基于新型三取代苯衍生物的雙極性主體材料及其高效藍(lán)色電致磷光
屈文山,朱慧文,李 偉,樊霞霞,魏 斌,3,高志翔*(.山西大同大學(xué) 微結(jié)構(gòu)電磁功能材料省市共建山西省重點(diǎn)實(shí)驗(yàn)室,山西 大同 037009;2.清華大學(xué) 化學(xué)系,北京 00084;3.上海大學(xué) 新型顯示技術(shù)及應(yīng)用集成教育部重點(diǎn)實(shí)驗(yàn)室,上海 200072)
1 Introduction
As one of the most promising techniques for multi-color displays and white lighting in the future,organic light-emitting diodes(OLEDs)have become the focus of research in both academic and industrial communities because of many merits[1-10].Organic luminescent materials play important roles in the developments of highperformance organic electroluminescence[11-15].The OLEDs based on triplet emitters including transitionmetal based phosphorescent emitters as well as pure organic thermally activated delayed fluorescent(TADF)emitters that could fully use both two kinds of excitons(triplet and singlet)have become the research focus[11-15].Besides the emitters,the high-performance host materials are also important for serving as the matrixes to suppress the triplettriplet annihilation of the dopants and the energy donor in phosphorescent or TADF OLEDs[16-25].
Host materials are the necessary component for highly efficient blue phosphorescent or TADF OLEDs[16-25].To design the high-performance host material suitable for blue phosphorescent or TADF OLEDs,the following factors should be well considered[7-9],the first one is the triplet energy level(T1)match of the host material and the dopant,because the highT1can ensure the efficient energy transfer from the energy donor(host material)to the energy accepter(blue emitter).The second factor of the host materials is their carrier transport ability,the host showing bipolar carrier transport properties(both electron and hole transport abilities)can balance the carrier transport behavior within the light-emitting layer,thereby increasing the efficiency of device and suppressing the efficiency roll-off.Thus,the rational construction of the host materials will accelerate the development of high-efficiency OLEDs[7-8].The most effective method in the design of bipolar host materials showing high energy level of triplet state is to reduce the π conjugation of the skeleton by connecting the electron transport moiety and the hole transport unit together in large torsion angle[7-8,14].Many kinds of host materials have been successfully designed by using this molecular design strategy[7-8].Recently,a family of bipolar host materials showing linear configuration by connecting carbazole(hole transport moiety)and diphenylphosphine oxide(electron transport moiety)units were developed to fabricate blue phosphorescent electroluminescence with high efficiency(peak external quantum efficiency(EQE)of 15.8%)[14].To further promote the progress of high-performance host materials,it is still necessary to develop new molecular design strategy to explore high-performance bipolar host materials with diverse structures(e.g.,the star shape,etc.).
In this work,the trisubstitution molecular design strategy is reasonably proposed for realizing high-performance blue electroluminescence.A pair of high-performance bipolar host materials(1 and 2)based on trisubstituted benzene are designed and prepared by incorporating cyanophenyl as the electron transport moiety and carbazole and dibenzo[b,d]furan as the hole transport moieties.Both materials show intense violet fluorescence in the non-polar or less polar solvents.The introduction of cyano unit into the different position on phenyl moiety could effectively adjust the energy levels of the triplet states of the bipolar host materials,especially for material 1 with much higherT1,which is more suitable for efficient blue phosphorescent OLEDs.In addition,the trisubstitution molecular design strategy also provides the bipolar host materials with the steric hindrance in favor of OLEDs.The peak current efficiency(CE),power efficiency(PE),and EQE of the blue phosphorescent OLED using material 1 as host realize 31.53 cd/A,26.93 lm/W,and 16.17%,respectively.
2 Experiments and Computation
The details of experimental information inculding OLEDs fabrication can be found in Supplementary Information.The synthetic route of bipolar host materials(1 and 2)is depicted in Fig.1

Fig.1 Chemical structures and the synthetic routes of new trisubstituted benzene-based bipolar host materials 1 and 2
2.1 Synthesis of the intermediates
2.1.1 4-(3-bromo-5-chlorophenyl)dibenzo[b,d]furan
A mixture of 1,3-Dibromo-5-chlorobenzene(11 mmol),dibenzo[b,d]furan-4-ylboronic acid(10 mmol),Pd(PPh3)4(0.55 mmol),2.0 mol/L K2CO3aqueous solution(20 mL),ethanol(20 mL),and toluene(80 mL)were stirred at 100℃for 6 h under N2.After the reaction was finished,the mixtures were diluted with CH2Cl2and washed with water,dried over anhydrous Na2CO3,then the solvent was evaporated.The crude product was purified by column chromatography to give as white solid(1∶20 mixture of ethyl acetate and petroleum ether as the eluent)(60% Yield).1H NMR(400 MHz,Chloroform-d)δ:8.03~7.93(m,3H),7.88~7.84(m,1H),7.65(d,J=8.2 Hz,1H),7.59~7.35(m,5H).
2.1.2 3-(3-chloro-5-(dibenzo[b,d]furan-4-yl)phenyl)-9-phenyl-9H-carbazole
A mixture of 4-(3-Bromo-5-chlorophenyl)dibenzo[b,d]furan(11 mmol),(9-phenyl-9H-carbazol-3-yl)boronic acid(10 mmol),Pd(PPh3)4(0.55 mmol),2.0 mol/L K2CO3aqueous solution(20 mL),ethanol(20 mL),and toluene(80 mL)were stirred at 100℃for 18 h under N2.After the reaction was finished,the mixtures were diluted with CH2Cl2and washed with water,dried over anhydrous Na2CO3,then the solvent was evaporated.The crude product was purified by column chromatography to give as white solid(1∶40 mixture of ethyl acetate and petroleum ether as the eluent)(85% Yield).1H NMR(400 MHz,Chloroform-d)δ:8.43(d,J=1.3 Hz,1H),8.22(d,J=7.7 Hz,1H),8.12(t,J=1.5 Hz,1H),8.04~7.98(m,2H),7.88(t,J=1.7 Hz,1H),7.77(t,J=1.7 Hz,1H),7.73(dt,J=8.5,2.1 Hz,1H),7.70~7.59(m,6H),7.53~7.43(m,6H),7.41~7.37(m,1H),7.35~7.31(m,1H).
2.2 Synthesis of the target materials
2.2.1 3′-(dibenzo[b,d]furan-4-yl)-5′-(9-phenyl-9H-carbazol-3-yl)-[1,1′-biphenyl]-3-carbonitrile(1)
A mixture of 3-(3-chloro-5-(dibenzo[b,d]furan-4-yl)phenyl)-9-phenyl-9H-carbazole(11 mmol),(3-cyanophenyl)boronic acid(10 mmol),Pd(OAc)2(0.55 mmol),K3PO4(30 mmol),SPhos(1.1 mmol),and toluene(80 mL)were stirred at 100℃for 24 h under N2.After the reaction was finished,the mixtures were diluted with CH2Cl2and washed with water,dried over anhydrous Na2CO3,then the solvent was evaporated.The crude product was purified by column chromatography to give as white solid(1∶40 mixture of ethyl acetate and petroleum ether as the eluent)(75%Yield).1H NMR(600 MHz,Chloroformd)δ:8.50(dd,J=1.8,0.7 Hz,1H),8.28(t,J=1.6 Hz,1H),8.24(dt,J=7.8,1.0 Hz,1H),8.10~8.08(m,1H),8.06(t,J=1.7 Hz,1H),8.04~8.00(m,3H),7.95(t,J=1.7 Hz,1H),7.80(dd,J=8.5,1.8 Hz,1H),7.75(dd,J=7.5,1.2 Hz,1H),7.70(dt,J=7.7,1.3 Hz,1H),7.67~7.60(m,6H),7.55~7.53(m,1H),7.52~7.50(m,2H),7.50~7.48(m,1H),7.47~7.43(m,2H),7.39(td,J=7.5,1.0Hz,1H),7.36~7.31(m,1H).MALDI-TOF-MS(m/z):calcd for C43H26N2O,586.20;found,586.20.Anal.calcd for C43H26N2O:C 88.03,H 4.47,N 4.77;found:C 88.07,H 4.81,N 4.72.
2.2.2 3′-(dibenzo[b,d]furan-4-yl)-5′-(9-phenyl-9H-carbazol-3-yl)-[1,1′-biphenyl]-4-carbonitrile(2)
The synthetic method is similar to that of 1,except the(4-cyanophenyl)boronic acid is used.White powder(80% yield).1H NMR(600 MHz,Chloroform-d)δ:8.49(dt,J=1.9,1.0 Hz,1H),8.28(t,J=1.6 Hz,1H),8.23(dt,J=7.8,1.0 Hz,1H),8.08(t,J=1.7 Hz,1H),8.03(ddd,J=7.7,1.4,0.7 Hz,1H),8.01(dd,J=7.6,1.2 Hz,1H),7.97(t,J=1.7 Hz,1H),7.90~7.89(m,1H),7.89~7.87(m,1H),7.81~7.78(m,3H),7.74(dd,J=7.5,1.2 Hz,1H),7.67~7.63(m,2H),7.63~7.60(m,3H),7.54~7.52(m,2H),7.52~7.50(m,2H),7.50~7.48(m,1H),7.47~7.45(m,2H),7.40(td,J=7.5,0.9 Hz,1H),7.33(ddd,J=7.9,4.4,3.6 Hz,1H).MALDI-TOF-MS(m/z):calcd for C43H26N2O,586.20;found,586.20.Anal.calcd for C43H26N2O:C 88.03,H 4.47,N 4.77;found:C 88.10,H 4.75,N 4.80.
3 Results and Discussions
3.1 Preparation and characterizations
Two target materials are prepared via multistep Suzuki coupling reactions[14].3-(3-Chloro-5-(dibenzo[b,d]furan-4-yl)phenyl)-9-phenyl-9Hcarbazole as the key intermediate was obtained by two-step Suzuki coupling reactions.In the first step,the reaction between 1,3-dibromo-5-chlorobenzene and dibenzo[b,d]furan-4-ylboronic acid gave the intermediate 4-(3-bromo-5-chlorophenyl)dibenzo[b,d]furan.In the second step,the 3-(3-chloro-5-(dibenzo[b,d]furan-4-yl)phenyl)-9-phenyl-9Hcarbazole can be obtained by the coupling reaction between carbon-bromine bond of 4-(3-bromo-5-chlorophenyl)dibenzo[b,d]furan and carbon-boron bond of(9-phenyl-9H-carbazol-3-yl)boronic acid.At last,by using SPhos as the ligand,the coupling reaction between carbon-chlorine bond of the key intermediate and(3-cyanophenyl)boronic acid or(4-cyanophenyl)boronic acid will gave the target host materials in high yield.The target materials can be isolated as white powders,and the chemical structures were further confirmed fully by nuclear magnetic resonance spectrometry(NMR),elemental analysis as well as mass spectrometry(MS).Both host materials show high solubility in organic solvents in spite of the lack of the flexible chains within the materials.Due to the asymmetric features in both materials,1H NMR spectra show complex resonance signals,as shown in Supplementary Information.The mass spectra were recorded for two new host materialsviaMALDI-TOF-MS.The experimental values of the molecular weight of both materials are perfectly consistent with their theoretical values.These characterizations clearly indicate the structures of the target host materials.
The glass transition temperature(Tg)of both compounds was also evaluated under N2by using differential scanning calorimetry(DSC)analysis.As shown in Fig.S1(Supplementary information),the glass transition temperature of materials 1 and 2 are up to 101oC and 105oC,respectively,implying that both materials show high temperature in glass transition.
We found that the functionalization in 4-position of dibenzo[b,d]furan is intended to generate the large torsion angle between dibenzo[b,d]furan and phenyl core,thereby reducing the degree of the π conjugation and rising the energy level of the triplet states target materials.So,this trisubstitution molecular design strategy also provides the bipolar host materials with the steric hindrance in favor of OLEDs.Moreover,different positions of cyano group introduced into the phenyl moiety can also further tune the energy levels of the triplet states of two host materials.
3.2 Photophysical and electrochemical properties
As shown in Fig.2,3,S2(Supplementary information)and Tab.1,the photophysical behaviors of both host materials were investigated in both solution states and neat film in detail.From the absorption spectra(Fig.2)for materials 1 and 2 in toluene,the intense absorption band ranging from 250 nm to 300 nm is assigned to the π→π*electronic transition of the dibenzo[b,d]furan unit.It should also be noted that the profiles of the absorption spectrum of material 1 is not similar to that of material 2,indicating the position of cyano group could show remarkable influence on the absorption spectrum of two isomers.The weak absorption ranging from 300 nm to 350 nm is the results of the π-π*and n-π*electronic transitions of the carbazole moiety[14,17].

Fig.2 UV-visible absorption,photoluminescence spectra of materials 1(a)and 2(b)in toluene and neat film at 25℃.

Tab.1 Photophysical and electrochemical properties for materials
In toluene,under the ultraviolet light of 300 nm,both materials show intense violet fluorescent emission peaked at 383 nm for 1 391 nm for material 2.In neat film,they emit at 398 nm for 1 412 nm for material 2.Compared with the emission in toluene,the slight redshift in emission maximum in neat film was attributed to the aggregation of material.At 77 K,the lowtemperature phosphorescence spectra(Fig.S2,Supplementary information)for two materials were obtained in 2-methyltetrahydrofuran(2-MeTHF).According to the vibronic sub-band with the highest energy of the low temperature emission spectra,the energy levels of the triplet states for both materials can be estimated to be 2.78 eV for material 1,2.70 eV for material 2[17].The different triplet energy levels reveal that the position of cyano group also have great influence on the triplet state of two isomers.
The solvent-dependent emission behaviors of two compounds were further investigated in various solvents with different polarities at room temperature.We can see from Fig.3,the room temperature PL spectra for two materials were obtained in hexane(Hex),toluene(Tol),tetrahydrofuran(THF),dichloromethane(DCM),N,N-dimethylformamide(DMF),and acetonitrile(ACN).As expected,both materials show the red-shift in emission maximum upon increasing the polarity of solvent.For example,for material 1,the emission maxima are 380 nm in hexane,383 nm in toluene,390 nm in THF,395 nm in DCM,425 nm in DMF,and 420 nm in ACN.For material 2,the emission maxima are 384 nm in hexane,391 nm in toluene,426 nm in THF,445 nm in DCM,475 nm in DMF,and 475 nm in ACN.The emission energies of material are well consistent with the order of the polarity of the solvents.The solvent-dependent emission behaviors of two materials are the result of the donor-acceptor structure[23-25],in which the carbazole and dibenzo[b,d]furan moieties are electron-donor,and the cyanophenyl moiety is electron-acceptor.
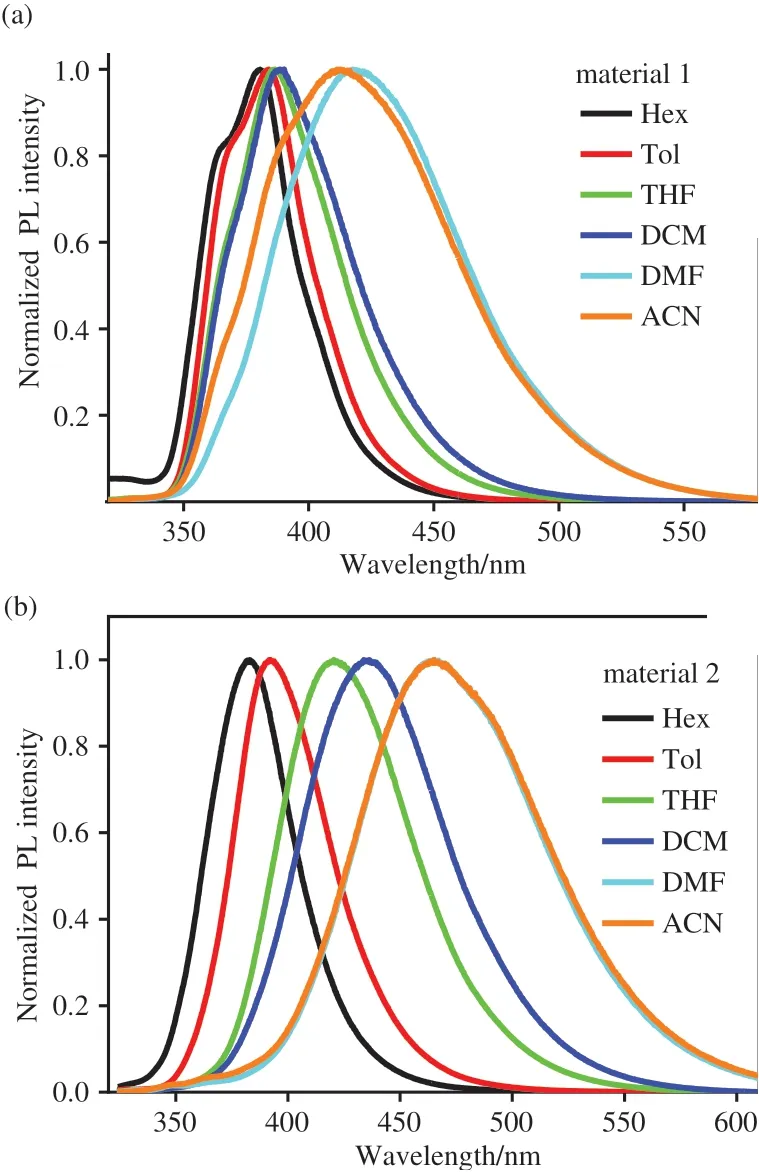
Fig.3 Photoluminescence spectra of the host materials 1(a)and 2(b)in different solvents at room temperature
In addition,the electrochemical property of materials 1 and 2 were evaluated in CH3CN by the cyclic voltammetry.Both materials exhibit reversible wave in oxidation process,and the potential for oxidation is estimated to be 1.22 V and 1.15 V.The oxidation process of carbazole moieties is responsible for the observed positive oxidation potentials[14].On the basis of the cyclic voltammetry,the energy levels of the lowest unoccupied molecular orbital(LUMO)and the highest occupied molecular orbital(HOMO)of material 1 are calculated to be-2.90 eV and-6.02 eV,respectively.The LUMO and the HOMO of material 2 are determined to be-2.94 eV and-5.95 eV,respectively.
3.3 Theoretical calculations
Aiming to further reveal the structure-property relationships of both new materials,the theoretical calculations for two new bipolar host materials were conducted by using the Gaussian 09 package at B3LYP/6-31G(d,p)level[26].The energy levels and electron cloud distributions of the materials at ground state(S0),singlet state and triplet state were calculated,as depicted in Fig.4 and summarized in Table S1(Supplementary information).We can clearly see from Fig.4,for two compounds,the HOMOs distributed mainly over the carbazole units and phenyl core,while the LUMOs located mainly in cyanophenyl units,which is in accordance with the fact that the cyanophenyl moiety is electron-deficient and the carbazole moiety has large electron density[7,14].Thus,the position of cyano group has little influences on the distributions of HOMOs and LUMOs of two isomers.The calculated oscillator strengths and energies for the lowest triplet and singlet transitions are summarized in Tab.S1(Supplementary information).For material 1,the electronic transition from S0to S1contributes 92.4% of the electronic transition from HOMO to LUMO.For material 2,the electronic transition from S0to S1contributes 96.6% of the electronic transition from HOMO to LUMO.The energy difference(ΔEST)of theT1and the S1of two compounds are 0.468 4 eV and 0.3247 eV,respectively.

Fig.4 Calculated electron cloud distributions and energy levels of LUMOs,HOMOs of the host materials 1(a)and 2(b).
3.4 Blue phosphorescent electroluminescence
The two new materials were further used as host materials to evaluate their performance in blue phosphorescent electroluminescence.As shown in Fig.S3(Supplementary information),by selecting the traditional blue phosphorescent iridium(Ⅲ)complex(FIrpic)as the dopant,the blue electrophosphorescent devices were constructed,the device structures are as follows:indium-tin-oxide(ITO)/molybdenum trioxide(MoO3)(3 nm)/di-(4-(N,N-di-p-tolyl-amino)-phenyl)cyclohexane(TAPC)(40 nm)/4,4',4"-tris(carbazol-9-yl)triphenylamine(TCTA)(10 nm)/host material 1 or 2:15%(mass fraction)FIrpic(20 nm)/1,3,5-tri((3-pyridyl)-phen-3-yl)benzene(TmPyPB)(40 nm)/lithium fluoride(LiF)(1 nm)/aluminum(Al)(100 nm).MoO3is used as hole injection layer,and ITO is used as anode.TmPyPB layer and TAPC layer serve as electron transport layer(ETL)and hole transport layer(HTL),respectively.TCTA is also used as hole transport layer.Materials 1 or 2 was used as the host for triplet emitter FIrpic,and the emissive layer(EML)is composed of materials 1 or 2 doped with triplet emitter FIrpic.The doping concentration of 15% in weight(15%)are chosen to test the performance of the OLED devices.Fig.S3(Supplementary information)illustrates the energy levels of the prepared blue OLEDs.The energy level of the triplet state for FIrpic(2.68 eV)is lower than these of the host materials 1 or 2[27-28].Thus,the energy transfer from the energy donor(host material)to the energy accepter(blue emitter)can be realized[29-31].Fig.5,6 and Tab.2 present the electrophosphorescence performances of the blue OLEDs using materials 1 and 2 as host material.For two material 1 and 2,the blue devices show low turn-on voltages(as low as 3.1 V for material 1 and 3.4 V for material 2),and the intense blue electroluminescence located at 472 nm with 499 nm as the emission shoulder can be observed from the prepared OLEDs.For both host materials,the normalized EL spectra with the 1931 Commission Internationale de L’Eclairage(CIE)coordinates of(0.16,0.33)(Fig.5(a))are well matched at the doping concentration of 15% under the applying voltage of 5 V,indicating that the two host materials have no effect on the electroluminescence,and the blue electrophosphorescence is produced from the unimolecular FIrpic[19].
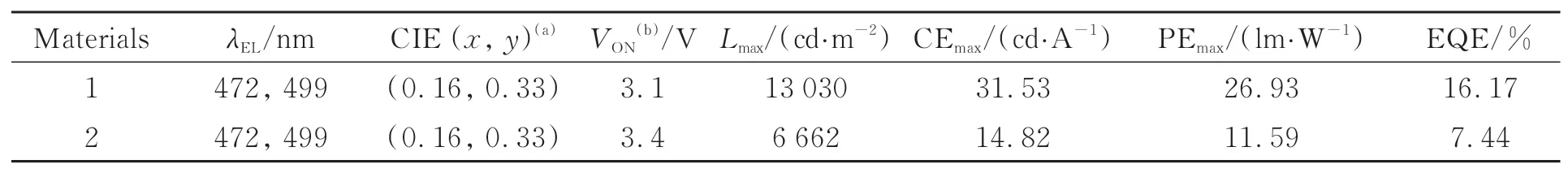
Tab.2 EL data for the blue light-emitting devices by using materials 1 and 2 as host materials

Fig.5 EL spectra of FIrpic(a),CE-L-EQE curves(b)of the blue devices at doping concentration of 15%by using materials 1 and 2 as host materials.Inset:EL photograph of OLED at 5 V.
Fig.6(a)depict the CE-luminance(L)-EQE curves of the prepared blue devices based on host materials 1 and 2.Fig.6(b)shows the PE-Lcurves and current density(J)-voltage(V)-Lcurves of the blue devices.As presented in Table 2,at this doping concentration,for host material 1,the maximum luminance can reach 13 030 cd/m2.The peak CE,PE and EQE of the blue device based on new host material 1 can reach 31.53 cd/A,26.93 lm/W and 16.17%,respectively.At the same doping concentration,for host material 2,the maximum luminance can reach 6 662 cd/m2.The peak CE,PE and EQE of the blue device based on new host material 2 are 14.82 cd/A,11.59 lm/W and 7.44%,respectively.It is worth noting that the performance of device based on host material 1 is much higher than that of host material 2,which is because that the triplet energy level of material 2(2.70 eV)is lower than that of material 1(2.78 eV),leading to the insufficient energy transfer from the host material to the triplet dopant FIrpic(T1=2.68 eV)[28-31].Thus,their device performances are well consistent with the order of the triplet energy levels of two host materials.

Fig.6 J-V-L curves(a)and PE-L curves(b)of the blue devices by using materials 1 and 2 as host materials
4 Conclusions
To conclude,two new and bipolar host materials showing tunable triplet energy levels are well proposed and prepared by incorporating carbazole and dibenzo[b,d]furan as the hole transport moieties and cyanophenyl as the electron transport moiety.Two materials also show intense violet fluorescence in the less polar or non-polar solvents.Owing to the the trisubstitution molecular design strategy and the pisotion-controlling of the cyano group on the phenyl moiety,the triplet energy levels and the energy gap of the prepared host materials could be well adjusted,especially for material 1 with much higherT1,which has been proved to be suitable for efficient blue phosphorescent OLEDs.Moreover,the trisubstitution molecular design strategy also provides the bipolar host materials with the steric hindrance in favor of OLEDs.The peak current efficiency,power efficiency,and external quantum efficiency of the blue phosphorescent OLED using material 1 as host reach 31.53 cd/A,26.93 lm/W,and 16.17%,respectively.The preliminary results suggest that the trisubstitution molecular design strategy has great potential in developing novel host materials with high-performance for the blue electrophosphorescence in the future.
Supplementary information:The online version contains supplementary information avaliable at https://cjlcd.lightpublishing.cn/thesisDetails#10.37188/CJLCD.2022-0142&lang=zh

