinct Alterations in Adipose Mitochondrial Bioenergetics.
2020; 21 [PMID: 32872407 DOI: 10.3390/ijms21176255]
28 McPherson PA, McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress.
2012; 68: 141-151 [PMID: 21983804 DOI: 10.1007/s13105-011-0112-4]
29 Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes.
1999; 15: 412-426 [PMID: 10634967 DOI: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8]
30 Sherf Dagan S, Goldenshluger A, Globus I, Schweiger C, Kessler Y, Kowen Sandbank G, Ben-Porat T, Sinai T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice.
2017; 8: 382-394 [PMID: 28298280 DOI: 10.3945/an.116.014258]
31 Feinman RD, Fine EJ. Nonequilibrium thermodynamics and energy efficiency in weight loss diets.
2007; 4: 27 [PMID: 17663761 DOI: 10.1186/1742-4682-4-27]
32 Fine EJ, Feinman RD. Insulin, carbohydrate restriction, metabolic syndrome and cancer.
2015; 10: 15-24 [PMID: 30289045 DOI: 10.1586/17446651.2014.960392]
33 Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review.
2004; 23: 373-385 [PMID: 15466943 DOI: 10.1080/07315724.2004.10719381]
34 Brehm BJ, Spang SE, Lattin BL, Seeley RJ, Daniels SR, D'Alessio DA. The role of energy expenditure in the differential weight loss in obese women on low-fat and low-carbohydrate diets.
2005; 90: 1475-1482 [PMID: 15598683 DOI: 10.1210/jc.2004-1540]
35 Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial.
2007; 297: 969-977 [PMID: 17341711 DOI: 10.1001/jama.297.9.969]
36 Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M, Stumvoll M, Stampfer MJ; Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet.
2008; 359: 229-241 [PMID: 18635428 DOI: 10.1056/NEJMoa0708681]
37 Herring LY, Stevinson C, Davies MJ, Biddle SJ, Sutton C, Bowrey D, Carter P. Changes in physical activity behaviour and physical function after bariatric surgery: a systematic review and metaanalysis.
2016; 17: 250-261 [PMID: 26783103 DOI: 10.1111/obr.12361]
38 Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, Mapuskar KA, Schoenfeld JD, Buatti JM, Spitz DR, Fath MA. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism.
2014; 2: 963-970 [PMID: 25460731 DOI: 10.1016/j.redox.2014.08.002]
39 Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials.
2013 ; 110: 1178-1187 [PMID: 23651522 DOI: 10.1017/S0007114513000548]
40 Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, Accurso A, Frassetto L, Gower BA, McFarlane SI, Nielsen JV, Krarup T, Saslow L, Roth KS, Vernon MC, Volek JS, Wilshire GB, Dahlqvist A, Sundberg R, Childers A, Morrison K, Manninen AH, Dashti HM, Wood RJ, Wortman J, Worm N. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base.
2015; 31: 1-13 [PMID: 25287761 DOI: 10.1016/j.nut.2014.06.011]
41 Feinman RD, Volek JS. Carbohydrate restriction as the default treatment for type 2 diabetes and metabolic syndrome.
2008; 42: 256-263 [PMID: 18609058 DOI: 10.1080/14017430802014838]
42 Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet.
2006; 17: 431-439 [PMID: 16940764 DOI: 10.1097/00008877-200609000-00009]
43 Hartman AL. Neuroprotection in metabolism-based therapy.
2012; 100: 286-294 [PMID: 21872441 DOI: 10.1016/j.eplepsyres.2011.04.016]
44 Moreno CL, Mobbs CV. Epigenetic mechanisms underlying lifespan and age-related effects of dietary restriction and the ketogenic diet.
2017; 455: 33-40 [PMID: 27884781 DOI: 10.1016/j.mce.2016.11.013]
45 Paoli A. Ketogenic diet for obesity: friend or foe?
2014; 11: 2092-2107 [PMID: 24557522 DOI: 10.3390/ijerph110202092]
46 Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets.
2013; 67: 789-796 [PMID: 23801097 DOI: 10.1038/ejcn.2013.116]
47 Seyfried TN, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Is the restricted ketogenic diet a viable alternative to the standard of care for managing malignant brain cancer?
2012; 100: 310-326 [PMID: 21885251 DOI: 10.1016/j.eplepsyres.2011.06.017]
48 Simone BA, Champ CE, Rosenberg AL, Berger AC, Monti DA, Dicker AP, Simone NL. Selectively starving cancer cells through dietary manipulation: methods and clinical implications.
2013; 9: 959-976 [PMID: 23837760 DOI: 10.2217/fon.13.31]
49 Vidali S, Aminzadeh S, Lambert B, Rutherford T, Sperl W, Kofler B, Feichtinger RG. Mitochondria: The ketogenic diet--A metabolism-based therapy.
2015; 63: 55-59 [PMID: 25666556 DOI: 10.1016/j.biocel.2015.01.022]
 World Journal of Diabetes2022年1期
World Journal of Diabetes2022年1期
- World Journal of Diabetes的其它文章
- Gut microbiota-derived metabolites are novel targets for improving insulin resistance
- High doses of catecholamines activate glucose transport in human adipocytes independently from adrenoceptor stimulation or vanadium addition
- Management of diabetic foot ulcers and the challenging points: An endocrine view
- Polycystic ovary syndrome and type 2 diabetes mellitus: A state-oftheart review
- Acarbose is again on the stage
