Photothermal-chemical synthesis of P–S–H ternary hydride at high pressures
Tingting Ye(葉婷婷) Hong Zeng(曾鴻) Peng Cheng(程鵬) Deyuan Yao(姚德元)Xiaomei Pan(潘孝美) Xiao Zhang(張曉) and Junfeng Ding(丁俊峰)
1Key Laboratory of Materials Physics,Institute of Solid State Physics,HFIPS,Chinese Academy of Sciences,Hefei 230031,China
2University of Science and Technology of China,Hefei 230026,China
3Frontiers Science Center for Transformative Molecules,School of Chemistry and Chemical Engineering,Shanghai Jiao Tong University,Shanghai 200240,China
Keywords: hydride,superconductor,high pressure,Raman spectroscopy
1. Introduction
Hydrides are materials in which hydrogen is combined with other elements to form ionic,covalent,or interstitial systems. They initially came to prominence because of their ability to reversibly store large amounts of hydrogen under moderate conditions.[1–4]Hydrides are also important in storage battery technologies such as nickel-metal hydride battery.[5,6]Recently, hydrides are promising materials for the realization of high temperature superconductivity as they can combine the unique prerequisites for superconductivity such as high-frequency phonons, strong electron–phonon coupling,and a high density of the electronic states.[7]Superconductivity in hydrides has been reinvigorated since discovery of a remarkably high superconducting transition temperatureTCof 203 K at 150 GPa in H3S in 2015.[8]Many new hydrides are synthesized,[9–11]and record of the highestTCis broken by hydrides in rapid succession.[12–15]
To explore new hydrides withTCabove room temperature, the investigation into the mechanism of the highTCof H3S has been a hot topic and fruitful.[16–19]It is suggested that the superconductivity of H3S can be attributed to a conventional phonon-mediated mechanism,as it exhibits strong covalent bonds giving rise to large electron–phonon coupling.[16,20]Increasing the covalent character of the sulfur-hydrogen bond by replacing S atoms with chalcogens or other atoms is a good choice to further enhance the superconducting properties of H3S.[21]Several ternary hydrides based on the H3S structure are predicted to exhibit highTCbehavior.[21–24]Very recently, a carbonaceous sulfur hydride(C–S–H)synthesized through a photochemical reaction at high pressure exhibits room-temperature superconductivity up to 283 K.[14]In addition,first-principles calculations predict a relative higherTCup to 280 K via 2.5% P doping in SH3owing to P being a covalent atom lighter than S.[25]However,the P–S–H system has never been observed in experiments,and its physical properties are still awaiting investigation.
The room-temperature superconductor C–S–H is synthesized by a photochemical method and has a molecular guest–host structure based on its Raman spectra before metallization.[14]A guest–host compound(H2S)2H2with the same stoichiometry as H3S is found at low pressures[26]and is transferred to superconducting H3S above 100 GPa.[27,28]The familiar way to synthesize (H2S)2H2is by compressing H2S and H2together. This strategy is believed to be useful for synthesizing ternary guest–host compounds.[29]Our previous work reported the observation of H–S–Se ternary compounds using this method.[30]
Here, we report the synthesis of a ternary van der Waals compound of P–S–H through a photothermal-chemical reaction under pressure. For reference, H2S, PH3, and PH3–H2samples were also synthesized and investigated by Raman spectroscopy. The pressure dependent Raman spectra suggest that the P–S–H compound is in a guest–host structure and has H2molecule with prolonged H–H distance. P–H and S–H modes coexist in the new hydride and are different from known compounds.
2. Experimental details
Symmetric diamond anvil cells (DACs) equipped with anvils with central culets 250 μm in diameter were employed in the experiments. Re gaskets were indented to 40 μm thickness and laser-drilled into a hole with a 110 μm diameter as the sample chamber. Small pieces of S and red phosphorus (>99.99%) Predordered from Sigma-Aldrich were positioned separately in the chamber and then filled with H2gas at~200 MPa.
Because H2S will solidify at a low pressure of approximately 1.1 GPa,to obtain fluid H2S,[26]laser heating was performed at pressures of 0.9 GPa. A relatively high power above 200 mW from a 532 nm solid-state laser is essential to melt the powder precursor and prepare various hydrides. Higher laser power can dramatically increase the synthesis rate, and 460 mW was adopted in the experiments. The laser sharply focused to a spot 2–3 μm in diameter and irradiated Predand S successively and moved back and forth. Synthesis of H2S and PH3was judged from Raman spectra measured. After finishing the synthesis of H2S and PH3,the sample was compressed up to 36 GPa at room temperature in the DAC.
Pressure was determined using the ruby fluorescence.[31]For the Raman experiments, a backscattering geometry was adopted for confocal measurements with incident laser wavelengths of 532 nm.[32,33]The Raman notch filters were of a very narrow bandpass (Optigrate) allowing Raman measurements down to 10 cm-1in the Stokes and anti-Stokes. One of these notch filters was used as a beam splitter to inject the laser into the optical path.
3. Results and discussion
After laser heating on red phosphorus(Pred)and H2,Predis melted by the laser under visual observation.However,solid Predwill generate back when we shot a moderate laser on the H2region to measure the Raman spectrum. This can indicate that the synthesized compounds of P and H are unstable and in a fluid state. A new sharp Raman peak arises at approximately 2330 cm-1after we heat Predagain for a long time (a few minutes) using a 460 mW laser, as shown in Fig. 1(b). This peak can be assigned to the P–H stretching mode,[34]indicating the synthesis of the stable compound PH3. In Fig. 1(a),PH3is fluid and can only be detected on and near P. On the other hand,H2S is easily obtained by heating S and H2. H2S with a Raman peak at approximately 2620 cm-1fills the whole chamber.

In Fig. 1(c), H2S solidifies into a transparent crystal at 3 GPa. PH3remains in a fluid state at such pressures. At 4.6 GPa,solid H2S turns black opaque and disappears quickly.Instead, some new crystals grow out near or attached on one side of the gasket, as labeled in Fig.1(d). They are all transparent but appear to have lower transparency than solid H2S.The Raman spectrum of these new crystals in Fig.1(b)shows the coexistence of slightly broad peaks of the P–H stretching mode, high-intensity S–H stretching mode, and H–H vibron. In particular, the H–H vibron splits into two peaks at approximately 4200 cm-1. One peak with a higher intensity originates from pure H2, and the other peak has a lower frequency and implies the synthesis of H2molecule guest–host compounds, such as (H2S)2H2.[26]It is plausible that these new crystals are ternary compounds composed of P, S, and H.The P–H stretching mode is no longer detected at other positions, which suggests that PH3solidifies into new crystals.On the other hand,some fluid H2S still exists in the chamber,as a high-frequency S–H mode can be detected everywhere.At 7.8 GPa, crystals change into two types according to virtual observation in Fig.1(e). The first kind of crystal turns to black opaque(marked as A),and the other one is rather bright(marked as B).
To further reveal the components of the two kinds of crystals, the Raman spectra of both crystals were investigated at different pressures, as shown in Fig. 2. At 7.8 GPa, the S–H stretching modes near 2500 cm-1and H–H vibron near 4200 cm-1of the two types of crystals are the same. Crystal A has only one weak peak of P–H stretch at approximately 2400 cm-1in the Raman spectra,similar to a shoulder in the S–H stretching mode. Crystal B has two clear peaks in the P–H stretching region in Fig.2. Meanwhile,two sharp peaks appear at approximately 600 cm-1and 1050 cm-1in crystal B, showing prominences in the roton mode of H2. In Fig. 2,the P–H stretching mode at 9.9 GPa disappears in crystal A,and all the Raman peaks could be attributed to S–H stretching modes and H–H vibrons,which indicates that crystal A is van der Waals compound (H2S)2H2with a molecular guest–host structure,as reported in earlier research on S–H compounds at high pressures.[26]For crystal B,the asymmetric broad band of the S–H stretching mode at approximately 2500 cm-1results in two clear peaks on top. The intensity of the P–H stretching mode near 2400-1increases remarkably. Some weak peaks below 220 cm-1originate from the lattice mode of H2S, indicating orientational ordering. The Raman spectra figure out transferring and gathering of P–H compound in crystal B coexisting with S–H and H–H bonds,which implies the formation of P–S–H ternary hydride.


In the following,let us focus on crystal B,which is plausible to be a P–S–H ternary compound. The pressure dependencies of the Raman spectra of crystal B up to 35.6 GPa are measured as shown in Fig. 3. According to the Raman active frequency of different bonds in the literature,[14,28,35–37]the Raman spectra are divided into three regions,namely,the lattice and bending modes below 1200 cm-1, the stretching modes of P–H and S–H between 1600 cm-1and 2800 cm-1,and the H–H vibron above 4050 cm-1. The origin of the Raman modes in crystal B is discussed in detail as follows.
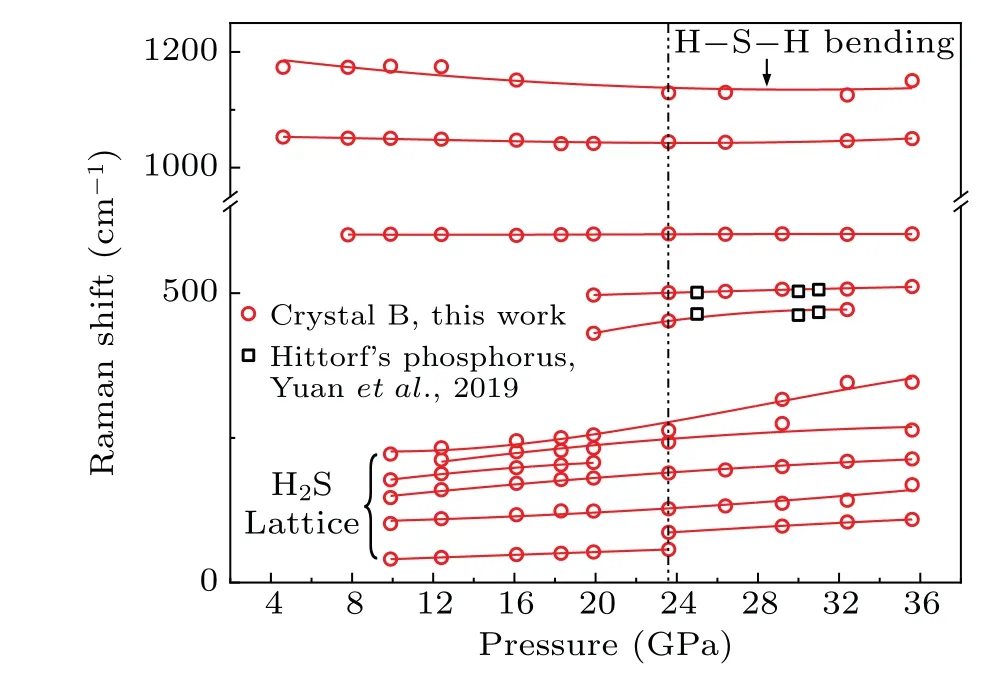
In Fig. 4, the lattice modes under 400 cm-1of crystal B could be attributed to the H2S molecule.[14,35]The highestfrequency peak near 1200 cm-1can be distributed to the H–S–H bending mode. Two peaks at 601 cm-1and 1050 cm-1are beyond example[38,39]and exhibit extraordinarily little change with pressure. To the best of our knowledge, authentic attribution of these peaks is not found from the earlier literature,suggesting the synthesis of a new compound at 4.6 GPa. Upon compression,two new peaks near 400 cm-1and 500 cm-1appear at approximately 20 GPa because the sample decomposes and generates Hittorf’s phosphorus.[36]Meanwhile, H2S lattice modes show disappearance for the peak at 180 cm-1and discontinuousness for the peak at 30 cm-1,which indicates a phase transition in crystal B near 23.6 GPa. The two new Raman modes in Fig.4 suggest the synthesis of a new compound differing from H–S or P–H compounds at 4.6 GPa.
Pressure dependencies of stretch modes for crystal B are shown in Fig.5. For reference, H2S,PH3, and PH3–H2samples were also synthesized individually by a photothermalchemical method. The Raman modes of crystal B under pressure are compared with those of the H2S, PH3, and PH3–H2samples and analyzed as follows. First, the dotted lines in Fig.5 show that four peaks of S–H stretching modes in crystal B are similar to (H2S)2H2,[28]which suggests that crystal B adopts the framework of (H2S)2H2. Second, the two Raman peaks at 2300 cm-1and 2350 cm-1are in correspondence with PH3[36]or(PH3)2H2[37]at low pressures,indicating that these two peaks could be attributed to the P–H stretching mode. Another Raman peak at 2380 cm-1is in good accord with the earlier report of (PH3)2H2at 4.6 GPa.[37]As(PH3)2H2is insufficiently studied by Raman spectroscopy for pressures above 7 GPa in the literature, we synthesized PH3-H2samples and investigated their Raman spectra in detail to further reveal the origin of the Raman peaks for crystal B at higher pressures, as shown in Fig. 5. In the low-pressure region below 7 GPa, the P–H bond of the reference sample matches the earlier reports of (PH3)2H2(squares in Fig. 5),indicating good sample quality and an authentic experimental setup. The pressure dependencies of the P–H stretching mode of crystal B are similar to those of the reference (PH3)2H2sample in the low-pressure region and show notable deviation above 7 GPa. Thus, the three peaks between 2300 cm-1and 2450 cm-1of crystal B belong to the P–H stretching mode but do not originate from (PH3)2H2or PH3. Third, the other three peaks(circles with solid lines)diverge from any known S–H or P–H compounds,which indicates that crystal B is not a mixture of S–H and P–H compounds. Two phase transitions could be defined according to the disappearance and appearance of Raman peaks,one at approximately 23.6 GPa and the other one at approximately 32.8 GPa. The stretching modes in Fig.5 suggest that crystal B is a van der Waals compound that contains PH3molecules.
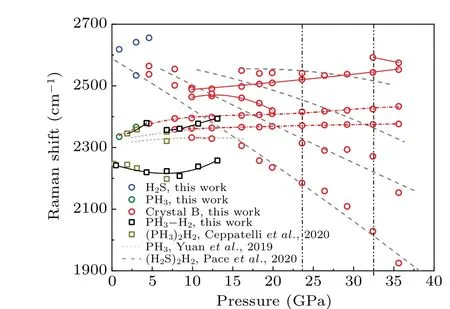
The H–H vibron modes in crystal B are consistent with(H2S)2H2[28]in Fig.6,indicating that crystal B has(H2S)2H2as a framework. We note that there are still some small distinctions between crystal B and (H2S)2H2. The first splitting of the H–H vibron arises at 23.6 GPa for crystal B, higher than 16.7 GPa for(H2S)2H2. In addition,two vibron modes at approximately 4140 cm-1and 4170 cm-1for(H2S)2H2cannot be clearly observed in crystal B. The discrepancies may originate from the insertion of PH3molecular in(H2S)2H2for crystal B.It is also possible that the unobserved splitting and vibron modes hide in the broad main peak near 4150 cm-1.On the other hand,the H–H vibron of(PH3)2H2does not exist in crystal B. Two phase transition pressures determined from the H–H vibron are consistent with the values of 23.6 GPa and 32.8 GPa in Fig.5. The H–H vibron in crystal B supports that the ternary compound is a mixed alloy with the PH3molecule inserted into the(H2S)2H2framework.

According to the pressure dependencies of Raman modes,the three features of crystal B are listed as follows. First,crystal B is a new compound formed at 4.6 GPa, which can persist up to 35.6 GPa with at least two phase transitions under pressure. Second, the P–H stretching modes in crystal B are similar to those in PH3or(PH3)2H2in the low-pressure region and differ above 7 GPa,suggesting that crystal B contains PH3molecules.Third,crystal B adopts the framework of(H2S)2H2with notable differences in the H–H vibron above 23.6 GPa.Thus, crystal B is very likely to be a P–S–H ternary hydride with the guest–host structure, namely, the PH3molecule inserts into the (H2S)2H2host lattice as a guest similar to the room-temperature superconductor C–S–H.[14]Accurate structural analysis is always extremely challenging for hydride superconductors because the light elements in hydrides lead to a very weak signal in x-ray scattering techniques.[8,12,14,40]Raman spectroscopy is a powerful and frequently used tool to probe the chemical and structural transformations of hydrides under pressure.[41,42]In fact, the crystal structure for the well-known C–S–H is still in debt. Theoretical calculations have hypothesized several different crystal structures for C–S–H.[14,43]To further reveal the structural properties of the P–S–H ternary hydride,synchrotron x-ray diffraction and theoretical analysis are needed in the future.
The guest–host structures for both (H2S)2H2and C–S–H hydrides at low pressures become the building blocks of superconducting compounds with strong covalent character at high pressures, which exhibit high transition temperature superconductivity driven by strong electron–phonon coupling to high-frequency hydrogen phonon modes.[14,44,45]An earlier theoretical study has prophesied that the P–S–H ternary hydride shows a superconducting transition temperatureTCup to 280 K with a cubic structure in theImˉ3mphase.[25]Although the P–S–H in this report is a van der Waals compound with a guest–host structure,the new ternary hydride is also plausible to transfer into a covalent compound through atomic substitution at high pressures.
4. Conclusion
P–S–H ternary hydride was synthesized at high pressures,and the Raman spectra were investigated in detail. The coexistence of the P–H stretching mode, S–H stretching mode and H–H vibration suggests the formation of a P–S–H compound at 4.8 GPa. Pressure dependencies of the Raman mode up to 35.6 GPa indicate that the ternary hydride is a van der Waals compound with a guest–host structure similar to the room-temperature superconductor C–S–H. Two phase transitions appear at approximately 23.6 GPa and 32.8 GPa for the P–S–H ternary hydride.
Acknowledgements
Project supported by the National Natural Science Foundation of China(Grant Nos.52002372,51672279,51727806,11874361, and 11774354), Science Challenge Project (Grant No.TZ2016001), and Chinese Academy of Sciences Innovation Grant(Grant No.CXJJ-19-B08).
- Chinese Physics B的其它文章
- Switchable terahertz polarization converter based on VO2 metamaterial
- Data-driven parity-time-symmetric vector rogue wave solutions of multi-component nonlinear Schr¨odinger equation
- Neutron activation cross section data library
- Multi-phase field simulation of competitive grain growth for directional solidification
- A novel similarity measure for mining missing links in long-path networks
- Effects of electrical stress on the characteristics and defect behaviors in GaN-based near-ultraviolet light emitting diodes