抑制MCL-1可逆轉PD-L1敲除引起的結直腸癌細胞對司美替尼化療敏感性下降*
孫磊, 洪楚原, 尹雪霞, 郭雄波, 張實, 鐘北平, 王國強
抑制MCL-1可逆轉敲除引起的結直腸癌細胞對司美替尼化療敏感性下降*
孫磊, 洪楚原, 尹雪霞, 郭雄波, 張實, 鐘北平, 王國強△
(廣州醫科大學附屬第二醫院胃腸外科,廣東 廣州 510260)
明確程序性死亡配體1programmed death ligand-1,-敲除是否可引起結直腸癌(colorectal cancer, CRC)細胞對司美替尼化療敏感性下降,并探尋逆轉-敲除引起的司美替尼化療敏感性下降的方法。人結腸癌RKO細胞及敲除-的RKO細胞接受司美替尼處理后,Western blot檢測兩組細胞中活化的胱天蛋白酶3(cleaved caspase-3, C-Casp-3)蛋白水平的差異,流式細胞術及細胞集落形成實驗分別檢測兩組細胞的凋亡率及細胞存活率。沉默髓細胞白血病基因1(myeloid cell leukemia-1,-)或AZD5991聯合司美替尼處理后,Western blot檢測RKO及敲除-的RKO細胞中C-Casp-3蛋白水平,流式細胞術及細胞集落形成實驗分別檢測細胞的凋亡率及細胞存活率。司美替尼聯合AZD5991處理小鼠結腸腺癌MC38細胞及沉默-的MC38細胞,Western blot檢測細胞中C-Casp-3蛋白水平,流式細胞術及細胞集落形成實驗分別檢測細胞的凋亡率及細胞存活率。Western blot結果發現,司美替尼可顯著降低磷酸化細胞外信號調節激酶(phosphorylated extracellular signal-regulated kinase, p-ERK)蛋白水平,敲除-可顯著降低細胞中司美替尼誘導產生的C-Casp-3,而-shRNA或聯合使用AZD5991可顯著增加細胞中司美替尼誘導產生的C-Casp-3。流式細胞術結果發現,敲除-顯著降低了司美替尼誘導產生的細胞凋亡,而-shRNA或聯合使用AZD5991均可顯著增加司美替尼誘導產生的細胞凋亡。細胞集落形成結果發現,敲除-顯著提高了低濃度司美替尼條件下細胞的存活率,而-shRNA或聯合使用AZD5991均可顯著降低細胞的存活率。更重要的是,-shRNA或聯合使用AZD5991均有效逆轉了-敲除引起的C-Casp-3、細胞凋亡及細胞存活率的改變。敲除-可引起CRC細胞對司美替尼化療敏感性下降,沉默-基因或聯合使用AZD5991可逆轉-敲除引起的CRC細胞對司美替尼化療敏感性下降。
髓細胞白血病基因1;程序性死亡配體1;司美替尼;結直腸癌;化療敏感性
在我國,結直腸癌(colorectal cancer, CRC)的發病率在所有惡性腫瘤中排第三位,且發病率逐年升高。靶向治療是CRC綜合治療的重要組成部分,然而藥物耐藥嚴重影響了患者的治療效果,成為影響患者預后的重要因素之一。因此,明確CRC化療耐藥的相關機制并探尋相應的治療靶點,這對改善CRC患者的預后具有重要的研究意義。
腫瘤細胞表面的程序性死亡配體1(programmed death ligand-1, PD-L1;又稱為CD274或者B7H1)是重要的免疫檢查蛋白,可通過與T細胞表面受體程序性死亡蛋白1(programmed death-1, PD-1)特異性結合引起免疫逃逸,而阻斷PD-L1/PD-1之間的相互作用可有效抑制多個腫瘤的發展。目前針對PD-L1/PD-1的免疫治療在多個腫瘤中顯示出巨大的臨床價值,其中腫瘤組織PD-L1蛋白表達陽性被認為是可采取抗PD-L1/PD-1治療的重要標記物[1]。然而,在CRC中僅有約10%的患者腫瘤組織PD-L1蛋白表達陽性,絕大部分患者難以從抗PD-L1/PD-1免疫治療中獲益[2-3]。PD-L1除了發揮其免疫檢查點的功能外,其自身可通過調節相關信號通路在腫瘤發生發展中扮演重要角色。研究發現,PD-L1在多個腫瘤細胞中發揮抗凋亡作用并參與腫瘤的化療耐藥[4]。在卵巢癌中,PD-L1可通過抑制自噬及激活mTOR信號通路抑制卵巢癌細胞的增殖,并在免疫正常的小鼠中發現沉默-可顯著抑制腫瘤的生長[5]。在黑色素瘤中,PD-L1通過mTOR信號通路促進腫瘤細胞的增殖[6]。在乳腺癌中,PD-L1可促進細胞增殖并通過JAK2/STAT3信號通路參與腫瘤的化療耐藥[7];在胃癌中,沉默-可影響腫瘤細胞的增殖及遷移[8]。以上研究結果表明PD-L1除了發揮其免疫功能外,在腫瘤的增殖、凋亡及化療耐藥中均可發揮重要的作用。因此,明確PD-L1在CRC中的非免疫功能有著重要的研究價值和臨床意義。本研究擬探討在CRC細胞中敲除-對司美替尼化療敏感性的影響,并探尋逆轉-敲除引起的司美替尼化療敏感性下降的治療方法。
材料和方法
1 細胞、主要試劑和儀器
人結腸癌RKO細胞、小鼠結腸腺癌MC38細胞及敲除-的RKO和MC38細胞株均由美國梅奧診所秦波教授贈送。
慢病毒感染相關試劑購自Sigma;所有抗體均購自Cell Signaling Technology;Annexin V和PI購自Becton Dickinson;Gimasa試劑購自Sigma;細胞培養箱購自Thermo;流式細胞儀購自Bio-Techne。
2 方法
2.1慢病毒感染細胞培養于6 cm培養皿中,當細胞達到80% 融合時更換含有6 mg/L polybrene的1 mL新鮮培養基,并加入2 mL含髓細胞白血病基因1(myeloid cell leukemia-1,-) shRNA質粒的慢病毒懸液,培養箱孵育12 h后,更換含嘌呤霉素(1∶5 000)的新鮮培養基進行篩選,待細胞數量足夠時采用Western blot測定感染效率。
2.2Western blot收集培養皿中的所有細胞至15 mL離心管,1 500 r/min離心5 min,棄去上清后冷的PBS洗滌細胞,加入SDS裂解液裂解細胞并100 ℃煮沸20 min。樣本上樣到SDS-PAGE凝膠孔中,100 V電泳1 h,蛋白轉至PVDF膜后用5%牛奶室溫封閉30 min。PBST洗膜3次后加入Ⅰ抗4 ℃孵育過夜,PBST洗膜3次后加入Ⅱ抗孵育1 h,PBST洗膜3次后加顯影劑顯影曝光并掃描拍照。
2.3流式細胞術每個樣本設置3個重復孔,藥物處理48 h后,收集培養皿中所有細胞至15 mL離心管,1 500 r/min離心5 min,棄去上清,冷的PBS洗細胞2次,100 μL 1× Binding Buffer重懸細胞,每管分別加入Annexin V和PI染色,以上操作全部在冰上完成。室溫靜置30 min后上流式細胞儀測定結果。
2.4細胞集落形成實驗細胞融合度約為80%時收集細胞至15 mL離心管,用0.25%胰蛋白酶消化后吹打成單個細胞,1 500 r/min離心5 min,PBS洗滌后無血清培養基重懸細胞,細胞計數后使用不同藥物濃度的無血清培養基將細胞密度稀釋至5×105/L,2 mL細胞懸液加入6孔板中,每個濃度設3個重復孔。14 d后,棄培養基,PBS洗滌細胞后加入甲醛固定細胞5 min,吸去甲醛加入Gimsa染常溫染色30 min,自來水緩流洗去染色液,孔板干燥后計數細胞集落的數量。
3 統計學處理
采用SPSS 16.0軟件行統計分析。所有數值均采用均數±標準差(mean±SD)表示。組間均數比較采用獨立樣本檢驗。以<0.05為差異有統計學意義。
結果
1 敲除PD-L1降低CRC細胞對司美替尼的化療敏感性
RKO細胞及敲除-的RKO細胞經過司美替尼的處理后,Western blot檢測相關蛋白的表達,結果發現司美替尼可顯著降低p-ERK蛋白水平,表明司美替尼可有效阻斷MEK/ERK信號通路,并且司美替尼可顯著抑制PD-L1蛋白的表達,這與之前的研究結果一致[9];重要的是,敲除-可顯著降低細胞中C-Casp-3蛋白水平(圖1A)。流式細胞術結果發現,敲除-可顯著降低司美替尼引起的細胞凋亡(圖1B)。細胞集落形成實驗證實,在低濃度的司美替尼培養基中,敲除-可顯著提高RKO細胞的存活率(圖1C)。以上結果表明,敲除-可降低CRC細胞對司美替尼的化療敏感性。
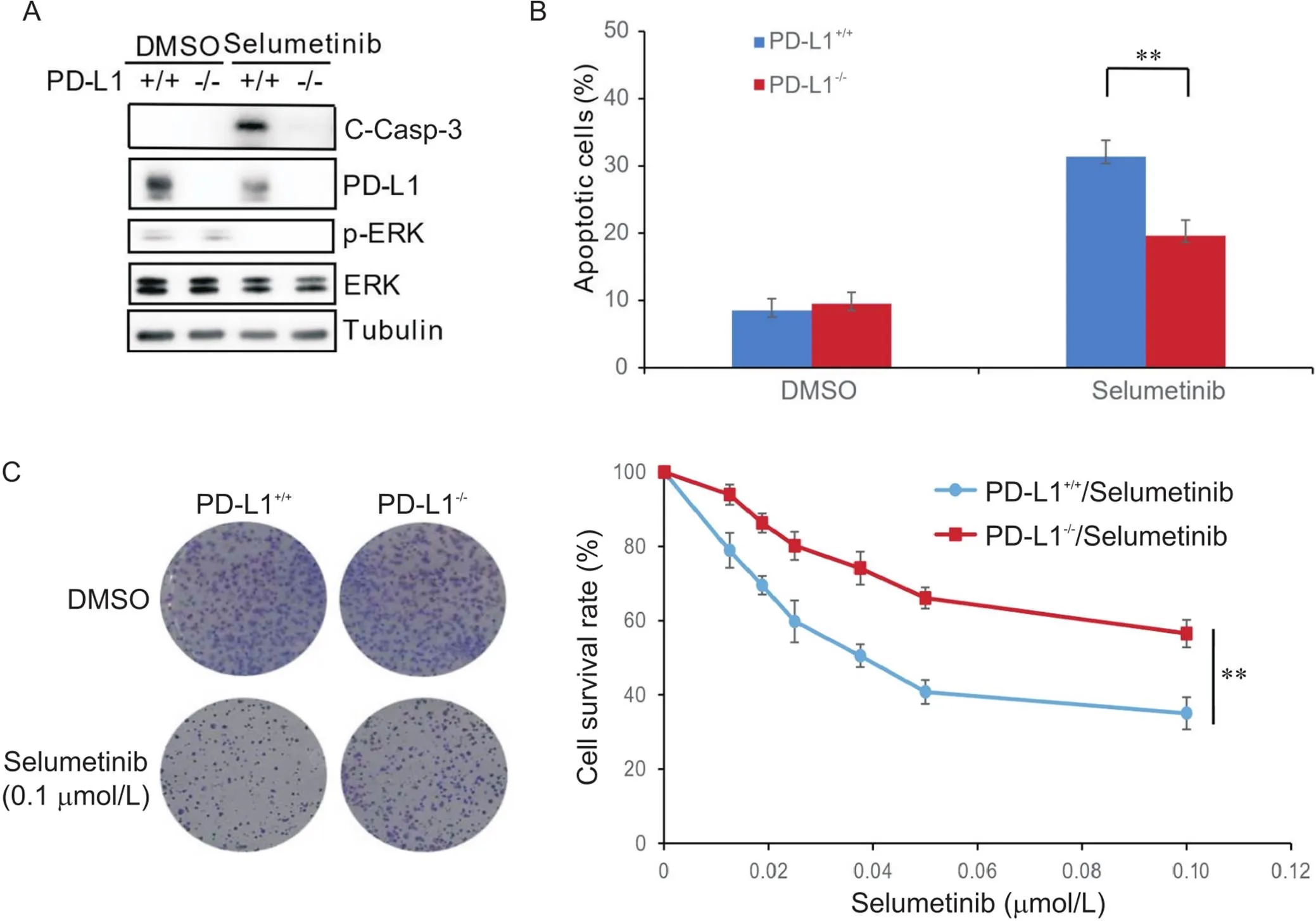
Figure 1. Knockout of PD-L1 decreased chemosensitivity of human colon carcinoma RKO cells to selumetinib. A: Western blot results showed that selumetinib significantly decreased p-ERK protein level, and knockout of PD-L1 significantly decreased selumetinib-induced cleaved caspase-3 (C-Casp-3) protein level; B: flow cytometric results showed that knockout of PD-L1 significantly increased the apoptotic rate after selumetinib treatment; C: colony formation assay results showed that knockout of PD-L1 significantly increased the cell survival rate after selumetinib treatment. Mean±SD. n=3. **P<0.01.
2 沉默MCL-1可逆轉PD-L1敲除引起的CRC細胞對司美替尼化療敏感性下降
MCL-1可通過結合Bim蛋白抑制線粒體途徑的細胞凋亡,而司美替尼可通過激活caspase通路誘導細胞凋亡。因此,我們推測沉默-可能會逆轉敲除-引起的CRC細胞對司美替尼的耐藥性。首先,在RKO及敲除-的RKO細胞中慢病毒感染沉默-,然后加入司美替尼處理細胞48 h。Western blot結果發現,沉默-可顯著升高兩組細胞中C-Casp-3蛋白水平,且兩組細胞之間C-Casp-3蛋白水平未見顯著差異(圖2A)。流式細胞術結果發現,沉默-可顯著增加司美替尼引起的細胞凋亡,并逆轉了-敲除引起的兩組細胞凋亡的差異(圖2B)。

Figure 2. Knockdown of MCL-1 reversed PD-L1 knockout-decreased chemosensitivity of human colon carcinoma RKO cells to selumetinib. A: Western blot results showed that knockdown of MCL-1 significantly increased selumetinib-induced C-Casp-3 protein level in both RKO and PD-L1 knockout RKO cells, and no significant difference was shown in the two cell lines; B: flow cytometric results showed that knockdown of MCL-1 significantly increased the apoptosis of both RKO and PD-L1 knockout RKO cells induced by selumetinib, and no significant difference was shown in the two cell lines. n=3. **P<0.01.
3 聯合使用MCL-1抑制劑AZD5991可逆轉PD-L1敲除引起的司美替尼化療敏感性下降
AZD5991是MCL-1的特異性抑制劑,我們聯合使用AZD5991和司美替尼共處理RKO細胞48 h。Western blot結果發現,聯合使用AZD5991可顯著升高兩組細胞中C-Casp-3蛋白水平,兩組細胞之間C-Casp-3蛋白水平未見顯著差異(圖3A)。流式細胞術結果表明,聯合使用AZD5991可顯著增加司美替尼引起的細胞凋亡,并逆轉了-敲除引起的細胞凋亡的差異(圖3B)。細胞集落形成實驗結果發現,聯合使用AZD5991可顯著降低低司美替尼濃度下的細胞存活率,并逆轉了-敲除引起的細胞存活率的差異(圖3C)。

Figure 3. Combination treatment with AZD5991 reversed PD-L1 knockout-decreased chemosensitivity of human colon carcinoma RKO cells to selumetinib. A: Western blot results showed that combination treatment with AZD5991 significantly increased selumetinib-induced cleaved caspase-3 (C-Casp-3) protein level in both RKO and PD-L1 knockout RKO cells, and no significant difference was shown in the two cell lines; B: flow cytometric results showed that combination treatment with AZD5991 significantly increased selumetinib-induced apoptosis of both RKO and PD-L1 knockout RKO cells, and no significant difference was shown in the two cell lines; C: colony formation assay results showed that combination treatment with AZD5991 significantly decreased the survival rate of both RKO and PD-L1 knockout RKO cells, and no significant difference was shown in the two cell lines. Mean±SD. n=3. **P<0.01.
為進一步證實以上研究結果,我們在小鼠結腸腺癌MC38細胞中重復上述實驗,結果發現,沉默-可顯著降低細胞中C-Casp-3蛋白水平及司美替尼引起的細胞凋亡,增加了低司美替尼濃度下的細胞存活率;聯合使用AZD5991可逆轉-敲除引起的C-Casp-3、細胞凋亡及細胞存活率的差異(圖4)。
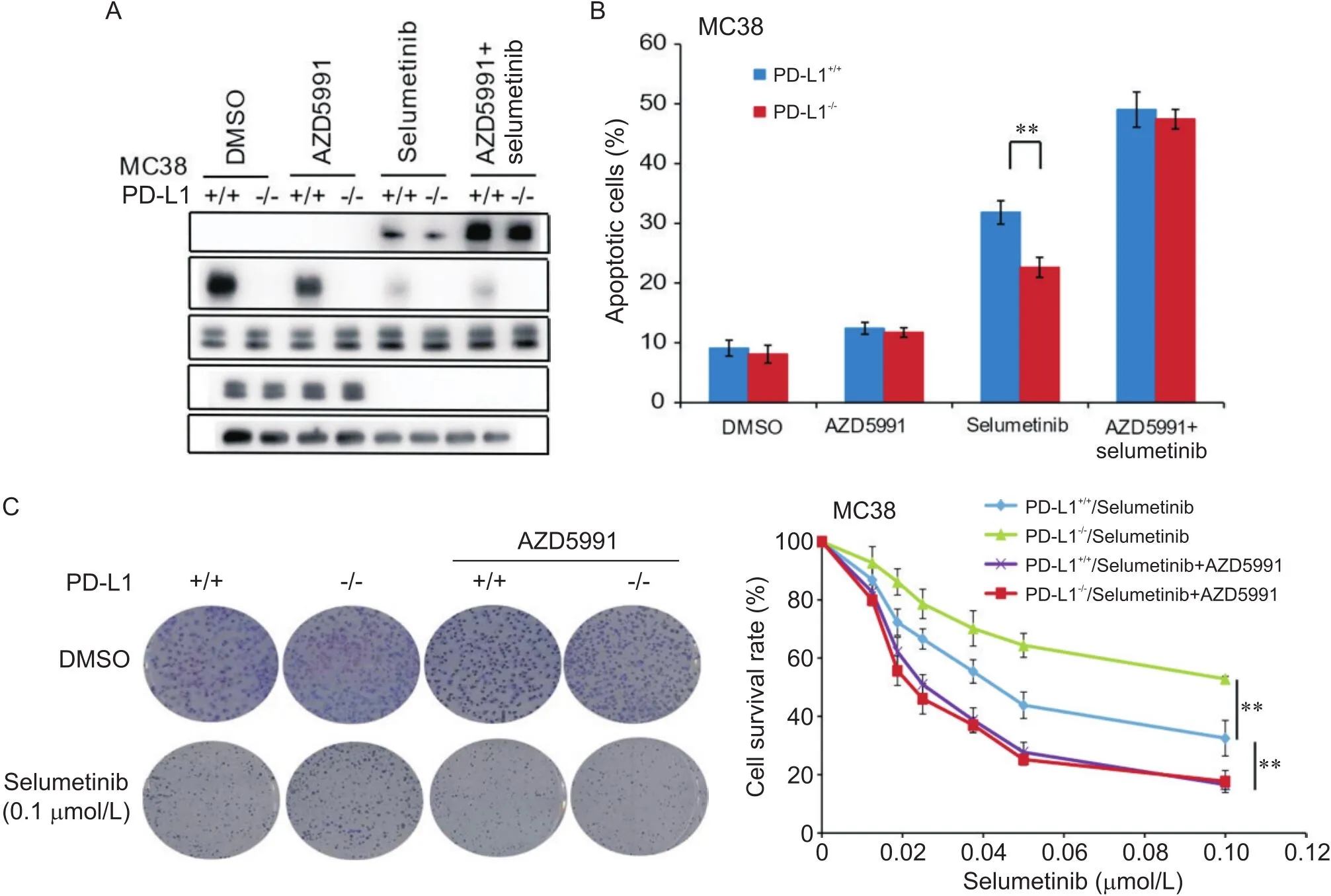
Figure 4. Combination treatment with AZD5991 reversed PD-L1 knockout-decreased chemosensitivity of mouse colon adenocarcinoma MC38 cells to selumetinib. A: Western blot results showed that combination treatment with AZD5991 significantly increased selumetinib-induced cleaved caspase-3 (C-Casp-3) protein level in both MC38 and PD-L1 knockout MC38 cells, and no significant difference was shown in the two cell lines; B: flow cytometric results showed that combination treatment with AZD5991 significantly increased selumetinib-induced apoptosis of both MC38 and PD-L1 knockout MC38 cells, and no significant difference was shown in the two cell lines; C: colony formation assay results showed that combination treatment with AZD5991 significantly decreased the survival rate of both MC38 and PD-L1 knockout MC38 cells, and no significant difference was shown in the two cell lines. Mean±SD. n=3. **P<0.01.
以上實驗結果表明,聯合AZD5991可顯著提高兩組細胞對司美替尼的化療敏感性,并逆轉了-敲除引起的細胞對司美替尼化療敏感性下降。
討論
PD-L1是重要的免疫檢查點,并且抗PD-L1/PD-1治療在部分腫瘤患者中取得令人矚目的療效,因此,PD-L1與PD-1結合后誘導的免疫逃逸機制成為目前的研究熱點。然而,除了免疫檢查點的功能外,PD-L1自身在腫瘤的發展中同樣發揮著重要的作用。PD-L1在腫瘤細胞或者免疫細胞中的表達水平被認為是抗PD-L1/PD-1治療有效的預測因子[10],然而,在CRC組織中,PD-L1表達的陽性率較低[2-3],并且部分表達陽性患者對抗PD-L1/PD-1治療無效。因此,明確PD-L1自身在腫瘤發展中的機制同樣有著重要的作用。在胰腺癌小鼠模型中發現,抑制PD-L1可通過抑制PI3K/Akt/mTOR信號通路抑制腫瘤的生長及轉移[11];在人頭頸部鱗癌及肺腺癌組織中發現,PD-L1可促進上皮間質變,細胞中E-cadherin表達下降,而vimentin表達增加[12-13]。此外,PD-L1在腫瘤化療耐藥中同樣發揮重要的作用,在卵巢癌細胞中,沉默-通過抑制P-gp及細胞周期蛋白D1進而增加細胞對順鉑的化療敏感性[14],在頭頸部鱗癌中,沉默-可顯著增加順鉑耐藥細胞的化療敏感性[15]。目前,PD-L1在CRC化療耐藥中的作用及機制的研究較少。司美替尼是MEK1/2信號通路特異性抑制劑,目前已批準應用于多個腫瘤的治療,其作用機制主要是通過抑制ERK1/2的磷酸化阻斷下游通路,并通過激活caspase通路誘導細胞凋亡。本研究首次發現,沉默-可引起CRC細胞對司美替尼化療耐藥,進一步證實了PD-L1除了發揮免疫檢查點的功能外,還可通過其自身的表達水平影響CRC的化療敏感性。
MCL-1是Bcl-2家族中重要的抗凋亡蛋白,主要通過與相關蛋白相互作用參與細胞凋亡的調節。經典的線粒體途徑凋亡中,腫瘤細胞在外部刺激下可通過多個信號通路激活Bim蛋白,活化的Bim蛋白從微管蛋白復合體上釋放,移位于線粒體膜,活化BAK/BAX促凋亡因子,使得寡聚化的BAX/BAK插入線粒體外膜,導致其通透性改變并釋放細胞色素C,細胞色素C與凋亡蛋白酶活化因子1形成凋亡小體,凋亡小體與凋亡蛋白caspase作用并進一步激活caspases級聯反應,最終導致細胞凋亡[16]。正常情況下,腫瘤細胞內的抗凋亡蛋白MCL-1通過與Bim蛋白結合抑制腫瘤細胞的凋亡,但在某些藥物的作用下,MCL-1與Bim蛋白的結合力下降并促進Bim的釋放,進而激活線粒體途徑凋亡信號通路促進細胞凋亡[17],研究證實,MCL-1與多個腫瘤的化療耐藥相關。在乳腺癌中,MCL-1的過表達與化療耐藥明顯相關[18]。在胰腺癌中,MCL-1與細胞對吉西他濱化療耐藥相關,沉默-可顯著增加細胞對吉西他濱的化療敏感性[19]。在卵巢癌中,MCL-1與細胞對卡鉑化療耐藥明顯相關[20]。因此,MCL-1在腫瘤的化療耐藥中發揮重要作用。本研究首次發現,無論PD-L1的表達水平如何,沉默-或聯合使用AZD5991均可顯著增加CRC細胞對司美替尼的化療敏感性,并可逆轉-敲除引起的對司美替尼化療敏感性下降,這對于PD-L1表達陰性的CRC患者提高對司美替尼化療敏感性有著重要的臨床指導價值。
綜上所述,我們研究發現沉默-可引起CRC細胞對司美替尼化療敏感性下降,沉默-或者聯合使用AZD5991均可逆轉-敲除引起的CRC細胞對司美替尼化療敏感性下降。PD-L1/MCL-1影響CRC細胞司美替尼化療敏感性的分子機制尚待進一步明確。目前,MEK抑制劑已應用于V600E突變型CRC的三線姑息治療,鑒于CRC組織中PD-L1的低表達率,MEK抑制劑聯合MCL-1抑制劑有可能成為部分CRC患者新的治療策略。
[1] Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy[J]. Nat Rev Cancer, 2019, 19(3):133-150.
[2] Inaguma S, Lasota J, Wang Z, et al. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas[J]. Mod Pathol, 2017, 30(2):278-285.
[3] Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, et al. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes[J]. Mod Pathol, 2016, 29(9):1104-1112.
[4] Azuma T, Yao S, Zhu G, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells[J]. Blood, 2008, 111(7):3635-3643.
[5] Clark CA, Gupta HB, Sareddy G, et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma[J]. Cancer Res, 2016, 76(23):6964-6974.
[6] Kleffel S, Posch C, Barthel SR, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth[J]. Cell, 2015, 162(6):1242-1256.
[7] Liu H, Tekle C, Chen YW, et al. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation[J]. Mol Cancer Ther, 2011,10(6):960-971.
[8] Li J, Chen L, Xiong Y, et al. Knockdown of PD-L1 in human gastric cancer cells inhibits tumor progression and improves the cytotoxic sensitivity to CIK therapy[J]. Cell Physiol Biochem, 2017, 41(3):907-920.
[9] Feng D, Qin B, Pal K, et al. BRAF(V600E)-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts[J]. Oncogene, 2019, 38(41):6752-6766.
[10] 陳淑芬,侯婧瑛,吳淑云,等. IFN-γ上調人胃癌細胞株PD-L1表達[J]. 中國病理生理雜志, 2013, 29(11):1952-1956.
Chen SF, Hou JY, Wu SY,et al. Interferon-γ up-regulates programmed death ligand1 in human gastric cancer cells[J]. Chin J Pathophysiol, 2013, 29(11):1952-1956.
[11] Zhao L, Li C, Liu F, et al. A blockade of PD-L1 produced antitumor and antimetastatic effects in an orthotopic mouse pancreatic cancer model via the PI3K/Akt/mTOR signaling pathway[J]. Onco Targets Ther, 2017, 10:2115-2126.
[12] Ock CY, Kim S, Keam B, et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma[J]. Oncotarget, 2016, 7(13):15901-15914.
[13] Kim S, Koh J, Kim MY, et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung[J]. Hum Pathol, 2016, 58:7-14.
[14] Zuo Y, Zheng W, Liu J, et al. MiR-34a-5p/PD-L1 axis regulates cisplatin chemoresistance of ovarian cancer cells[J]. Neoplasma, 2020, 67(1):93-101.
[15] Shen B, Huang D, Ramsey AJ, et al. PD-L1 and MRN synergy in platinum-based chemoresistance of head and neck squamous cell carcinoma[J]. Br J Cancer, 2020, 122(5):640-647.
[16] 黃曄,廖陽,沈楊炳,等. 槲皮素通過抑制c-Jun的表達水平增強5-氟尿嘧啶對胃癌細胞凋亡的誘導活性[J]. 中國病理生理雜志, 2018, 34(2):6.
Huang Y, Liao Y, Shen YB, et al. Quercetin enhances 5-fluorouracil-induced apoptosis in gastric cancer by down-regulating c-Jun expression[J]. Chin J Pathophysiol, 2018, 34(2):6.
[17] Fletcher JI, Huang DC. Controlling the cell death mediators Bax and Bak: puzzles and conundrums[J]. Cell Cycle, 2008, 7(1):39-44.
[18] Balko JM, Giltnane JM, Wang K, et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets[J]. Cancer Discov, 2014, 4(2):232-245.
[19] Wei SH, Dong K, Lin F, et al. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell[J]. Cancer Chemother Pharmacol, 2008, 62(6):1055-1064.
[20] Wu X, Luo Q, Zhao P, et al. MGMT-activated DUB3 stabilizes MCL1 and drives chemoresistance in ovarian cancer[J]. Proc Natl Acad Sci U S A, 2019, 116(8):2961-2966.
Inhibition of MCL-1 reverses poor chemosensitivity of colorectal cancer cells to selumetinib induced by-knockout
SUN Lei, HONG Chu-yuan, YIN Xue-xia, GUO Xiong-bo, ZHANG Shi, ZHONG Bei-ping, WANG Guo-qiang△
(,510260,)
To determine the effects and mechanism of programmed death ligand-1-on chemosensitivity to selumetinib in colorectal cancer (CRC) cells.After human colon carcinoma RKO cells and-knockout RKO cells were treated with selumetinib, cleaved caspase-3 (C-Casp-3) protein level was detected by Western blot, and the apoptosis rate and cell survival rate were tested by flow cytometry (FCM) and colony formation assay, respectively. The RKO and-knockout RKO cells were treated with selumetinib after myeloid cell leukemia-1(-) knockdown, or co-treated with selumetinib and AZD5991, C-Casp-3 protein level was detected by Western blot, and the apoptosis rate and cell survival rate were tested by FCM and colony formation assay, respectively. After mouse colon adenocarcinoma MC38 cells and-knockout MC38 cells were co-treated with selumetinib and AZD5991, C-Casp-3 protein level was detected by Western blot, and the apoptosis rate and cell survival rate were tested by FCM and colony formation assay, respectively.Western blot showed that selumetinib significantly decreased phosphorylated extracellular signal-regulated kinase (p-ERK) protein level. Knockout of-significantly reduced selumetinib-induced C-Casp-3 protein level.-shRNA or co-treatment with AZD5991 significantly increased selumetinib-induced C-Casp-3 protein level. The FCM results showed that knockout of-significantly reduced the apoptosis induced by selumetinib, and-shRNA or co-treatment with AZD5991 significantly increased apoptosis induced by selumetinib. Colony formation assay showed that knockout of-significantly increased cell survival rate after treatment with low concentration of selumetinib, and-shRNA or co-treatment with AZD5991 significantly reduced cell survival rate. More importantly,-shRNA or co-treatment with AZD5991 eliminated the differences in C-Casp-3, apoptosis and cell survival rate caused by-knockout.Knockout of-decreases the chemosensitivity of CRC cells to selumetinib. Both genetic and pharmaceutical inhibition of MCL-1 reverses poor chemosensitivity of CRC cells to selumetinib induced by-knockout.
Myeloid cell leukemia-1; Programmed death ligand-1; Selumetinib; Colorectal cancer; Chemosensitivity
R363; R73-36; R735.3+5
A
10.3969/j.issn.1000-4718.2022.06.007
1000-4718(2022)06-1008-07
2021-11-25
2022-01-25
廣州市屬高校科研項目(No.1201630159);廣州市科技計劃市校聯合項目(No.202102010062);廣州醫科大學附屬第二醫院精英人才配套項目(No. 010M07060)
Tel: 020-34153251; E-mail: wgqjyh@163.com
(責任編輯:余小慧,李淑媛)

