利用空間位阻和氫溢流協同作用促進5-羥甲基糠醛選擇性加氫制備5-甲基糠醛
李少鵬,杜靖,張彬,劉艷貞,梅清清,孟慶磊,,董明華,2,杜鵑,趙志娟,鄭黎榮,韓布興,2,5,趙美廷,*,劉會貞,2,5,*
1中國科學院化學研究所膠體界面與熱力學重點實驗室,北京分子科學國家實驗室,北京 100190
2中國科學院大學化學科學學院,北京 100049
3天津大學分子聚集態科學研究院,天津 300072
4中國科學院高能物理研究所,北京 100049
5懷柔綜合性國家科學中心物理科學實驗室,北京 101407
1 Introduction
Biomass is an abundant renewable carbon resource and can be transformed into different kinds of valuable chemicals because they contain various oxygen-containing functional groups1,2.Selective hydrogenation is a commonly used strategy to achieve the transformation of biomass and its derivatives3,4. Since most biomass or its derivatives contain a variety of reducible functional groups, it is a great challenge to control the activity or selectivity in many cases.
5-(Hydroxymethyl)furfural (HMF), which can be prepared from cellulose, is an important biomass platform compound5,6.There are different reducible chemical bonds (C=O, C-OH,C=C) in HMF. Various products (Scheme 1a) such as 2,5-dihydroxymethylfuran (DHMF), 2,5-tetrahydrofurandimethanol(DHTHF), 2,5-dimethylfuran (DMF), 2,5-hexanediol (HDO)have been obtained by the HMF selective hydrogenation7-14. 5-Methylfurfural (5-MF) is a very important chemical that can be used as a food additive. It is also a commonly used intermediate for synthesizing tobacco flavor, pyrethroid, prallethrin,agricultural chemicals, anti-cancer products, etc15-18. In addition, it is an important chemical intermediate of synthesis high-quality diesel19-21. Preparation of MF from the selective hydrogenation of HMF has always been desirable. However, it is very difficult from both thermodynamic and kinetic aspects constraints. In our previous work, the direct hydrogenation of HMF to MF without additives has been realized by Pt1/Nb2O5catalyst22.
In this work, the selectivity hydrogenation of HMF to MF has been controlled with a novel strategy, which elegantly combined the steric hindrance, the electron effect, and the hydrogen spillover. Based on this strategy, Pt@PVP/Nb2O5was designed for selective hydrogenation of HMF to MF (Scheme 1b, this work). It was found that the catalyst could catalyze the reaction efficiently and the selectivity of MF could reach 92% when the HMF was converted completely.
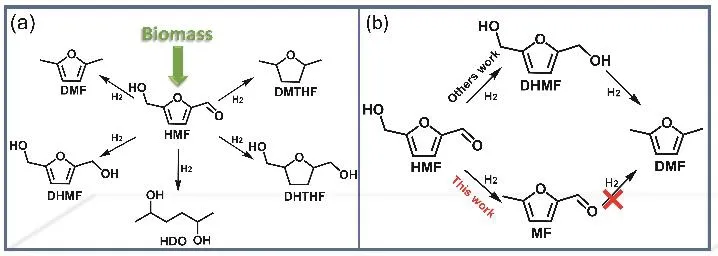
Scheme 1 The reaction path of HMF.
2 Experimental
2.1 Chemicals
Niobium oxalate hydrate (99%), polyvinylpyrrolidone (99%),hexadecylazaniumbromide (99%), H2PtCl6.6H2O (99%) were supplied by Sinopharm Chemical Reagent Co. Ltd.Tetrahydrofuran (99%), 5-hydroxymethylfurfural (98%), 2-furylmethanol (98%), furfuryl alcohol (98%) and n-decane(98%) were obtained from J&K Ltd. 2,5-Dimethylfuran (99%),5-methylfurfural (99%) were provided by TCI (Shanghai)Development Co., Ltd. Pt black (1%, mass fraction) and TiO2(99%) were purchased from Sigma-Aldrich. Deionized water was used during the experimental process. H2(> 99.99%) was purchased from Beijing Analytical Instrument Company. All chemicals involved in the synthesis of catalysts were used directly.
2.2 Preparation of catalysts
2.2.1 Synthesis of Nb2O5
Niobium pentaoxide nanoparticles were prepared according to the documented method22. In the experiment, niobium oxalate hydrate (0.538 g) was dissolved in deionized water. 10 mL of CTAB (0.2 mol·L-1) surfactant aqueous solution was added.Then the mixture was loaded into a dried Teflon autoclave at room temperature, followed by hydrothermal treatment of the above solution at 180 °C for 10 h. And then the white precipitate was washed with distilled water and ethanol three times. The grinded powder was calcined in a muffle oven at 500 °C for 4 h.
2.2.2 Synthesis of PVP-modified Pt nanoparticles(Pt@PVP NPs)
Pt@PVP NPs were synthesized according to the method reported in other literature. 5.0 mL H2PtCl6aqueous solution was added into 95 mL PVP solution. The solution was refluxed in a flask for 3 h, and then the PVP-stabilized Pt NP solution was obtained. The mole ratio of PVP and Pt was adjusted by changing the amount of H2PtCl6and PVP added.
2.2.3 Synthesis of supported Pt@PVP/Nb2O5
12 mL as-synthesized Pt nanoparticles solution was added into Nb2O5aqueous solution and stirred for 2 h. Subsequently,the solvent was removed at 80 °C. The obtained powder was washed with deionized water and dried at 80 °C. Then, the grinded dark grey powder was calcined in a tube furnace (Anhui Kemi Machinery Technology Co., Ltd.; Model TFV-1200-50-Ⅰ-220) to obtain Pt@PVP/Nb2O5.
2.2.4 Synthesis of conventional Pt/Nb2O5
In a typical procedure, an appropriate amount of H2PtCl6aqueous solution was slowly added into the 100 mg Nb2O5solution with magnetic stirring for 5 h. Then, under the ice bath temperature, the NaBH4(freshly prepared) was added dropwise into the solution. After 2 h, the obtained grey granules were washed and dried at 80 °C for 12 h.
2.3 Catalyst characterizations
The powder X-ray diffraction (XRD) patterns were obtained on Rigaku D/max 2500 equipped with nickel-filtered Cu-Kα(D/MAX-RC, Japan). The microstructure and size of Pt/Nb2O5,Pt@PVP NPs, and Pt@PVP/Nb2O5were obtained by using transmission electron microscopy (TEM) on JEM-2100F at 120 kV (JEOL, Japan). The Pt contents of different catalysts were analyzed by ICP-AES (Thermo, America). XAFS experiments were operated at the 1W2B beamline at Beijing Synchrotron Radiation Facility (BSRF). The X-ray photoelectron spectroscopy (XPS) was collected on the Thermo Scientific ESCA Lab 250Xi electron spectrometer (Thermo, America).
2.4 Hydrogenation reaction
In a typical experiment, appropriate amount of catalyst was dispersed in 2 mL THF and then 0.3 mmol HMF was added to the mixture in a Teflon-lined stainless-steel reactor of 20 mL.The reactor was purged with H2to remove the excess air. The reaction was carried out at desired temperature. After completion of the hydrogenation reaction, n-decane was added to the reactor.The solution was centrifuged and then analyzed by gas chromatography-mass spectrometry (GC-MS, Agilent 7890B-5977A) with a flame ionization detector (FID) (Agilent,America).
3 Results and discussion
The catalyst Pt@PVP/Nb2O5(10/1) was synthesized by the reduction-deposition method and the overall synthesis strategy was illustrated in Fig. 1a, in which 10/1 is the mole ratio of Pt and PVP. Detailed preparation processes for catalysts were described in the Experimental Section. Briefly, Nb2O5was fabricated from niobic acid roasting at 500 °C in air23. Known amounts of PVP and H2PtCl6were added into ethanol under stirring and Pt@PVP was obtained. Then, the Pt@PVP nanoparticle suspension was added to Nb2O5aqueous solution under stirring (Fig. 1a).
The XRD patterns of the Nb2O5and Pt@PVP/Nb2O5are given in Fig. 1b. The diffraction peaks at 2θ = 22.6°, 28.5°, and 36.6°are assigned to the pseudohexagonal Nb2O5crystal. The TEM images of Nb2O5and Pt@PVP/Nb2O5are shown in Fig. 1c and Fig. 1d. Uniform Pt nanoparticles (NPs) with average diameter of 2.8 nm were immobilized on Nb2O5. The high-resolution TEM (HRTEM) image (Fig. 1e) showed that the interplanar distance of Pt NPs on Nb2O5is 0.23 nm, which corresponds to the standard lattice fringe of Pt (200) surface. High-angle annular dark-field scanning transmission electron microscopy(HAADF-STEM) and energy-dispersive X-ray (EDS) elemental mapping (Fig. S1) revealed that all elements were uniformly distributed throughout the Pt@PVP/Nb2O5. The content of Pt in Pt@PVP/Nb2O5was 0.75% (mass fraction) (Table S1).
The catalytic performance of Pt@PVP/Nb2O5was studied.Interestingly, the selectivity of MF could reach 92% with > 99%conversion of HMF in 24 h (Table 1, entry 1). To get some information about the roles of the components in the catalyst, the catalytic performances of Nb2O5, Pt/Nb2O5, and Pt@PVP were also checked (Table 1). Although the conversion of HMF over Nb2O5was only 7%, the selectivity of MF could reach 63%(Table 1, entry 2). The by-product was from the C-C coupling reaction during the process of dehydroxylation because of the strong acidity of Nb2O5and no DHMF was detected which was from the hydrogenation of C=O. It means that Nb2O5was active for the dehydroxylation and inactive for the hydrogenation of C=O in HMF. The low activity of the Nb2O5may be attributed to its low ability to split H2. The cat alytic activity of Pt/Nb2O5was much higher than pure Nb2O5because Pt nanoparticles can efficiently activate H2. However, DHMF, which was generated from hydrogenation of the C=O group of HMF, was the main product over Pt/Nb2O5(Table 1, entry 3). The conversion of HMF and the selectivity of MF was 16% and 37% over Pt@PVP in 12 h, respectively (Table 1, entry 4). PVP also improved the selectivity of MF over Pt/C. No MF was obtained over commercial Pt/C catalyst (Table 1, entry 5) and the selectivity increased to 32% over Pt@PVP/C catalyst (Table 1, entry 6).The Pt@PVP/TiO2had low catalytic activity for the target product (Table 1, entry 7). The results indicate that Pt, PVP, and Nb2O5were indispensable for the highly selective hydrogenation of HMF to MF.
The effect of the mole ratio of Pt and PVP was checked for the Pt@PVP/Nb2O5catalyst (Fig. S2). The TEM images of the modified Pt nanoparticles with different Pt/PVP ratios are shown in Fig. S3. The activity of the catalyst gradually decreased with the increase of the amount of PVP due to the covering of the metal surface, while the selectivity of MF increased. When the ratio of Pt/PVP was greater than 10/1, the selectivity of MF could reach above 90%. The selectivity of MF was constant when further increase the amount of PVP. When the mole ratio of PVP/Pt was less than 10/1, HMF could still be adsorbed by Pt nanoparticles, and the selectivity of MF was low. When the Pt nanoparticles were coated with plenty of PVP, a large amount of PVP inhibits the adsorption of HMF by Pt nanoparticles, and the selectivity of MF could remain at 90%. These results indicate that PVP could improve the selectivity of MF significantly and the effect was kept unchanged after the amount of PVP adsorbed on the Pt particles was large enough. The effect of reaction temperature on the Pt@PVP/Nb2O5catalytic performances was researched (Fig. S4). When we increase the temperature from 100 °C to 160 °C, the conversion of HMF increases. Although the selectivity of MF was the highest at 120 °C (> 99%), the conversion was very low. The most suitable reaction temperature was 140 °C. To verify the durability of the Pt@PVP/Nb2O5catalyst, the reusability of the catalyst was also tested for the hydrogenation of HMF to MF. After the reaction, the catalyst was washed with THF. Next, the catalyst was dried in vacuum oven at 80 °C overnight before being reused in the next run. The results in Fig. S5 clearly showed that the Pt@PVP/Nb2O5catalysts could be reused at least 4 times without obvious decrease in activity and product selectivity.
To further study the reaction pathway, some control experiments were conducted. The effect of the reaction time on the catalytic performance over Pt@PVP/Nb2O5was studied and the result was demonstrated in Fig. S6. Fortunately, the selectivity of MF could reach 92% and the selectivity was independent of conversion. Even if the reaction time was prolonged to 30 h, MF was not further hydrogenated to DMF,indicating that the by-product was not from the hydrogenation of C=O. In addition, MF was also used as the substrate to check the catalytic performance of Pt/Nb2O5and Pt@PVP/Nb2O5. The Pt/Nb2O5could efficiently catalyze the hydrogenation of MF to DMF with almost complete conversion. However, no MF was converted over the Pt@PVP/Nb2O5(Table S2). The results indicate that Pt@PVP/Nb2O5is inert for the hydrogenation of C=O, which explains the high selectivity of MF. The variation trend of the catalytic performance with the reaction time over the Pt/Nb2O5was also checked and the result was shown in Fig. S7.The conversion of HMF was 80% with 12% selectivity of MF in 3 h. However, MF was converted to DMF with the prolongation of the reaction time, demonstrating that PVP was crucial for the high selectivity.
It is well known that the electronic state often affects the performance of catalysts significantly. The electron interaction among the three interfaces in Pt@PVP/Nb2O5was studied by X-ray photoelectron spectroscopy (XPS), and the results are presented in Fig. 2. The binding energy of Nb in Pt/Nb2O5,Pt@PVP/Nb2O5shifts toward lower binding energy compared with that of Nb in pure Nb2O5, indicating that the electrons transferred from Pt to Nb2O5and then Nb in Pt@PVP/Nb2O5get more electrons. The binding energy of Pt 4f in Pt@PVP/Nb2O5displayed a slight shift toward higher binding energy compared with Pt@PVP and Pt/Nb2O5, demonstrating the lowest electron density of Pt in Pt@PVP/Nb2O5compared with Pt@PVP and Pt/Nb2O5(Fig. 2b). All the results above show that Pt was more electron-deficient and Nb2O5more electron-rich in Pt@PVP/Nb2O5.
Considering the difference of binding energy of Pt in Pt/Nb2O5and Pt@PVP/Nb2O5was very small, Pt L3-edge X-ray absorption near edge structures (XANES) analysis was performed to further characterize the electronic state of Pt, and the results are given in Fig. 3. XANES analysis showed an increase in the Pt d-band vacancy for Pt@PVP/Nb2O5compared with Pt/Nb2O5(Fig. 3a). Namely, the electron density of Pt was lower in Pt@PVP/Nb2O5than that in Pt/Nb2O5. This is consistent with the results of XPS characterization that there exist strong electronic interactions between Pt and PVP or Nb2O5. Fourier transformation of extended X-ray absorption fine structure (FT-EXAFS) further showed weakening of Pt-Pt coordination shells while emerging Pt-N shells24(Fig. 3b and Fig. S8). It means that exists interaction between PVP and Pt.Wavelet transform (WT) oscillations further display visually the Pt-N throughout the whole catalyst. In Fig. 3c, WT plots of Pt/Nb2O5showed intensity maximum near 2.5 ? (1 ? = 0.1 nm),which is associated with the Pt-Pt. However, as shown in Fig.3d, another intensity maximum can be observed at 1.9 ?, which can be attributed to the existence of Pt-N coordination. These results further confirmed the formation of Pt-N in Pt@PVP/Nb2O5.
The electronic states of Pt were further characterized by in situ CO-diffuse reflectance infrared Fourier transform (CO-DRIFT)spectra measurements. It can be known from in situ CO-DRIFT spectra in Fig. S9 that the band for CO adsorbed on metallic Pt(CO-Pt0) in the prepared Pt@PVP/Nb2O5was observed at 2083 cm-1. By comparison, the Pt/Nb2O5catalyst showed substantial CO chemisorption at 2072 cm-1, and the band for CO adsorbed on metallic Pt (CO-Pt0) in the synthesized Pt@PVP was observed at 2066 cm-1. These results showed that electron transfer from Pt nanoparticles to Nb2O5support. The characterization results are consistent with the above XPS.
The adsorption mode of the substrate on the solid surface of the catalyst is a key factor to affect the selectivity of the reaction.The chemisorption of C-OH, and C=O can be studied by FTIR spectroscopy usingn-propanal and methanol as model molecules25,26. The bands of the methoxy species observed at 1211 cm-1and 1153 cm-1were assigned to theν(C-O) bands of the on-top and bridged sites, respectively (Fig. S10). This is the direct evidence of chemical adsorption of C-OH by the surface of Nb2O5. However, Nb2O5could not adsorb C=O groups chemically. It means that Nb2O5could activate C-OH selectively. It has been reported that Nb2O5can promote the deprotonation of the phenol molecules and activate Caromatic-OH27,28. So, the phenomena are consistent with those reported in the literature.
It has been reported the activated hydrogen species on metal surfaces can migrate to the supports to take part in the hydrogenation reaction, which seemed to be hydrogen spillover29-31. The hydrogen spillover can be studied by mixing catalyst with a support32. The physically mixed commercial Pt/C catalyst and Nb2O5were used to study the hydrogen spillover and the results are given in Table 2. Although the HMF conversion was very low, the selectivity of MF can reach 61% in a short time (Table 2, entry 1). The conversion of HMF was 65% while no MF was produced over commercial Pt/C catalyst (Table 2, entry 2). However, MF was produced over the physically mixed commercial Pt/C catalyst and Nb2O5. With the decrease of the amount of Pt/C catalyst, the selectivity of MF increased gradually (Table 2, entries 3-5). When the dosage of Nb2O5was 40 mg and the Pt/C was 3 mg, the selectivity of MF could reach 43% with 8% conversion of HMF (Table 2, entry 5). When only3 mg Pt/C was used as the catalyst, no MF was detected (Table 2, entry 6). The result shows that hydrogen spillover exists in the reaction system. The hydrogenation of C=O occurred on the surface of Pt NPs and the formation of MF can be attributed to the surface of Nb2O5. In addition, the physical mixture of WO3and Pt@PVP/Nb2O5catalyst showed the transformation from WO3with bright yellow color to WO3-xwith light blue color at room temperature, confirming the occurrence of hydrogen spillover (Fig. S11). The hydrogen spillover can also be proved by pulse adsorption of hydrogen and the results are shown in Table S3. The metal dispersion of Pt@PVP was only 2.33% while it increased to 46.63% for Pt@PVP/Nb2O5. Due to the presence of hydrogen spillover, the increase in the number of active sites for H2activation. The metal dispersion of Pt/Nb2O5was 71.85%, suggesting that some active sites of H2were blocked by PVP in Pt@PVP/Nb2O5. The higher activity of Pt@PVP/Nb2O5over Pt@PVP (Table 1, entries 1 and 4)suggests that the reaction proceeded mainly via the hydrogen spillover pathway, namely the activated hydrogen on Pt NPs migrated to the surface of Nb2O5and reacted with C-OH activated by Nb2O5.
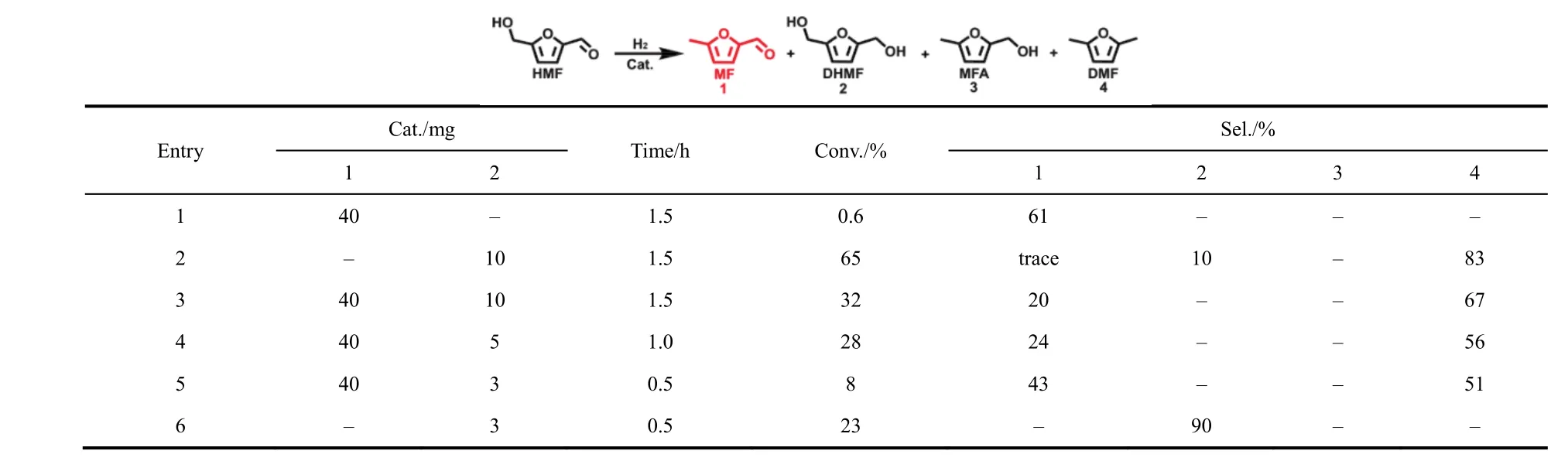
Table 2 Reaction results over different catalytic systems a.
The hydrogen spillover and the chemical adsorption of C-OH on the surface of Nb2O5have been confirmed in our experiments. In this work, we also studied the hydrogenation process of HMF to MF on the surface of Nb2O5in the presence of activated hydrogen with density functional theory (DFT). The calculation was conducted through the Vienna Ab-initio Simulation Package (VASP) in this work. The results (Fig. 4)show that the reaction contains three primary steps. Firstly, the C-OH bond of HMF is ruptured because of the strong interaction between Nb and OH to form methylene groups and the free energy barrier is 0.96 eV. Secondly, the Nb-OH reacts with activated hydrogen atoms of H2and forms Nb-OH2.Finally, one of the hydrogen atoms in Nb-OH2transfers to the methylene groups and generates MF, and meanwhile another active hydrogen atom of H2combines with the Nb-OH to form H2O. The free energy barrier of the whole process for the selectivity hydrogenation of HMF to MF on the surface of Nb2O5was 0.96 eV, which can be overcome at 140 °C. The DFT calculation also shows that the hydrogenation of HMF to MF can proceed on Nb2O5surface in the presence of activated hydrogen migrated from the surface of Pt.
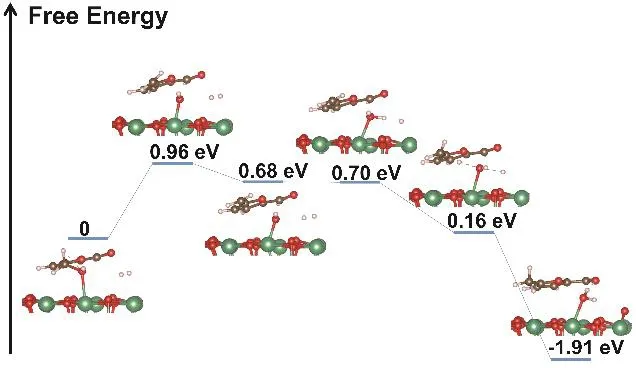
Fig. 4 The energy profiles for HMF hydrogenation into MF on Pt@PVP/Nb2O5.
According to the experimental and DFT calculation results,we can discuss the interesting phenomenon that HMF could be hydrogenated to MF selectively over Pt@PVP/Nb2O5, which is shown in Scheme 2. The activation of HMF on Pt was inhibited by the PVP layer, while Pt could activate hydrogen because it can penetrate the PVP layer due to its small size. The C-OH groups activated by Nb2O5were hydrogenated by the active hydrogen transferred to Nb2O5from Pt via hydrogen spillover.The selectivity was very high because the acidic surface of Nb2O5made it easier to adsorb C-OH, rather than C=O. In addition, the electron effect was also beneficial to the formation of MF. As discussed above, the electron interaction at the threephase interface in the Pt@PVP/Nb2O5made the Pt particles more electron-deficient and Nb2O5more electron-rich. The electron-rich Nb2O5decreased the C-C coupling reaction, and electron-deficient Pt was favorable to hydroxyl adsorption and unfavorable to C=O adsorption, which further enhanced the selectivity of MF33.
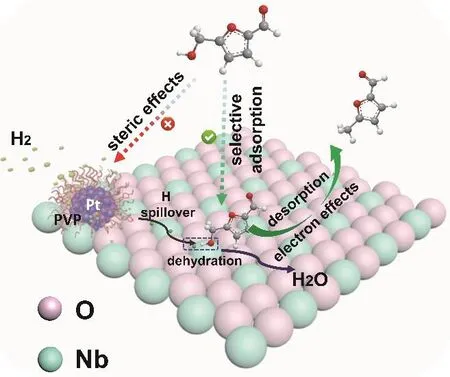
Scheme 2 A plausible mechanism for the HMF hydrodeoxygenation over the prepared Pt@PVP/Nb2O5.
4 Conclusions
In summary, Pt@PVP/Nb2O5could catalyze the selective hydrogenation of HMF to MF with a selectivity of up to 92%.The steric effect, hydrogen spillover, and electron transfer at the interface of the three-phase catalyst system contribute simultaneously to the reaction. Although PVP blocked the access of HMF to the surface of Pt, H2can be activated on the surface of Pt, which will migrate further to the Nb2O5surface through the hydrogen spillover pathway. When the HMF is adsorbed on Nb2O5, the C-OH will be hydrogenated with high selectivity.This work represents a new paradigm to control the hydrogenation selectivity, and we believe that the route to produce MF from renewable HMF has great potential for the green production of such important chemical intermediate.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.

