The investigation of NTO/HMX-based plastic-bonded explosives and its safety performance
Li-xiosong Du ,Sho-hu Jin ,Qing-hi Shu ,Li-jie Li ,Kun Chen ,Ming-lei Chen ,Jun-feng Wng
a School of Materials Science and Engineering,Beijing Institute of Technology,Beijing,China
b Gansu Yinguang Chemical Industry Group Co.Ltd,Baiyin,China
c State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing,China
Keywords:3-nitro-1,2,4-triazol-5-one (NTO)Plastic-bonded explosive (PBX)Safety performance Cook-off
ABSTRACT 3-nitro-1,2,4-triazol-5-one (NTO) is the main component of insensitive munitions (IM) formulation because of its outstanding insensitive properties.In this paper,a series of NTO/HMX-based composite explosives were prepared and characterized.The study focuses on the effect of NTO on the performance of the formulations,especially the safety performance.The results revealed that the mechanical sensitivity of formulations was associated with NTO content,as well as the thermal conductivity,specific heat capacity and Arrhenius parameters.Then,the high amount of NTO using in formulation was proved to be helpful for NTO/HMX-based formulation to exhibit good thermal safety.Besides,by accelerating rate calorimeter(ARC)and a modified cook-off equipment,the pressure and pressure rise rate were proved as the important indicator for judging the thermal safety performance in confined spaces.Finally,the numerical simulation was used as a credible method for predicting the respond temperature of cook-off experiment.
1.Introduction
Globally,the growing applications of insensitive munitions(IM)is in progress.The nitro heterocyclic compound 3-nitro-1,2,4-triazol-5-one (NTO),one of the promising insensitive candidates in high energy explosive formulations,is the main component of the famous IM formulation IMX-101 which was already approved by the U.S.Army as a safer,and yet equally effective replacement to 2,4,6-trinitrotoluene (TNT) [1-3].
Recently,NTO-based formulations used in IM were widely studied.For example,a series of NTO-based IM formulations named XF? [4] and XP? [5] were developed and paid attention by NATO(North Atlantic Treaty Organization).In addition,two NTO-based casted compositions containing spheroidal NTO and FOX-7 (1,1-diamino-2,2-dinitroethene) were prepared respectively on a laboratory scale in Poland[6,7].As for pressed plastic-bonded explosive(PBX) formulations,researchers studied the detonation performance and thermal performance of NTO/HMX-based PBX[8,9] (HMX:1,3,5,7-tetranitro-1,3,5,7-tetraazacyclo-octane).Moreover,the detonation properties of new DAAF/NTO-based PBX were evaluated by Los Alamos National Laboratory [10] (DAAF:3,3?-diamino-4,4?-azoxyfurazan).
At present,as the key ingredient of IM formulation,the effect of NTO in formulations,especially the safety performance,has been seldom reported.In this paper,a series of NTO/HMX-based molding powder and its PBX were prepared.The influences of NTO on performance parameters such as detonation properties,mechanical sensitivities and thermal safety were discussed.In the aspect of thermal safety performance,we paid more attention to the effect of pressure in confined spaces.Herein,the accelerating rate calorimeter (ARC) and a modified cook-off experiment equipment which have the function of pressure monitoring were used to investigate the thermal safety of PBX.As a good complementarity with cook-off experiment,finite element numerical simulations were performed to study the temperature characteristics of PBX cylinders during cook-off experiment.The current work provided new insight into the application of NTO in IM formulations.
2.Experiment
2.1.Formulations and materials
The main components of formulations in this study were NTO and HMX.In addition,a hydrocarbon polymer and a gem-dinitro compound with excellent thermal stability were used as the binder and the plasticizer.We also considered wax as the additional insensitive agents.Using the above raw materials,three kinds of NTO/HMX-based PBX molding powders(NH-1,NH-2 and NH-3 for short) were prepared by the water suspension method [11].The three NTO-based formulations were listed in Table 1.
All of the raw materials were purchased from the Gansu Yinguang Chemical Industry Group.Co.LTD.Moreover,the purities of NTO and HMX ≥99.5%.
2.2.Characterization
The morphologies of molding powders were studied by using scanning electron microscopy (SEM,Hitachi,S-4800).The crystal structure was investigated by using an X-ray diffractometer (XRD,Panalytical,Xpert Powder).
2.3.Detonation properties and mechanical sensitivity
According to the chemical formula,density and enthalpies,the detonation parameters and JWL equation parameters of samples were both predicted by EXPLO5 software [12].
To be safe,it is necessary to study the mechanical sensitivities of molding powders before pressed to PBX cylinders.Impact sensitivity and friction sensitivity of samples were tested according to the China National Military Standard GJB-770B-2005.The impact sensitivity was tested by using the WL-1 drop hammer apparatus,a mass of 50 mg sample was impacted by 10 kg hammer from 250 mm drop height.The friction sensitivity was recorded by using MGY-1 pendulum friction apparatus,a mass of 30 mg samples fixed between steel anvils and hit by a 1.5 kg pendulum hammer with a below 90tilt angle and 3.92 MPa pressure.100 times were needed to test both impact sensitivity and friction sensitivity.And the impact sensitivity and friction sensitivity were expressed by explosion probability(Pand P,%).The higher P is,the more sensitive appears.
2.4.Thermal characteristic and safety performance
The thermal conductivity(λ)of explosives was tested by DRL-II thermal conductivity measuring apparatus.The specific heat capacity (C) were determined by differential scanning calorimeter(DSC,NETZSCH Company),the sample mass was 12.95 mg,and the heating rate was 10C/min from 0 to 45C with nitrogen atmosphere.
To evaluate the thermal safety performance of samples in confined spaces,the Constant Heating Rate mode [13] in accelerating rate calorimeter (ARC,NETZSCH Company) was used.The samples were placed in Hastelloy vessel with an air atmosphere.In addition,a pressure transducer was attached to the vessel,and therange for the transducer(Honeywell,BD Sensors)was 0-20 MPa.In this paper,the experiments were conducted using a constant heating rate of 1C/min and started with 100.5 kPa (the local atmospheric pressure)at 30C.Moreover,the time-dependent values for pressure and temperature were written every 0.01 min.
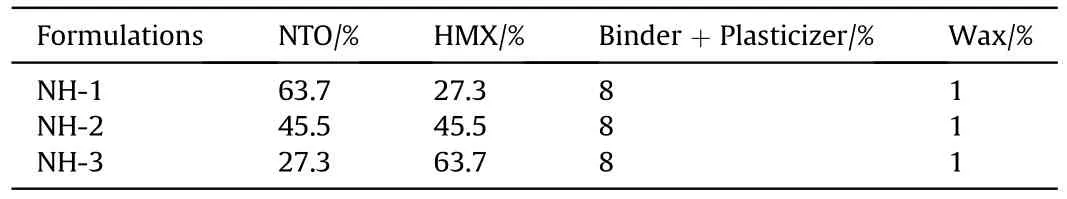
Table 1 The formula of the three composite explosives.
In most of countries and regions,slow cook-off test was required in the military standard of the explosive test to estimate the thermal vulnerability of the munition.The explosive cylinders (Fig.1)with the 40 mm diameter and 40 mm length were pressed by molding powders and loaded in a slow cook-off bomb.The cylinder bomb,80.5 mm internal diameter by 80.5 mm internal length,was made of stainless steel.In addition,heat tape and some control thermocouples were attached to the side of the bomb,which were used to control the external temperature of the experiment.In the process of heating,the safety of the bomb was affected by not only the exothermicity but also the fast pressure rise rate during the explosive decomposition and combustion.Therefore,it was necessary to continuously and directly monitor the pressure change during the cook-off process.In this paper,we made a vent at the top endcaps of bomb which can be fitted with tubing and connected to a pressure transducer to collect the pressure data.The schematic of the cook-off system was shown in Fig.2.In every single experiment,two explosive cylinders were used and heated at a heating rate of 1C/min until the explosive thermal explosion.
2.5.Simulation
According to the bomb and explosive cylinders of slow cook-off experiment,the corresponding finite element model was established by Ansys software to simulate the ignition location,ignition time,ignition temperature and transient temperature distribution during the slow cook-off experiment.In addition,the following theoretical assumptions were used in the calculation:(1) the explosive cylinders were homogeneous and phase change of components was ignoring;(2) thermo-physical parameters of explosives remained constant;(3) there was no gap between explosive cylinders and shell;(4) thermal decomposition reaction of explosives was described by the Arrhenius equation.The Fourier heat conduction equation was in the form (Eq.(1)) [14]:

where ?is Laplacian operator;ρ is density;Cis specific heat capacity;λ is thermal conductivity coefficient;T is temperature;t is time;S is the term of self-heating source for explosives which is described as Eq.(2)

where A is the pre-exponential factor;Eis activation energy;Q is decomposition heat of explosive;R is universal gas constant;f(α)is mechanism function.The all above parameters of formulations were shown in the Supplementary Materials.
3.Result and discussion
3.1.Morphology and structure
For the purpose of exploring the coating effect of additives on the main explosives,the SEM analysis was performed for the prepared molding powder.The surface morphology of different molding powder particles was shown in Fig.3.
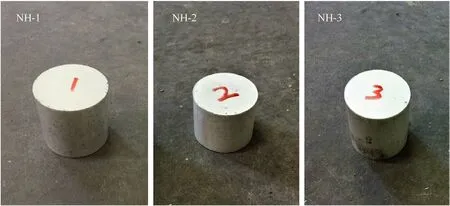
Fig.1. The explosive cylinders of different sample.
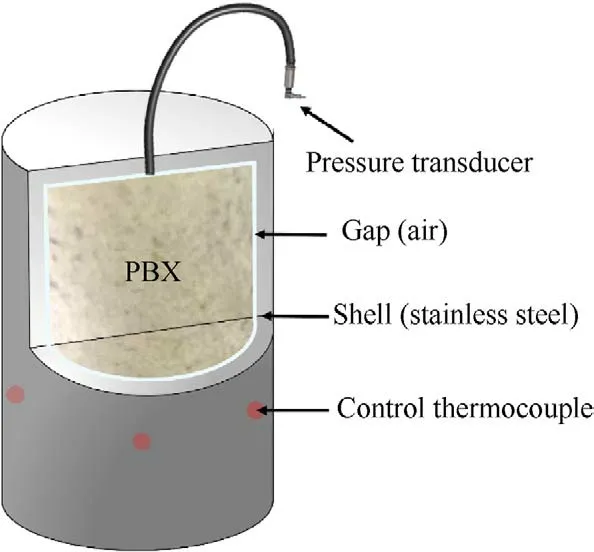
Fig.2. Then schematic of the cook-off system.
As shown in Fig.3,the shapes of molding powders were all relatively spherical and the surfaces of molding powders were compact and smooth.In addition,the additives were found everywhere on the surface of molding powders.Furthermore,the gaps between explosive crystals were filled with additives.That meant the NTO and HMX were well coated by the additive system.
The crystal structures of energetic components in three formulations were characterized by using a powder XRD patterns(Fig.4).The characteristic peaks positions of NTO/HMX-based molding powders were consistent with those of the NTO and HMX with no other characteristic peaks,which were indicating that the crystal type of NTO or HMX did not change during the preparation process of molding powders.
3.2.Detonation properties
The chemical formula,density,enthalpy of formation and detonation parameters of the three NTO/HMX-based formulations were listed in Table 2.In addition,the parameters of NTO and HMX were also listed for comparison.For the calculation details,please see the corresponding chapters in the Supplementary Materials.
In Table 2,the pressed density of samples showed a decreasing trend with the decrease of NTO content because the density of pure NTO was higher than pure HMX.However,the enthalpy of formation,detonation velocity,detonation pressure and detonation heat increased with the decrease of NTO content.Undoubtedly,it owed to these parameters of pure HMX were much higher than that of pure NTO.
Furthermore,the standard JWL-EOS[15](Eq.(3))parameters of the three formulations were calculated (Table 3),which could be used in the subsequent numerical simulation study.
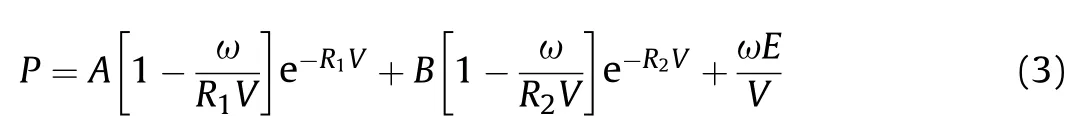
where V is relative specific volume;E is the internal energy of detonation production;A,B,R,Rand ω are the empirical parameters;Eis explosive detonation energy.
3.3.Mechanical sensitivities
As listed in Table 4,the mechanical sensitivities of pure HMX were extremely high in which the explosion percentage of impact and friction were all reaching 100%,it meant that the pure HMX was much sensitive to the external mechanical stimuli.But pure NTO was much safer than HMX because the impact sensitivity and friction sensitivity of pure NTO were only 25% and 30%,respectively.As for the three NTO/HMX-based formulations,the impact and friction sensitivity were decreasing with the increase of NTO content.Evidently,the high amount of NTO had a beneficial effect on mechanical sensitivities of NTO/HMX-based formulations.In addition,the impact and friction sensitivity of NH-1 and NH-2 were both lower than pure NTO,which indicated that the molding powder had lower mechanical sensitivity after coating with binder and wax.
3.4.Specific heat capacity and thermal conductivity
The specific heat capacity (C) and thermal conductivity (λ) of samples were shown in Table 5,which were the important thermal parameters and could be used in the following numerical simulation.Furthermore,the relationship between C,λ and mechanical sensitivities of the NTO-based formulations were shown in Fig.5.
From Fig.5,the value of Cwas decreasing and the value of λ was increasing with the increase of mechanical sensitivity.This phenomenon could be explained by “hot spot” theory:The explosive molding powder with the high value of Cwould need more heat accumulation to generate hot spots that may be existing between explosives particles or between explosive and contact materials.Besides,the low value of λ implied the explosive molding powder exhibited a stronger heat insulation effect,which had a better protective effect on the heat caused by external stimulation.Therefore,the above two elements could reduce the explosion probability caused by external mechanical stimuli in a cooperative way.
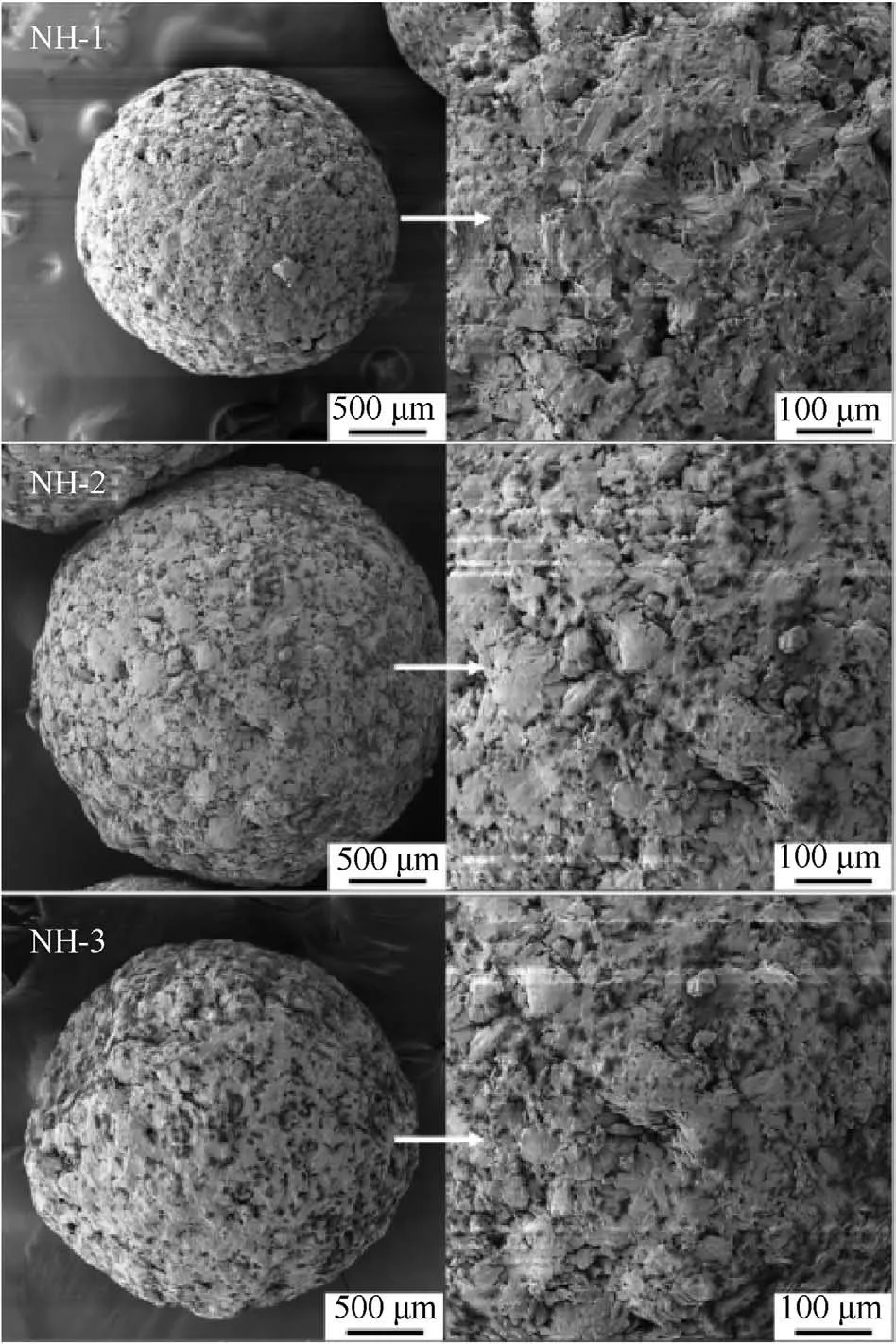
Fig.3. Surface morphology of molding powders.
The influence of thermal conductivity and specific heat capacity on the mechanical sensitivity was not the only remarkable phenomenon.Another noteworthy experimental phenomenon was that the mechanical sensitivities of NTO/HMX-based composite explosives had a positive correlation with Arrhenius parameters.As the NTO content decreased,the pre-exponential factor and activation energy of the composite explosive formulation increased,and its mechanical sensitivities increased.When hot-spots are generated by external mechanical stimuli,the formulation with higher Arrhenius parameters behaved faster reaction fractions.Coupled with higher λ and lower C,it led to faster diffusion of the high-temperature hot-spots areas.Therefore,Arrhenius parameters strongly influenced mechanical sensitivities of NTO/HMXbased composite explosives.
3.5.Thermal safety performance
In the vast majority of cases,the composite explosives were used in confined spaces.Therefore,the effect of heat and pressure on the sealed shell should be attended in the course of studying on the thermal safety of explosive.In this paper,the Constant Heating Rate Mode of ARC and slow cook-off experiment were used to explore the thermal safety performance of samples in grain and cylinder,respectively,which the pressure and temperature changes of samples under continuous external heating were dynamically collected.

Fig.4. XRD of composite explosives and corresponding energetic components.
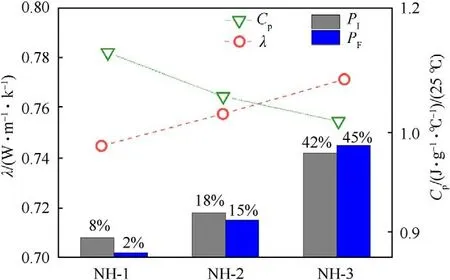
Fig.5. CP,λ and mechanical sensitivities of the formulations.
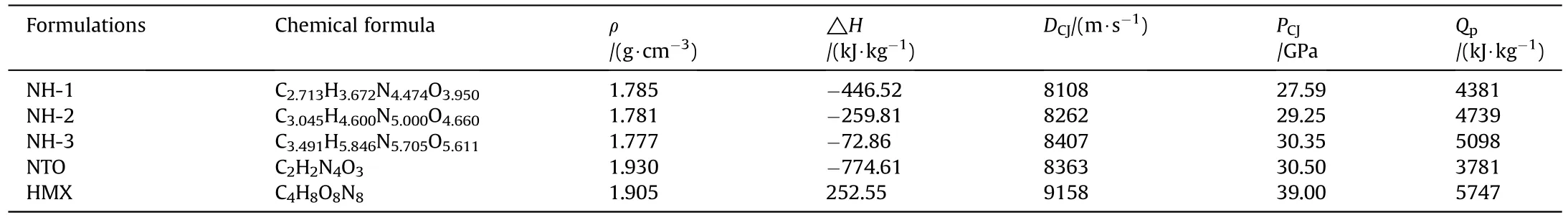
Table 2 Chemical formulas,densities,enthalpies of formation and detonation parameters of the formulations.
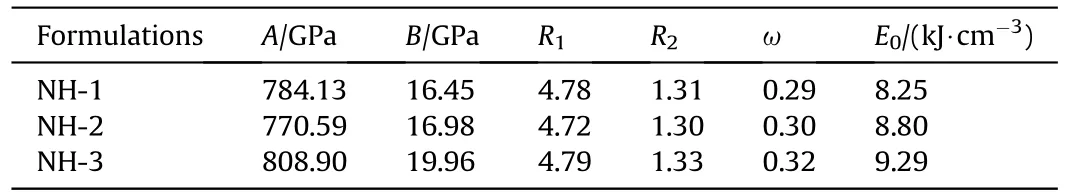
Table 3 JWL-EOS parameters of NTO-based composite explosives.
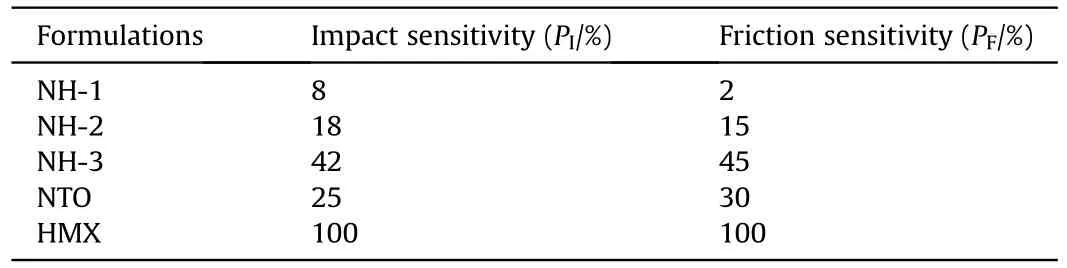
Table 4 Mechanical sensitivities of NTO-based composite explosives.
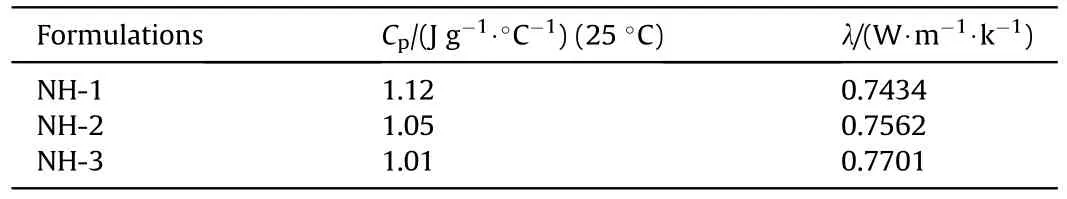
Table 5 CP and λ of NTO-based formulations.
Using ARC,the thermal decomposition processes of NH-1,NH-2 and NH-3 molding powder(100 mg)were recorded on the heating rates of 1 K/min.The pressure and the pressure rise rate as a function of temperature were displayed in Fig.6,and the detailed parameters were listed in Table 6.
As shown in Fig.6,the pressure was rising slowly after the initial decomposition temperature (T),which indicated that a tiny amount of gas was slowly produced.It could be interpreted as the sublimation of solid-phase NTO and thermal decomposition of gasphase NTO [16].Subsequently,the samples were igniting and undergoing autocatalysis reaction after a certain temperature (the temperature at ignition time,T) with different P(pressure at ignition time),thus the overpressure phenomenon and a fast pressure rise rate were observed in Fig.6.In this stage,samples were violently decomposed and a large amount of gas was generated rapidly.Undoubtedly,it was putting the munition at a risk for explosion and was undesirable for thermal safety of explosive.Moreover,the Tand Tof three formulas were both in the order of NH-1<NH-2<NH-3,which indicated that the thermal stability of formulations became better with the decrease of NTO content.Besides,the Pof NH-1was higher than that of NH-2 and NH-3.Because the NH-1 had the highest amount of NTO,more solidphase NTO could be sublimated and decomposed into gas.
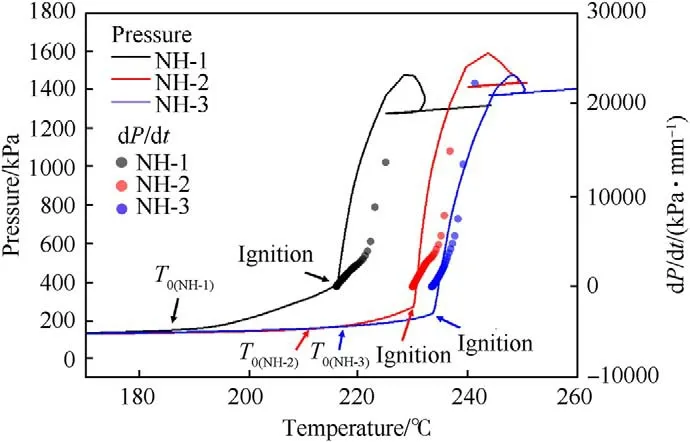
Fig.6. Plots of pressure and pressure rise rate versus temperature.
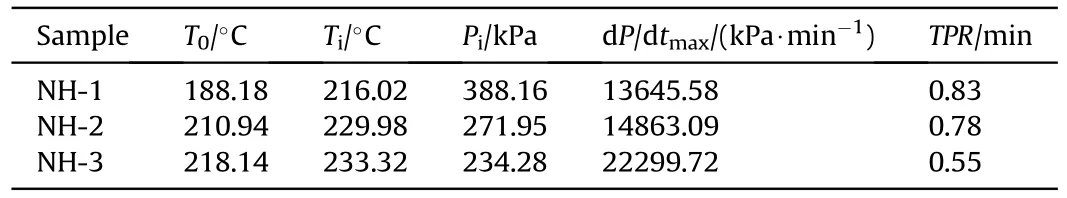
Table 6 The detailed decomposition parameters of explosives.
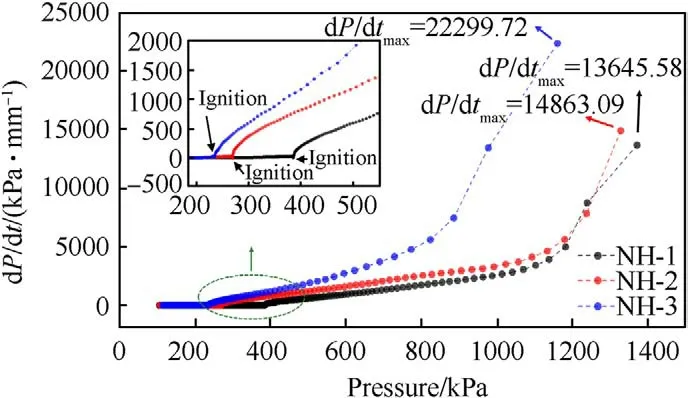
Fig.7. Plots of pressure rise rate versus pressure.
The shell bore a larger strain rate and easier to form shear crack under a faster pressure rise rate [17,18],thereby the pressure rise rate was an important parameter to describe the thermal decomposition process and the thermal safety performance.As shown in Fig.7,the pressure rise rates as a function of pressure were displayed.After ignition,the three samples exhibited different curve shapes in the plots of the pressure rise rate.The trend growth of the pressure rise rate of NH-3 was obviously faster than that of NH-1 and NH-2.In addition,the maximum pressure rise rate (dP/dt)of NH-3(22299.72 kPa/min)was also far higher than that of NH-1 (13645.58 kPa min) and NH-2 (14863.09 kPa/min).It meant the formulation with less NTO content would cause more serious destruction under the same constraint conditions,therefore reflecting a poor thermal safety performance.
Furthermore,the time to reach the maximum pressure rise rate after ignition (TPR) of NH-1,NH-2 and NH-3 were 0.83,0.78 and 0.55 min (listed in Table 6),respectively.TPR could also prove the intensity of thermal decomposition.The sample with a shorter TPR exhibited a faster decomposition reaction rate and a more violent response.Therefore,the decreasing TPR with the decrease of NTO content also made clear that the increase of NTO content improved the thermal safety of NTO/HMX composite explosives.
Slow cook-off experiment was also an important method to evaluate the thermal safety performance of explosives.The heating rate was 1C/min and experiments ended until the response occurred.We also took pictures of the post-experiment debris of NH-1,NH-2 and NH-3 (shown in Fig.8).

Fig.8. Post-experiment debris for NH-1,NH-2 and NH-3.

Fig.9. Plots of pressure versus time for NH-1,NH-2 and NH-3.
As shown in Fig.8,the weakest reaction was from NH-1.We found the slow cook-off bomb and heat tape were still approximately intact and a large number of yellow particles(uncompleted decomposition reactants) vented through the gap which was forming when one endcap of slow cook-off bomb was rushed out.After the response of NH-2,we found the shell wall was partly torn and one endcap was rushed out.Besides,the heat tape and pressure transducer were shocked away.Obviously,the most violent response was observed from NH-3.In Fig.8,not only the shell was blasted into several fragments,but also the tubing and pressure transducer were all destroyed.
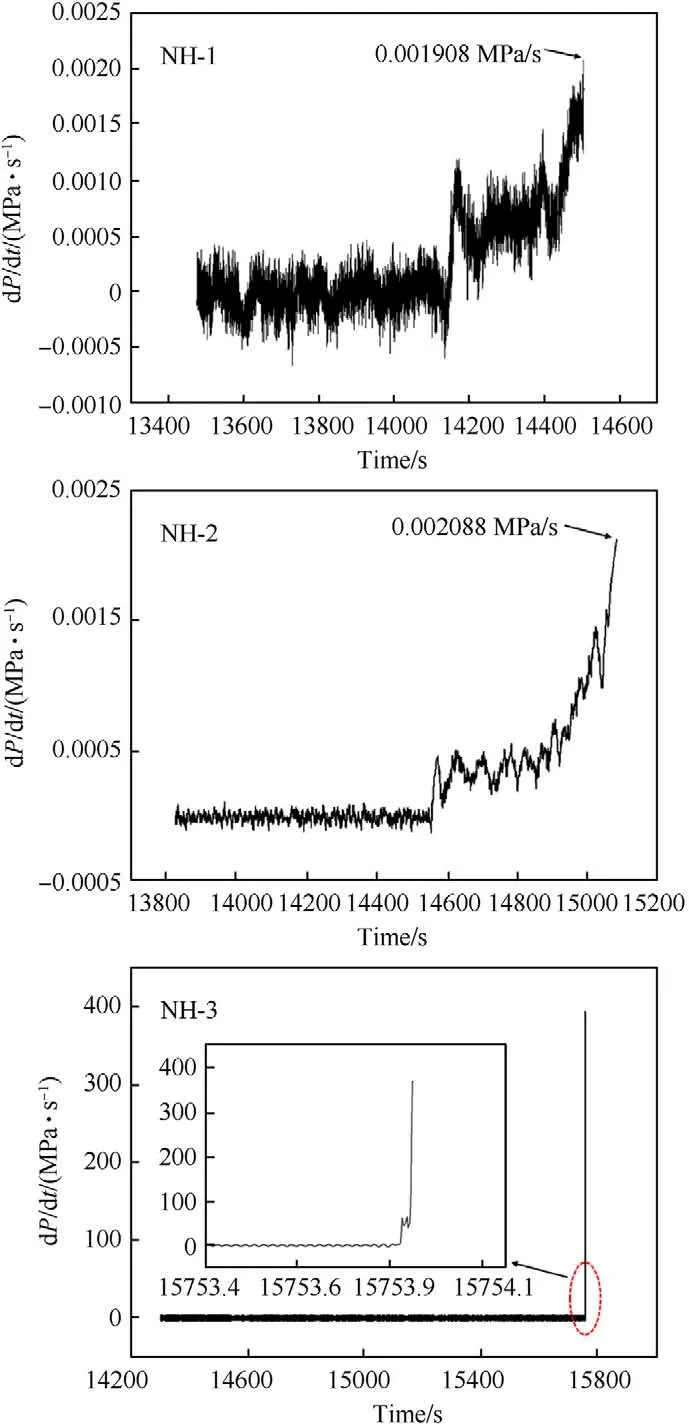
Fig.10. Plots of pressure rise rate versus time for NH-1,NH-2 and NH-3.
We expected the pressure playing an important role to affect the response.Fig.9 and Fig.10 presented the pressure and pressure growth rate versus time respectively,which were recorded by the pressure transducer before samples thermal explosion.
As shown in Fig.9,taking NH-1 as an example,the pressure kept rising from 217C(T)to 223C(T)with 515.30 s.In this stage,the decomposition and combustion of explosive lasted until the response occurred.By the end of this stage,the maximum pressure rise rate (dP/dt) of NH-1 reached 0.00198 MPa/s (shown in Fig.10).The pressure curve of NH-2 was similar to NH-1,however,it took a shorter time (339.93 s) from pressure rising to bomb response.In addition,the dP/dtof NH-2 was 0.002088 MPa/s which was slightly higher than that of NH-1.These results indicated that the intensity of NH-2 was more violent than that of NH-1.As to NH-3,an odd trend of pressure was presented,which increased dramatically in ultra-short time and reached an abnormally high value.Besides,the heat response temperature data were listed in Table 7.
In the cook-off experiments,the order of Tand Tof the three formulations were all NH-1<NH-2<NH-3.It meant Tand Tdecreased with the increase of NTO content,which indicated that the increase of NTO content became a disadvantage for thermal stability of NTO/HMX-based formulation.
As the results in this section,the formulation contained more NTO content would exhibit better thermal safety performance for NTO/HMX-based formulations,which were in good agreement by ARC analysis and slow cook-off experiment.
3.6.Slow cook-off simulation
The numerical simulations of slow cook-off processes for samples were conducted according to the experimental condition,in which the bomb was heated for 1C/min until the ignition reaction occurred.The temperature distributions at ignition time for NH-1,NH-2 and NH-3 were shown in Fig.11.
From Fig.11,the lowest temperature occurred at the endcaps of bomb,which owed the convection effect between endcaps and air.In addition,the ignition positions were all located at the volume center area of bomb and the temperature distributions were symmetrical at ignition time.To compare the results between experiment and simulation,the simulated ignition temperature at bombsurface(T)and center(T)of samples were summarized in Table 8.
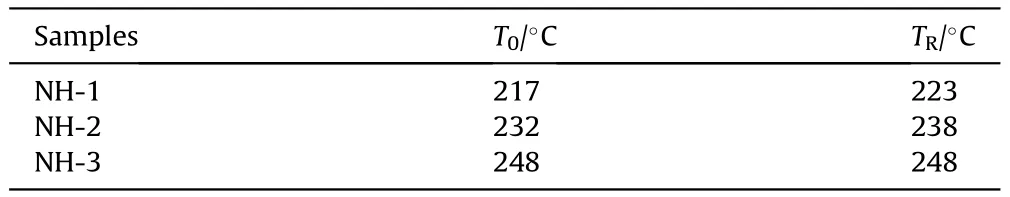
Table 7 Heat response temperatures of different formulations in cook-off test.
As listed in Table 8,Tof NH-1,NH-2 and NH-3 were 227.84C,239.53C and 245.43C,respectively,it showed a good agreement with the response temperature of cook-off experiment(within the error of 3%)and confirmed the validation of the selected simulation model.Simultaneously,it testified the accuracy of Twhich the value was all much higher than that of Tand was in reverse order(NH-3<NH-2<NH-1) comparing with T.As we expect,the numerical simulation we used in this paper was a good supplement for the experiments and a credible method to predict the response temperature.
4.Conclusion
A series of NTO/HMX-based composite explosives were well prepared and characterized via multiple experiments and simulation techniques.The study focuses on the effect of NTO to the performance of the formulations.The main conclusions were as follows:
(1) The density,enthalpy of formation,detonation velocity,detonation pressure and detonation heat increased with the decrease of NTO content in the NTO/HMX-based formulations.
(2) In the NTO/HMX-based formulations,the increase of NTO content in the formulation was beneficial to the decrease of mechanical sensitivities.In addition,the mechanical sensitivity of composite explosives was associated with the thermal conductivity,specific heat capacity and Arrhenius parameters.
(3) The results of ARC analysis and slow cook-off experiment were in a good agreement:the addition of NTO content in formulation will significantly enhance the thermal safety and reduce the thermal hazards of NTO/HMX-based formulation in confined spaces.Moreover,pressure and pressure rise rate could serve as an important indicator for judging the thermal safety performance of explosives in confined spaces.
(4) As a supplement for cook-off experiments,the numerical simulation obtained a good agreement with the response temperature of cook-off experiment.In addition,the numerical simulation was a credible method to predict theresponse temperature of cook-off experiment and visually displayed transient temperature distribution.
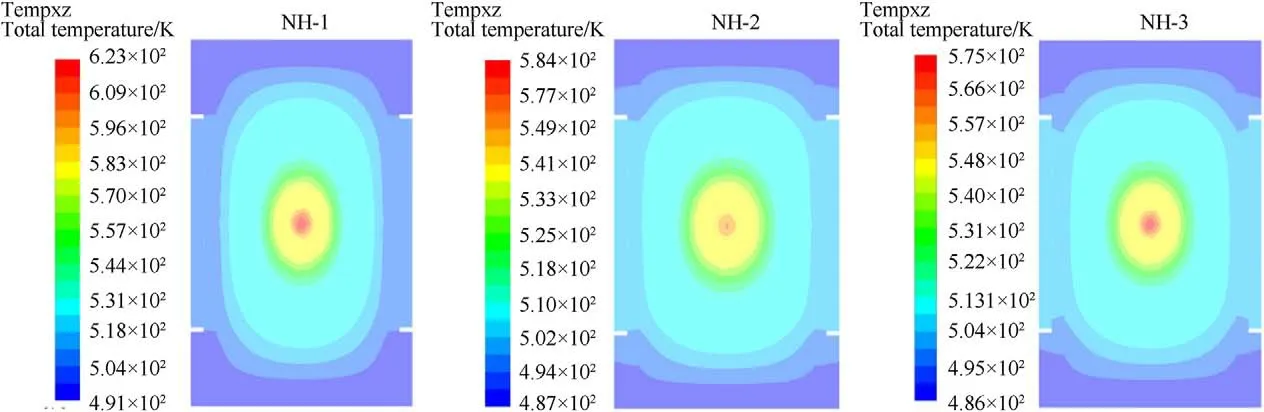
Fig.11. Temperature distribution at ignition time of NH-1,NH-2 and NH-3.
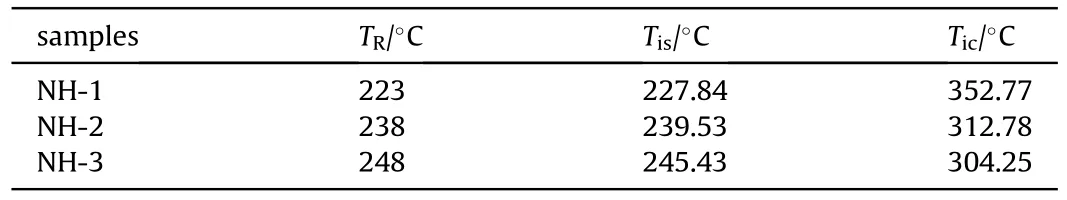
Table 8 The simulated temperature results of NH-1,NH-2 and NH-3.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors are grateful to the National Defense Foundation of China (3090021322001,3090020221912,3090021211903.) for financial support of this work.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.04.002.
- Defence Technology的其它文章
- Defence Technology
- A study on the surface overpressure distribution and formation of a double curvature liner under a two-point initiation
- Performances and direct writing of CL-20 based ultraviolet curing explosive ink
- One-step synthesis of FeO(OH) nanoparticles by electric explosion of iron wire underwater
- Driving force coordinated control of an 8×8 in-wheel motor drive vehicle with tire-road friction coefficient identification
- Monitoring and Prediction of the Vibration Intensity of Seismic Waves Induced in Underwater Rock by Underwater Drilling and Blasting

