基于5-羥甲基間苯二甲酸和咪唑衍生物的半導體型Ni-MOFs的合成、晶體結(jié)構(gòu)和光催化性質(zhì)
徐中軒 石明鳳 白旭玲 袁婷婷
(1遵義師范學院化學化工學院,遵義 563002)(2云南民族大學化學與環(huán)境學院,昆明 650504)
Metal-organic frameworks(MOFs)as a kind of relatively new crystalline materials possess intriguing architectures and some potential applications in heterogeneous catalysis,recognition,gas storage,separation,and so on[1-3].Therefore,designing and synthesizing MOFs to meet different needs have scientific significance and applied importance[4-6]. However, the obtained structures of MOFs are influenced by temperature,solvent system,metal ions,ligands,pH value,and even ratio of metal and ligand[7-10].So it is difficult to adjust reaction conditions to obtain MOFs with specific structures in crystal engineering.Fortunately,organic ligands play a critical role among such synthetic factors,and selecting an appropriate ligand to assemble with metal ions is a feasible method to build target MOFs[11-12].Aromatic carboxylic acids have been extensively used in the synthesis of MOFs owing to their rich coordination modes and diversified structures.Many functional MOFs based on aromatic carboxylic acids,such as HKUST-1,MOF-5,and MIL-101,have been used in different field[13-16].Furthermore,N-containing imidazole derivatives are also a kind of effective ligands.Their different shapes and nuclearity can form various MOFs as second ligands in the presence of aromatic carboxylic acids.Although these strategies have been wildly applied in obtaining MOFs for several years,it is still a promising direction for us to further study[17-23].
With the increasing textile industry and printing activities,a colossal amount of industrial wastewater containing organic dyes caused the deterioration of the aquatic ecosystem[24].However,it is a challenge to clear organic dyes from wastewater via conventional methods for their high solubility[23].Photocatalytic degradation can decompose most organic pollutants into non-polluting small molecules[25].TiO2,SnO2,ZnO,etc.as semi-conductive photo-catalysts have been used to degrade organic dyes.But shortcoming in low absorption efficiency for light limits their further applications[26].MOFs materials as a new class of photocatalysts have been developed for their tailored structures and high absorption efficiency for light in the ultravioletvisible region.Given that,some semi-conductive MOFs have been used to degrade organic dyes by many researchers[27-29].So it is a very significant work to further construct semi-conductive MOFs for disposing organic dyes.
As one of the aromatic carboxylic acids,5-(hydroxymethyl)isophthalic acid(H2HIPA)is an effective and versatile linker for its own advantages:(1)two rigid carboxylate groups with variable coordination modes;(2)5-hydroxymethyl group acting as hydrogen bonding donor and/or acceptor[30].Based on aforementioned search backgrounds,we selected H2HIPA to react with Ni(Ⅱ)to build MOFs under the help of imidazole derivatives 1,1′-(2,5-dimethyl-1,4-phenylene)bis(1H-imidazole)(2,5-DPBI)and 1,1′-(2,5-dimethyl-1,4-phenylene)bis(4-methyl-1H-imidazole)(2,5-DPBMI),respectively(Scheme 1).As a result,two 3D Ni-MOFs,{[Ni(HIPA)(2,5-DPBI)1.5(H2O)]·2.25H2O}n(1) and[Ni(HIPA)(2,5-DPBMI)(H2O)]n(2),were obtained via hydrothermal synthesis.Herein,we report their synthetic processes,crystal structures,semi-conductive characteristics,and photocatalytic properties in the degradation of organic dye.
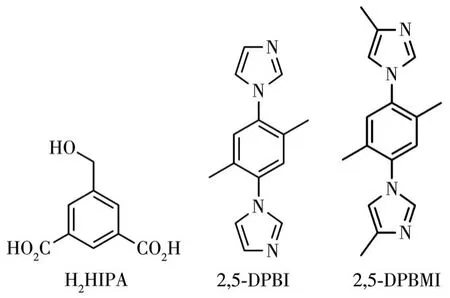
Scheme 1 Structures of ligands H2HIPA,2,5-DPBI,and 2,5-DPBMI
1 Experimental
1.1 Materials and methods
H2HIPA was obtained according to our previous work[30],and the other chemicals were commercially available and used without further purification.Powder X-ray diffraction(PXRD)patterns were gained via a Rigaku MiniFlex 600 diffractometer(U=40 kV,I=15 mA,CuKαradiation,λ=0.154 060 nm)from 5.00°to 50.00°.Thermogravimetric analyses(TGA)were performed on a NETSCHZ STA-F3 thermoanalyzer from room temperature to 800℃.Elemental analysis results were acquired by a PerkinElmer 240C elemental analyzer.Electrochemical tests were carried out on a CHI 760E electrochemical workstation equipped with a standard three-electrode system.UV-Vis absorption spectra and the content of dye were determined by a Shimadzu UV-3600 Plus spectrophotometer.
1.2 Synthesis of Ni-MOF 1
H2HIPA(20 mg,0.10 mmol),2,5-DPBI(40 mg,0.15 mmol),Ni(NO3)2·6H2O(0.15 mmol,45 mg)and NaOH(20 mg,0.25 mmol)were dissolved in 6 mL of deionized water.Then the reactive mixture was autoclaved at 130℃for 48 h and slowly cooled to room temperature,resulting in blue block-shaped crystals of 1 in 30% yield(based on H2HIPA).Anal.Calcd.for C30H29N6NiO6(%):C,57.35;H,4.65;N,13.38.Found(%):C,56.92;H,4.72;N,13.16.IR(KBr pellet,cm-1):3 304m,3 112w,1 640s,1 665s,1 571s,1 510s,1 429m,1 287m,1 225m,1 120w,1 064m,959w,934w,773w,736w,662w,581w,463w.
1.3 Synthesis of Ni-MOF 2
The synthetic procedure of 2 was similar to that of 1,except 2,5-DPBI was replaced by 2,5-DPBMI.Blue block crystals of 2 were collected with a yield of 40%(based on H2HIPA).Anal.Calcd.for C25H26N4NiO6(%):C,55.90;H,4.88;N,10.43.Found(%):C,56.20;H,4.92;N,10.12.IR(KBr pellet,cm-1):3 437m,3 162w,1621m,1547s,1522s,1543m,1373s,1200w,1113w,1 064w,1 027w,779w,724w,662w,594w,439w.
1.4 Photocatalytic degradation reaction
Methylene blue(MB)was selected as a model organic dye in water samples for photocatalytic degradation reactions.The reaction was according to the following processes:60 mg of complex 1 or 2 was mixed together with 60 mL of an aqueous solution of MB(5 mg·L-1)under stirring in the dark for an hour till adsorption-desorption equilibrium between catalyst and MB was established.Then the solution was exposed to UV light from a mercury lamp(300 W)and kept continuously stirring under irradiation.The samples of 3 mL were taken out every 30 min for the analysis.The content of MB was determined through UV-Vis absorption intensity on a Shimadzu UV-3600 Plus spectrophotometer.
1.5 Crystal-structural determination
Two single crystals with dimensions of 0.20 mm×0.20 mm×0.20 mm(1)and 0.30 mm×0.2 mm×0.20 mm(2)were selected,relatively,and the data were collected by a Rigaku 003 CCD diffractometer with a MoKαradiation(λ=0.071 073 nm)at room temperature.The final structures were determined via the SHELXT-2014 program and refined with full-matrix leastsquares techniques by the SHELXL-2017 program on Olex2-1.2 software[31-33].All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms from C atoms were positioned geometrically and refined using a riding model,and the H atoms from coordinated H2O moieties were located by difference maps and constrained to ride on their parent O atoms.The basic information for the crystal and structural refinement data and some selected bond length and angles for complexes 1 and 2 are listed in Table 1 and S1(Supporting information),respectively.
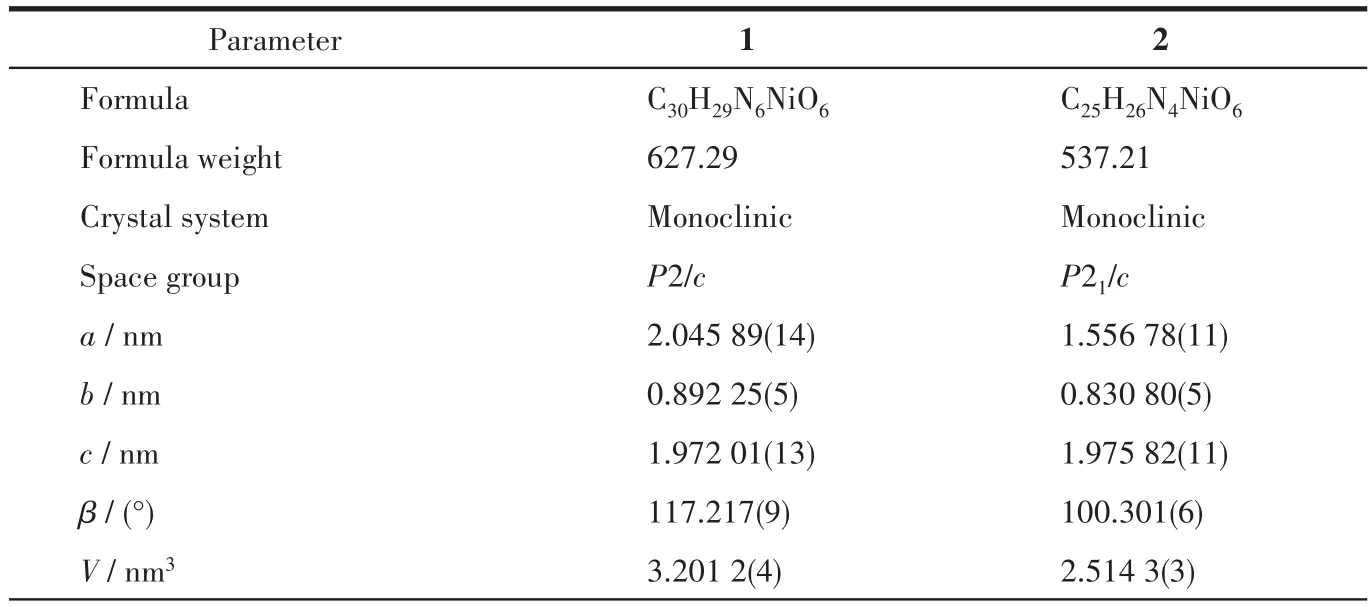
Table 1 Crystal data and structure refinement details for complexes 1 and 2
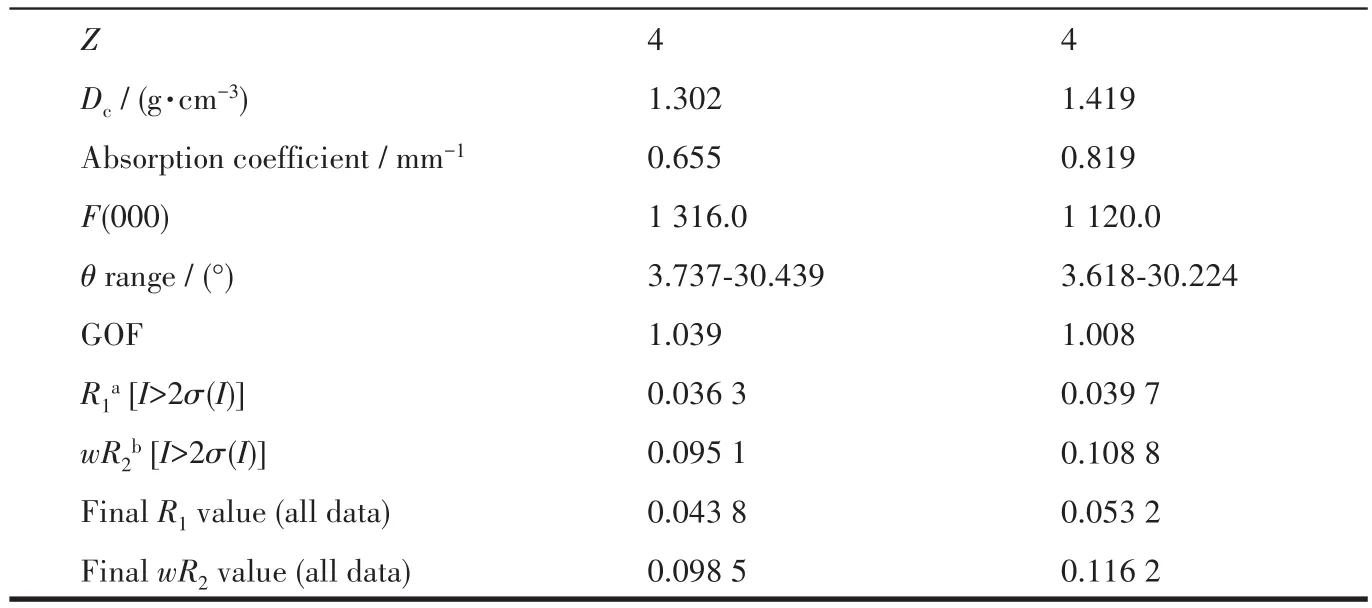
Continued Table 1
CCDC:2114481,1;2114482,2.
2 Results and discussion
2.1 Crystal structure of complexes 1 and 2
X-ray crystal structure analysis indicates that complex 1 crystallizes in the monoclinic system with theP2/cspace group.The asymmetric unit consists of one independent Ni(Ⅱ)ion,one complete deprotonated HIPA2-ligand,three halves of 2,5-DPBI,and one coordinated water molecule(Fig.1a).Additionally,some disordered guest molecules in the asymmetric unit could not be further identified and their reflection data are subtracted by SQUEEZE method from the corresponding single crystal structure.As a result,two and a quarter water molecules as guest molecules should exist in an asymmetric unit according to the SQUEEZE information.Ni(Ⅱ)ion adopts an octahedral configuration to coordinate with three oxygen atoms from two carbonyl groups of HIPA2-ions and one water molecule and three imidazole nitrogen atoms from two 2,5-DPBI molecules.Since complex 1 is constructed by two dis-tinct ligands 2,5-DPBI and HIPA2-,it is divided into two parts according to ligand to simply its complicated framework.If only 2,5-DPBI molecules and Ni(Ⅱ)centers are considered,a 3D 2,5-DPBI-Ni(Ⅱ)framework is formed(Fig.1b).In the 2,5-DPBI-Ni(Ⅱ)framework,each Ni(Ⅱ)ion is connected by three 2,5-DPBI molecules.Thus,the 2,5-DPBI-Ni(Ⅱ)framework can be reduced into the 3-connectedthsnet with the topological symbol of(103).Different from the 3D framework resulting from 2,5-DPBI and Ni(Ⅱ) centers,Ni(Ⅱ) ion and HIPA2-ions can only form HIPA-Ni(Ⅱ)chain.The HIPA-Ni(Ⅱ)chains are further filled in the 2,5-DPBINi(Ⅱ)framework resulting in the final framework of complex 1(Fig.1c).For the introduction of HIPA2-ligand,the whole framework of complex 1 is viewed as a 5-connected net with a topological symbol of(42.66.82)(Fig.1d).
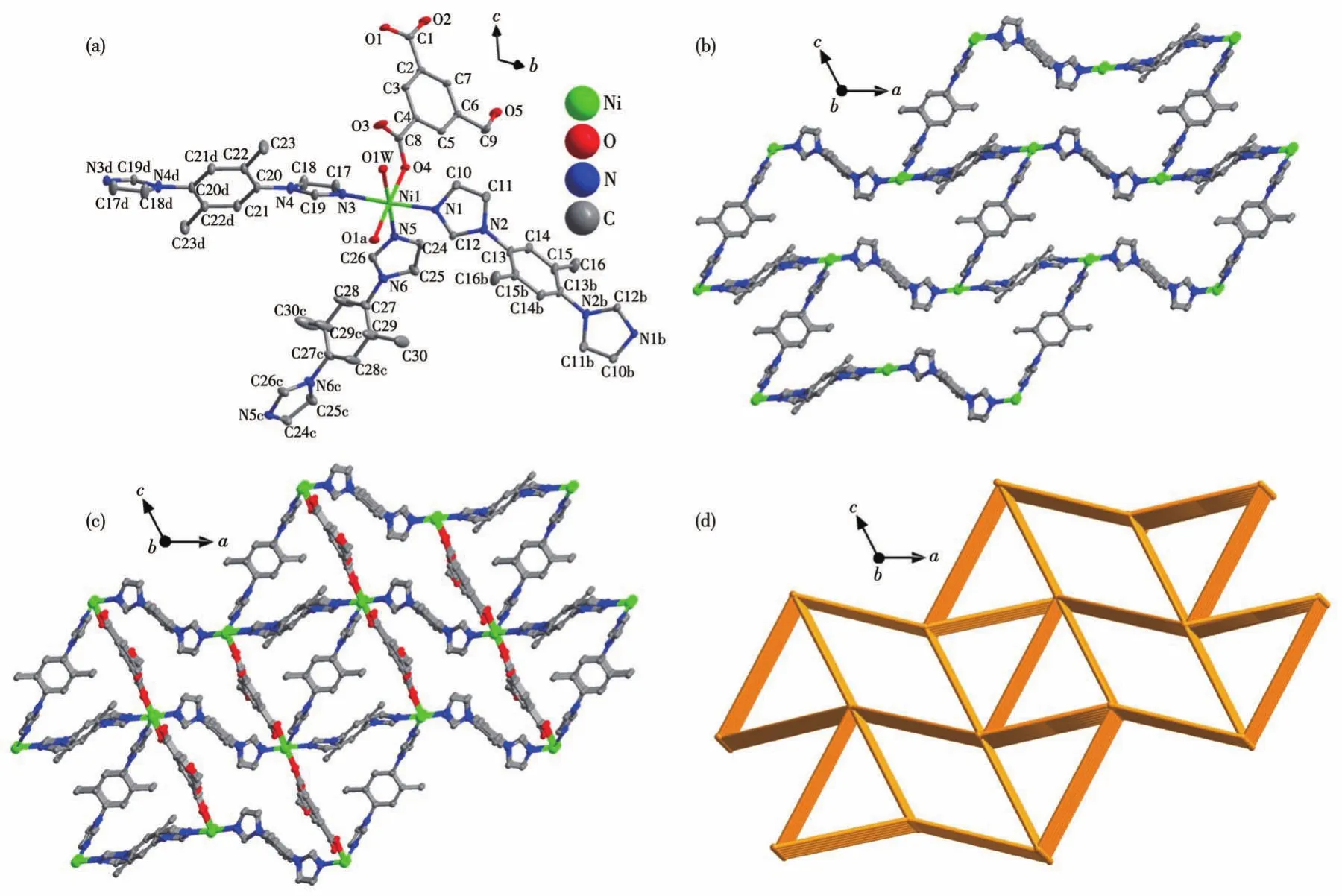
Fig.1 Schematic illustrations of 1:(a)coordination environments of Ni(Ⅱ) center;(b)2,5-DPBI-Ni(Ⅱ) framework;(c)3D framework;(d)5-connected net
Complex 2 crystallizes in the monoclinicP21/cspace group,and the asymmetric unit contains one Ni(Ⅱ)center,one deprotonated HIPA2-anion,two halves of 2,5-DPBMI,and one coordinated water molecule.As shown in Fig.2a,the octahedral Ni(Ⅱ)center is coordinated by three oxygen atoms from HIPA2-anion,two nitrogen atoms from 2,5-DPBMI,and one oxygen atom from the water molecule.Based on such coordination mode,Ni(Ⅱ)centers,HIPA2-anion,and 2,5-DPBMI are further connected together to construct a 3D framework with nano-channels along thea-axis(Fig.2b).The size of the honeycomb-like channel is up to 1.5 nm×1.5 nm,where each layer of the channel is comprised of six Ni(Ⅱ)centers,three HIPA2-anions and three 2,5-DPBMI molecules.Structural interpenetration is inevitable in such an empty framework,and further analysis reveals that the whole framework of complex 2 is a threefold interpenetration structure(Fig.2c).In the framework,each Ni(Ⅱ)center as a 4-connected node is linked by two HIPA2-anions and two 2,5-DPBMI molecules,while HIPA2-and 2,5-DPBMI are simple linker.Finally,the whole framework of complex 2 is a 4-connecteddianet from the view of topology(Fig.2d).
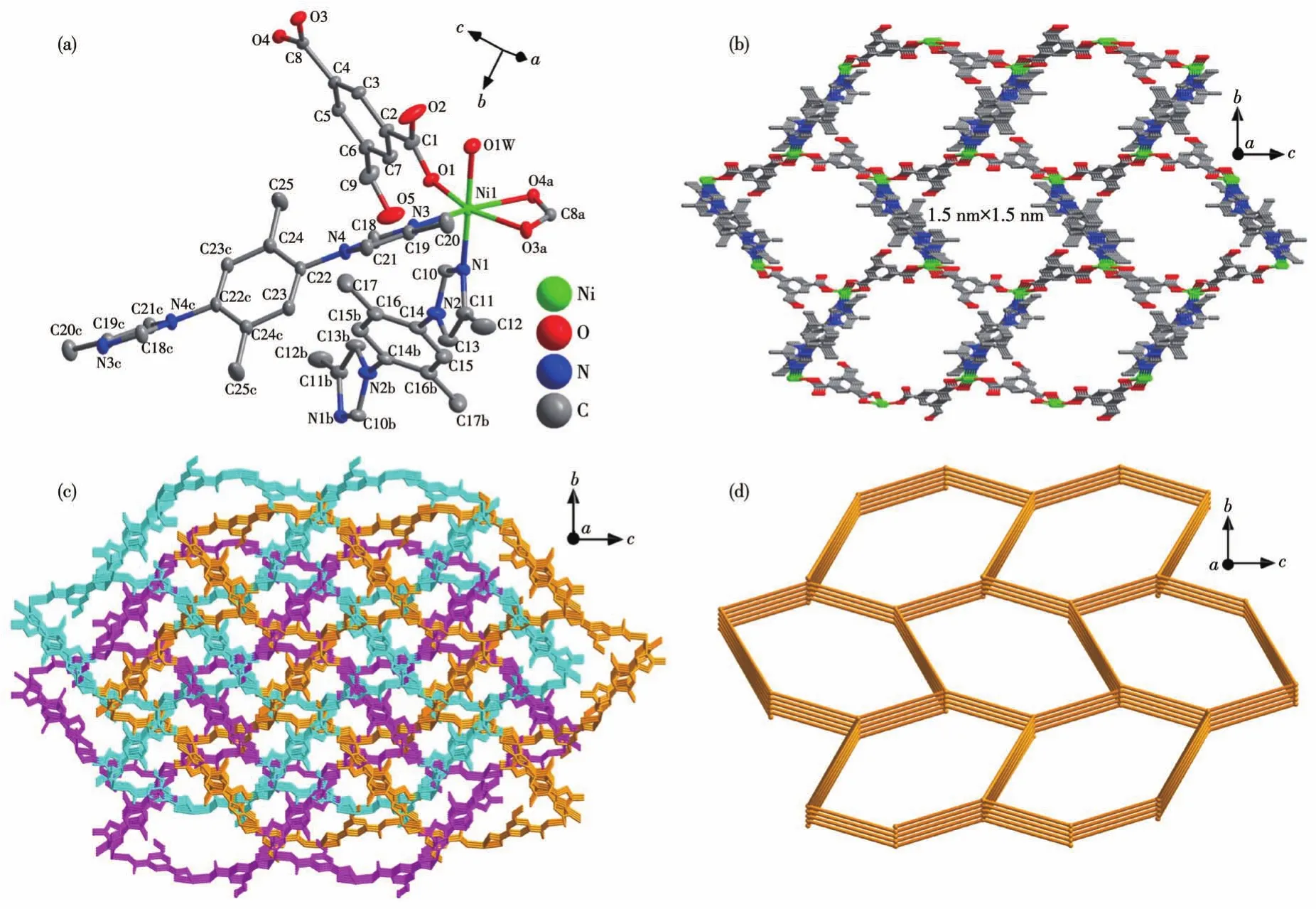
Fig.2 Schematic illustrations of 2:(a)coordination environments of Ni(Ⅱ) center;(b)empty 3D framework with nano-channels;(c)3-fold interpenetrating framework along the a-axis;(d)4-connected dia net
Complexes 1 and 2 were synthesized by the same aromatic carboxylic acids and metal ions under similar reaction conditions.Compared with 2,5-DPBI,2,5-DPBMI has two additional methyl groups in imidazole units.Methyl groups increase the volume of the auxiliary ligand and enhance the electron density of the N-donor atom.Different volumes and electron densities between 2,5-DPBI and 2,5-DPBMI bring about different sterical effects and nucleophilic reactivity in the reaction system.Effects from ligands lead to structural diversities between complexes 1 and 2,playing a key role in the synthetic processes.
2.2 PXRD and TG analysis
The PXRD experiments for complexes 1 and 2 were performed to check the phase purity of the bulk materials.The PXRD patterns match well with the simulated ones,indicating the phase purities of the samples(Fig.3a).The PXRD also firm complexes 1 and 2 are very stable in a normal organic solvent,such as methanol and ethanol.Moreover,after being soaked in water under ultraviolet(UV)irradiation,PXRD results show complexes 1 and 2 are still stable(Fig.S3,S4).To further investigate their thermal stabilities,the TGA experiments were carried out from room temperature(25℃)to 800℃under a nitrogen atmosphere(Fig.3b).Complex 1 showed no obvious weight loss up to 230℃,and then the whole frameworks began to collapse over 230℃.Complex 2 was stable in a temperature range of 25 to 170℃,then a weight loss(3.23%)took place between 170 and 220℃,which is attributed to the removal of coordinated water molecules (Calcd.3.35%).As the temperature further increased,the framework collapsed from 370℃and sharp weight loss continued to take place till the end of the TGA experiment.
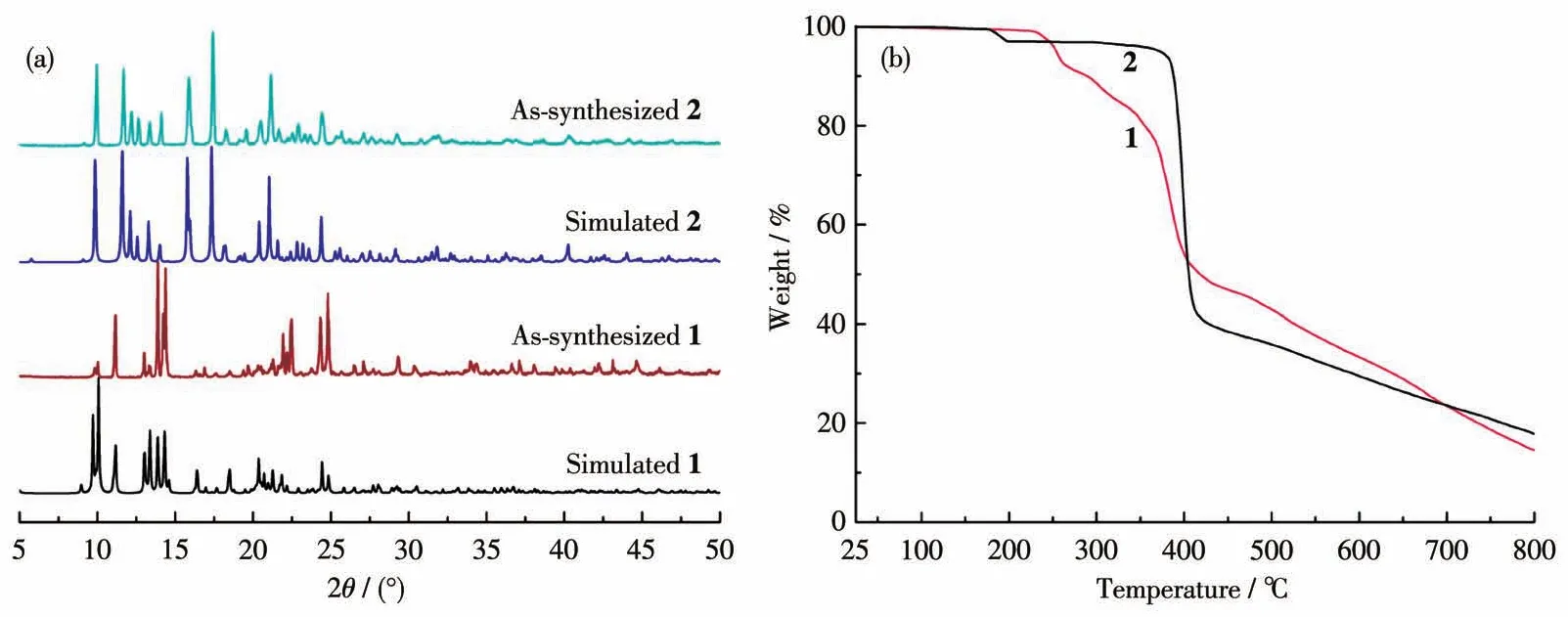
Fig.3 PXRD patterns(a)and TGA curves(b)of complexes 1 and 2
2.3 Semi-conductive properties and photocatalytic behaviors of complexes 1 and 2
Solid UV-Vis absorption tests reveal that complex 1 had two strong absorption bands in the regions of 200-500 nm and 550-850 nm(Fig.4a).Solid UV-Vis absorption spectrum of complex 2 was similar to that of complex 1,and the corresponding absorption bands located in 200-450 nm and 500-800 nm(Fig.4b).The diffuse reflection spectrum indicate that the bandgaps of complexes 1 and 2 were about 1.52 and 1.40 eV according to Kubelka-Munk method,respectively(Fig.S5,S6).To further clarify their semiconducting characters,the Mott-Schottky measurements were carried out at frequencies of 1 500,1 000,and 500 Hz,respectively.The experimental results reveal that 1 and 2 are typical n-type semiconductors,and their bottoms of the conduction band(LUMO)are about-0.61 and-0.38 eV(vs NHE),respectively(Fig.4c,4d).Furthermore,the experimental curves from electrochemical impedance spectroscopy(EIS)had a small radius,showing that complexes 1 and 2 should possess low resistance in charge transportation and high charge-separation efficiency(Fig.5a,5b).
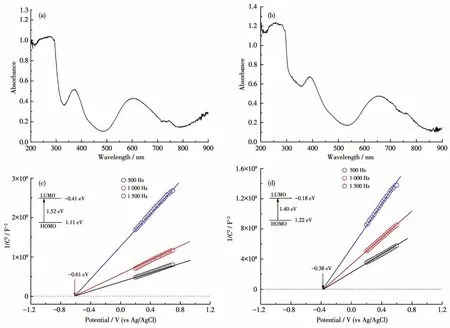
Fig.4 Solid UV-Vis absorption spectra of 1(a)and 2(b),and Mott-Schottky plots of 1(c)and 2(d)

Fig.5 EIS spectra for 1(a)and 2(b),and absorption spectra of the MB solution during photocatalytic degradation by 1(c)and 2(d)
Based on the above test results,complexes 1 and 2 should be candidate catalysts for some photocatalytic reactions.Therefore,the photocatalytic experiments were performed and the experiments revealed that the absorption peaks of MB obviously decreased under the exposure of UV-visible light in the presence of 1 or 2 along with increasing reaction time(Fig.5c,5d).The calculation results show that the degradation ratio of MB was 84% for 1 and 73% for 2 after three hours(Fig.6).Furthermore,the blank degradation experiment was also performed under UV irradiation without the catalyst.Compared with the blank experiment,complexes 1 and 2 had photocatalytic activity for the degradation of MB.Besides,to evaluate the stability of complexes 1 and 2 during the photocatalytic tests,recycling experiments were carried out(Fig.S8).The obtained results indicate that complexes 1 and 2 preserved their original catalytic activities after three reaction cycles,showing only slight changes in degradation efficiency.
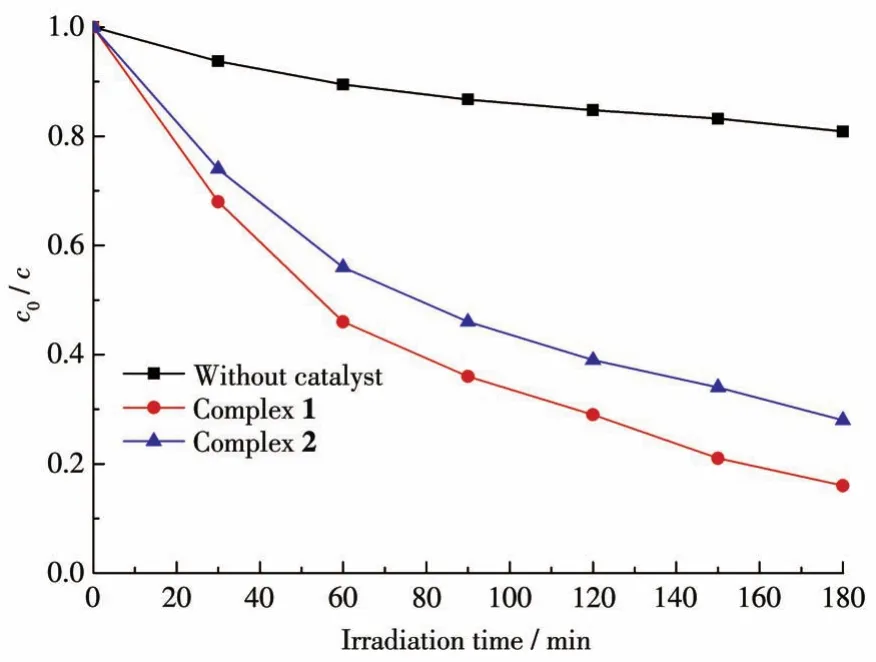
Fig.6 Photocatalytic degradation of MB solution with complexes 1,2,and without the catalyst
3 Conclusions
In summary,two Ni-MOFs were synthesized based on 5-(hydroxymethyl)isophthalic acid(H2HIPA)and different imidazole bridging linkers.Their structures exhibit different characteristics from a 5-connected framework to a 3-fold interpenetratingdianet,which reveals that bridging N-donor linkers have significant effects on the H2HIPA coordination modes and the final structures.Furthermore,the two complexes as typical n-type semiconductors are proved to be good candidates for the photocatalytic degradation of MB.
Supporting information is available at http://www.wjhxxb.cn
- 無機化學學報的其它文章
- 《無機化學學報》投稿須知(NOTICE TO AUTHORS)
- 基于吡啶基三聯(lián)吡啶配體的鋅(Ⅱ)和鎘(Ⅱ)配合物的晶體結(jié)構(gòu)和熒光性質(zhì)
- 一例基于5-((萘-1-基甲基)氨基)間苯二甲酸配體的Cd(Ⅱ)金屬有機骨架的合成、晶體結(jié)構(gòu)及對酸性氨基酸的檢測
- 一鍋法制備S型異質(zhì)結(jié)光催化劑Fe2O3/Fe2TiO5及其高效降解有機污染物性能
- 一種帶吡啶懸臂大環(huán)異雙核Zn(Ⅱ)-Ni(Ⅱ)配合物的合成、晶體結(jié)構(gòu)及其DNA結(jié)合/切割性質(zhì)
- 基于兩性離子配體構(gòu)筑的Eu(Ⅲ)配位聚合物的晶體結(jié)構(gòu)和對呋喃西林的檢測

