基于多金屬氧酸鹽和多壁碳納米管的雙酚A電化學傳感器的構建與性質
李 娜 張 聰 任聚杰*, 崔 敏 趙海燕張鴻悅 陳世旭 韓宏彥 張 煥
(1河北科技大學理學院,河北省表界面光電調控重點實驗室,石家莊 050018)(2河北工業職業大學,石家莊 050091)(3河北省中醫院,石家莊 050018)
0 Introduction
Polyoxometalates(POMs)as a kind of high negative oxygen clusters composed of a series of transition metal oxides arranged and connected through edge-,corner-or face-shared methods can be easily functionalized by metal ions or organic molecules[1-2].POMs can carry out multi-step,fast,and reversible multi-electron transfer reactions without the collapse of their structure.However,POMs had a low surface area and were difficult to separate from aqueous solution,so all kinds of POM-based inorganic-organic hybrid materials have been synthesized.More and more attention was attracted to this area to investigate their fascinating structures and properties,especially electrochemical activity[3-11].POM-based inorganic-organic hybrid materials in the electrochemical reaction can continue to rapid,reversible,and step-by-step multi-electron transfer under the mild condition without decomposition[12],which makes them show excellent electrocatalytic ability and stability.However,the poor conductivity of POMs limits their use as electrode modification materials in electrochemical sensors.
Bisphenol A(BPA)is widely used in the manufacture of epoxy/polycarbonate resin products,which are considerably used in baby bottles,plastic food containers,and medical devices.However,BPA is an endocrine-disrupting chemical,which does extremely harm to the healthy growth of infants[13-15].Since 2 March 2011,the production of baby bottles with the chemical BPA has been banned.So,it is necessary to propose a selective analysis procedure to monitor BPA in the different real samples[16-18],and most researchers are focusing on the use of electrochemical sensors for BPA detection[19-21].So the electrochemical method was a dopted to detect BPA in this work.Many inorganic compounds,organic compounds,and metal complexes have been used as electrocatalysts to detect BPA[22-26].Regrettably,the number of highly selective and longterm stable redox catalysts remains limited.
Herein,we synthesized a new compound:(H2L)2(HL)2L(PMo12O40)2·2H2O(marked as PMo12),as the active center to modify the glassy carbon electrode(GCE)to test BPA(PMo12/GCE).To improve the conductivity of the prepared working electrode,we also used multi-walled carbon nanotubes(MWCNTs)to modify the electrode.MWCNTs which maintain a fixed distance between layers have many unusual mechanical and electrochemical properties[27].MWCNTs are considered to be a typical 1D nanomaterial and are one of the frontier fields of international science in recent years.Therefore,to improve the conductivity of the modified electrode,we used the drop coating method to drop the MWCNTs on the electrode surface to modify the electrode.As far as we know,the structure reported in this paper is currently unreported,and the current study has not reported the introduction of these two compounds into electrochemical sensors at the same time.The differential pulse voltammetry(DPV)technique,cyclic voltammetry(CV),and electrochemical impedance spectroscopy(EIS)were used for the determination of BPA,and the test conditions were optimized,such as the pH value of the system,the amount of the MWCNTs,and the target compound.The detection performance of the modified electrode under the optimal parameters is as follows:in a range of 1-20 μmol·L-1,the detection limit of 0.5 μmol·L-1(S/N=3).The practicality of the sensor was verified by the application of actual samples.The results showed that the recovery rate of BPA was 95.5%-100.7%in the detection of actual samples,indicating that the sensor could be used for the detection of actual samples.
1 Experimental
1.1 Reagents and instruments
The reagents included MWCNTs (Shenzhen Nanotech Port Co.,Ltd.)and BPA(Tianjin Guangfu Fine Chemical Research Institute),and phosphate buffered saline(PBS,0.05 mol·L-1,pH 7.0)was prepared from K2HPO4-KH2PO4containing 0.1 mol·L-1KCl.Ligand L(L=1,3-bis(1-imidazolyl)propane)(Jinan Henghua Century Co.,Ltd.).The reagents were all analytically pure,and the water used for the electrochemical experiment was distilled water.
Instruments:CHI660D electrochemical workstation(Shanghai Chenhua Instrument Co.,Ltd.);BT1250 electronic balance(Sartorius Scientific Instruments Co.Ltd.); KH22200B ultrasonic cleaner (Kunshan Hechuang Ultrasonic Instrument Co.,Ltd.);Constant temperature magnetic heating stirrer(Jintan Honghua Instrument Factory,Jiangsu Province);Perkin-Elmer 2400 CHN Elemental analyzer.Powder X-ray diffraction(XRD)patterns were recorded in a Rigaku XRD-6000 diffractometer with CuKαradiation(λ=0.154 2 nm)at 40 kV and 30 mA with 2θ=5°-50°.Fourier transform infrared spectrum (FT-IR,400-4 000 cm-1,Nicolet6700,USA)was used to determine the composition of the compound.X-ray photoelectron spectroscopy(XPS)equipped with an AlKαmonochromated X-ray source(Thermo Scientific Escalab 250Xi,USA).Thermogravimetric(TG)analysis(STD-2960,USA)was applied with the temperature raised from room temperature to 1 000℃at 10℃·min-1under nitrogen.
1.2 Synthesis of polyacid compound
0.1 mmol of L,0.1 mmol of Cu(OAC)2·4H2O,and 0.1 mmol of H3PMo12O40were dissolved in 10 mL deionized water,the pH value of the mixture was adjusted to 4.56 by 1 mol·L-1of sodium hydroxide solution,and then the mixture was transferred into 25 mL Teflonlined autoclave and maintained at 160℃for 5 d.After cooling down to room temperature,the resulting green crystal was filtered and washed with distilled water and dried at room temperature.The calculated yield of PMo12was 76%.Elemental analysis:Calcd.(%):C 9.92,H 1.23,N 5.14.Found(%):C 9.88,H 1.45,N 5.22.
1.3 Determination of X-ray single-crystal diffraction
Crystals of good form and quality are glued to the capillary glass wire,then the crystal data of the compound was collected on a Bruker smart apex CCD areadetector diffractometer with a graphite monochromator and MoKα(λ=0.071 073 nm)radiation at room temperature.The diffraction data were collected byω-scan method at 298 K.The structure was solved by the direct method using SHELXTL-2019 on a legend computer and was modified using full-matrix least squares[28].All non-hydrogen atoms were refined anisotropically.Hydrogen atoms were located in the calculated positions and refined by using a riding model.The crystal and the structural refinement data for the compound are summarized in Table S1(Supporting information)and the hydrogen bonds are listed in Table S2.CCDC:1054587.
1.4 Preparation of modified electrode
0.01 g of PMo12was weighed and dissolved in 10 mL secondary water for ultrasonic dispersion for 30 min to evenly disperse to obtain suspension of PMo12.0.01 g of MWCNTs were weighed and dissolved in 10 mL dimethyl formamide(DMF)for ultrasonic dispersion for 30 min to obtain the suspension of MWCNTs.
A GCE was used as the basic electrode in this study.Firstly,the GCEs were polished with 1.0,0.3,and 0.05 μm aluminum oxide powder on the polishing plate successively,after each polishing,the electrodes were cleaned by ultrasonic in water,anhydrous ethanol,and water,then dried with nitrogen.2 μL of MWCNTs suspension was absorbed with a pipette gun and dripped onto the pretreated GCE.The suspension was dried and used as MWCNTs/GCE.PMo12/GCE with 3 μL of PMo12solution and PMo12/MWCNTs/GCE with 2 μL of MWCNTs suspension and 3 μL of PMo12solution were prepared by the same method.
1.5 Electrochemical test
A three-electrode system was used in the experiment:working electrode(modified electrode),reference electrode(Ag/AgCl electrode),and counter electrode(platinum plate).The electrochemical impedance was scanned in a solution of 5 mmol·L-1[Fe(CN)6]3-/[Fe(CN)6]4-(containing 0.1 mol·L-1KCl)at a frequency of 0.1-106Hz with an open circuit potential.After each use,the electrodes were rinsed with two times of distilled water.All experiments were carried out at room temperature.
2 Results and discussion
2.1 Crystal structure of compound
Single-crystal X-ray diffraction analysis reveals that the compound crystallizes in the triclinic crystal system.The unit cell parameters area=1.160 20(9)nm,b=1.208 01(11)nm,c=1.923 89(17)nm,α=82.607 0(10)°,β=87.961(2)°,γ=76.003 0(10)°(Table S1).
The compound contains two water molecules,two protonated ligands H2L,two protonated ligands HL,one L,and two Keggin [PMo12O40]3-(Fig.1a),the[PMo12O40]3-contains one[PO4]tetrahedron and twelve[MoO6]octahedron,the[PO4]located in the center of the cage formed by the twelve[MoO6].P1 is tetracoordinated,coordinating with the four surrounding oxygens(O1,O2,O3,O4),respectively,and the bond length of P1—O is 0.151 1-0.154 6 nm.Mo is hexacoordinated,and Mo—O bond length is 0.162 6-0.238 5 nm.Valence calculation indicates that all the P atoms are in+5 oxidation state,the Mo atoms are in+6 oxidation state,and the ligands are protonated to balance the charge of the entire molecule.
The adjacent polyacid anions are linked together by O—H…O,and then the two polyacid anions are linked with five ligands by N—H…O to form secondary building units.The adjacent secondary building units link each other to form 3D supramolecular structures by N—H…O and C—H…O(Fig.1b,Table S2).

Fig.1 (a)Structural units and(b)3D structure of the compound
2.2 XRD,IR,and TG of the compound
The diffraction peaks of the compound can match well with the simulated data in the key positions(Fig.2),this can indicate the phase purity of the compound.The peak at 2θ=7.6°,7.7°,8.4°,9.1°,10.1°,12.1°,15.2°,15.8°,16.6°,18.3,18.7°,19.3°,23.6°,24.1°,24.6°,25.0°,26.6°,27.9°,and 28.7°correspond to the(010),(100),(011),(10),(111),(10),(020),(12),(01),(20),(11),(123),(300),(115),(10),(320),(233),(006),and(331)planes,respectively.

Fig.2 XRD patterns of the compound
The peaks of IR(Fig.3)in the region of 700-1 100 cm-1are correlated to the polymetallic oxygen clusterνas(P—Oa),νas(Mo—Od),νas(Mo—Ob—Mo),andνas(Mo—Oc—Mo),and the peaks in a range of 1 200-2 000 cm-1correspond to the ligand.It is further proved that the compound is a polyacid structure.

Fig.3 IR spectrum of the compound
The compound is a two-step weight loss(Fig.4),the first step lost the ligand molecule at 20-494℃,and the weight loss of 17.7%(calculated 15.9%),the second step loss at 494-1 000℃is due to the entire structure collapse.

Fig.4 TG curve of the compound
2.3 XPS of the compound
The high-resolution XPS spectra were used to analyze the elements of the compound(Fig.5).The P2ppeaks were observed with the binding energy of 134.5 eV(2p1/2)and 133.7 eV(2p3/2)in Fig.5a,and the peaks of Mo were observed at 235.1 eV(3d3/2)and 232.0 eV(3d5/2)in Fig.5b,suggesting the existence of Mo6+ions in PMo12[29].

Fig.5 XPS spectra of P2p(a)and Mo3d(b)
2.4 Electrochemical characterization of the modified electrodes
EIS was employed to illustrate the electrical conductivity of the electrodes.Nyquist spectra were obtained by alternating-current impedance test of the working electrode in 0.1 mol·L-1KCl solution containing 5 mmol·L-1[Fe(CN)6]3-/[Fe(CN)6]4-.The EIS data were simulated with electrical equivalent circuit models by using ZSimp Win software.As shown in Fig.6,theRctof MWCNTs/GCE was about 57 Ω.While after modifying the GCE with PMo12,theRctincreased to 960 Ω mainly because of the intrinsic low conductivity of PMo12.Compared with PMo12/GCE,theRctof PMo12/MWCNTs/GCE decreased to 92 Ω suggesting the electrical conductivity of the working electrode was significantly improved by MWCNTs.The above results have confirmed that PMo12/MWCNTs/GCE had excellent electrochemical performance and was suitable to construct sensors[30].

Fig.6 EIS spectra of(a)PMo12/GCE,(b)MWCNTs/GCE,and(c)PMo12/MWCNTs/GCE
2.5 Optimization of experimental conditions
To obtain the high performance of the electrochemical sensor,the conditions of the modified electrode were discussed.The influence of the quantity of POMs and MWCNTs on the modified electrode was evaluated.
The results demonstrated the BPA oxidation peak current was the strongest when the amount of MWCNTs was 2.0 μL in 1.0-3.0 μL as shown in Fig.S1,and the peak current was the strongest when the amount of POMs was 3.0 μL in 1.0-5.0 μL as shown in Fig.S2.
As shown in Fig.7,0.05 mol·L-1PBS with different pH values were optimized.As can be seen from the figure,the oxidation peak current of BPA was the highest at pH 7.0,so PBS with pH 7.0 were selected for the experiment.And it can be seen that there is a good linear relationship between peak potential and pH(Fig.8),and the linear equation isy=-0.055x+0.92.According to the Nernst equation[31],Formula 1:

Fig.7 Relationship between peak current and pH

Fig.8 Linear relationship between peak potential and pH
Ep=0.059mpH/n+b(1)WhereEpis the peak potential,mis the number of protons participating in the reaction,andnis the number of electrons transferred in the reaction.It can be seen from the figure that the slope is close to the theoretical value of 0.059,indicating that the same number of electrons and protons are transferred in the electrocatalytic process of BPA.
2.6 Exploration of the reaction mechanism
In addition,the catalytic mechanism of the modified electrode on BPA was studied by CV.With the increase of the scanning speed(from 10 to 100 mV·s-1),the oxidation peak current intensity of the modified electrode is linearly related to the scanning speed(v),this shows that the electrode catalysis of BPA is an adsorption-controlled electrochemical reaction process(Fig.9,10).

Fig.9 CV curves of PMo12/MWCNTs/GCEs at different scan rates

Fig.10 Linear relationship between peak current and scanning rate

As shown in Fig.11,the peak potentialEpincreased with the logarithm of the scanning speed and had a good linear relationship in the scanning speed range.According to Laviron's theory[32],for irreversible oxidation reactions,the relationship betweenEpandvcan be expressed as Formula 2:Here,E?is the formal potential,αis the electron transfer coefficient,K?is the rate constant of the standard hetero-electron transfer,Tis the temperature(298 K),Fis the Faraday constant(96 500 C·mol-1),nis the number of transferred electrons,andRis the gas constant(8.314 J·mol-1·K-1).According to the linear equation in Fig.10:Ep=0.018 1lnv+0.493(αis assumed to be 0.5 for a completely irreversible electrode process),the slope shows that BPA has transferred two electrons in the oxidation reaction,and from theEp-pH relationship we know that the number of protons and electrons transferred is the same.Thus,the oxidation of BPA involves two protons and two electrons(Formula 3),which is consistent with the report[33-34].

Fig.11 Relationship between peak potential and the logarithm of the scanning speed

POMs anions have reversible redox activity,which can proceed with fast and reversible electron transfer[35]:
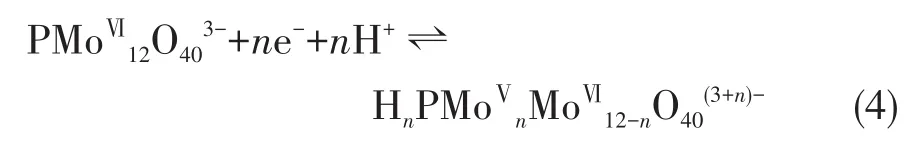
The electrocatalytic oxidation of BPA by PMo12was presumed as follows[36]:

2.7 Quantitative determination of BPA
The oxidation peak potential of BPA detected by the modified electrode was 0.5 V.As shown in Fig.12 and 13,the linear range was 1-20 μmol·L-1(1,4,7,12,15,and 20 μmol·L-1),I=0.711c+0.179(R2=0.989),and the detection limit was 0.5 μmol·L-1(S/N=3).These results indicate that the modified electrode is suitable for the electrochemical detection of BPA in PBS.The performance of the prepared sensor was compared with other BPA sensors reported in Table 1.It can be seen that the prepared sensor has the lowest detection limit and a wide linear range.

Fig.12 DPV of PMo12/MWCNTs/GCEs with different concentrations of BPA(1-20 μmol·L-1)

Fig.13 Linear relationship between concentration and current

Table 1 Comparison of different methods
2.8 Study on stability and anti-interference of the modified electrode
To investigate the stability of the modified electrodes,PMo12/MWCNTs/GCEs were placed in the fridge for 9 d before being tested.As shown in Fig.14,this data indicates that the electrochemical sensor for detecting BPA had strong stability.

Fig.14 Stability of PMo12/MWCNTs/GCEs
To evaluate the anti-interference performance of the prepared electrochemical sensor,10 μmol·L-1BPA was added to PBS and several potential interferers of 10 μmol·L-1(2-naphthol,catechol,p-nitrophenol,4-acetaminophen,hydroquinone)as shown in Fig.15.In the studied potentials,the distractors did not affect the current response of BPA.The results show that the modified electrode electrochemical sensor had a high anti-interference performance.

Fig.15 Anti-interference experiment of PMo12/MWCNTs/GCEs
2.9 Simulation of practical application detection
In previous tests,we have carried out a series of sensor performance tests on the PMo12/MWCNTs/GCE modified electrode under optimal experimental conditions,which proved that the modified electrode has the potential to become a sensor.To test the application of the modified electrode further in practice,5 and 10 μmol·L-1BPA were added to tap water(Sample 1)and lake water(Sample 2)respectively.DPV was used to determine the results,as shown in Table 2.The recovery rate was 95.5%-100.7%,and the results show that the sensor can be used in practical applications.

Table 2 Test results of actual sample
3 Conclusions
In conclusion,we synthesized new organicinorganic hybrids successfully based on POMs by a simple,eco-friendly route and utilized them as novel electrode materials for the fabrication of an ultrasensitive electrochemical sensor for BPA detection.As far as we know,the reports for BPA detection utilizing PMo12-MWCNTs-based electrochemical sensors were really rare.In the optimum conditions,this electrochemical sensor presented excellent electrochemical properties to BPA with a linear range from 1-20 μmol·L-1,and a detection limit of 0.5 μmol·L-1(S/N=3),and the electrochemical sensor exhibited satisfactory antiinterference and stability.Furthermore,the constructed sensor was successfully applied to measure the amount of BPA in real medicinal samples with satisfactory results.These results pave the way for utilizing POMs as structural components sensing platform design and extended POM applications in environmental pollution testing.
Supporting information is available at http://www.wjhxxb.cn
- 無機化學學報的其它文章
- Synthesis,Structure,and Properties of Rare-Earth Complexes Based on Pyridine Dicarboxylic Acid Ligands
- Facile Synthesis of Si@LiAlO2 Nanocomposites as Anode for Lithium-Ion Battery
- Solvent-Controlled Morphology of Ni-BTC and Ni-BDC Metal-Organic Frameworks for Supercapacitors
- A Fast-Responsive Mitochondria-Targeting Fluorescent Probe Detecting Hypochlorite in Living Cells and Zebrafish
- A Complex of Silver(Ⅰ)with N-O Chelating Agent and Phosphine Ligand:Design,Structures,and Biological Activity
- Synthesis,Structure,Magnetic,and Fluorescent Sensing Properties of Cobalt(Ⅱ)Coordination Polymer Based on 1-(3,5-Dicarboxybenzyl)-1H-pyrazole-3,5-dicarboxylic Acid

