On-surface synthesis of one-dimensional carbyne-like nanostructures with sp-carbon
Wenze Gao(高文澤), Chi Zhang(張弛), Zheng Zhou(周正), and Wei Xu(許維)
Interdisciplinary Materials Research Center,College of Materials Science and Engineering,Tongji University,Shanghai 201804,China
Keywords: on-surface synthesis,carbyne,scanning tunneling microscopy,atomic force microscopy
1. Introduction
The discovery and creation of new carbon allotropes have led to innovations in both chemistry and material science,which have enriched the applications of carbon-based materials and opened doors to new technologies.[1]Over the past decades, the precise control of carbon at the nanoscale has allowed continuous discoveries of low-dimensional carbon allotropes,including zero-dimensional(0D)fullerenes,[2]one-dimensional (1D) carbon nanotubes (CNTs),[3]and twodimensional(2D)graphene.[4]Compared to CNTs,a 1D carbon allotrope built with sp2-hybridized carbons whose length far exceeds the cross-sectional radius,carbyne is an infinite 1D carbon chain composed of sp-hybridized carbon atoms,which has the cross-sectional dimension reduced to a single carbon atom. As one of the most elusive carbon allotropes, carbyne has attracted significant attention since the 1960s.[5–7]In principle, carbyne can be either in a cumulenic form (with consecutive carbon–carbon double bonds) which exhibits metallic behavior,or in a polyynic form(with alternating single and triple bonds)showing semiconducting property(Fig.1),[8–10]and the latter form has been demonstrated to be energetically more favorable.[11]
As an infinite carbon chain, carbyne has been theoretically predicted to possess lots of intriguing properties, such as room-temperature superconductivity,[12,13]high hydrogen storage capability,[14]and nonlinear optical properties.[15]Moreover, Young’s modulus and the anticipated stiffness of carbyne are greater than most known carbon allotropes, including diamond, graphene, and CNTs.[16]Despite having these advantages, carbyne was much less explored compared to other carbon allotropes (e.g., graphene) due to its high chemical reactivity and extreme instability. Thus, it was postulated to be impossible to synthesize in the laboratory.[17]Due to the difficulties in synthesis and isolation of individual carbyne, chemists also turned to the exploration of polyynes and cumulenes as model compounds (Fig. 1).[10,18,19]In recent years, various feasible synthetic routes for the preparation of carbyne-like nanostructures have been reported,such as bottom-up synthesis,[17,20,21]arc-discharge,[22–24]laser ablation,[25–27]heat-treatment,[28–30]fusion inside carbon nanotubes,[31,32]etc.,which inspired chemists to reexamine the accessibility and stability of carbyne. In 2016, a long linear carbon chain comprising more than 6000 carbon atoms was successfully synthesized using thin double-walled carbon nanotubes as confining nanoreactors,[33]and it is considered to be the longest carbon chain ever reported.
In the past decade, on-surface synthesis has emerged as an extremely promising approach for atomically precise fabrication of novel nanostructures that can be hardly synthesizedviaconventional solution synthetic chemistry.[34,35]The rapid developments and combination of scanning tunneling microscopy(STM),[36,37]noncontact atomic force microscopy(nc-AFM),[38]and x-ray photoelectron spectroscopy (XPS)have allowed us to design andin situcharacterize novel carbon nanostructures with unprecedented resolution. Thus, elusive low-dimensional carbon allotropes have been successfully fabricated on surfaces, including these built with sp2-hybridized carbons (e.g., graphene nanoribbons[39,40]and biphenylene network[41])and sp-hybridized carbons(e.g.,polyynes[42]and cyclo[n]carbons[43,44]). Herein,we highlight recent works regarding the on-surface synthesis of 1D carbyne-like nanostructures (1D carbon nanostructures containing carbyne fragments),including polyyne compounds,cumulene compounds,and organometallic polyynes (i.e., metalated carbynes). We believe this review would shed light on the precise fabrication and characterization of ultimate single strands of carbyne by on-surface synthesis strategy.
2. On-surface synthesis of polyynes
Polyynes have been widely investigated as models for carbyne and have great potential as molecular wires for charge transport[45]owing to their nonlinear optical properties.[15]As the number of carbon atoms increases,long polyynes become inherently unstable because they tend to cross-link with each other in an exothermal reaction.[46]Notably,the development in the field of on-surface synthesis provides a promising strategy for atomically precise fabrication and in-depth investigation of long polyynes under highly controllable conditions.

Fig. 2. On-surface synthesis of polyynes by atomic manipulations. [(a)–(j)] On-surface synthesis of [(a)–(d)] triyne, [(e)–(h)] hexayne, and [(i) and(j)]octayne on bilayer NaCl on Cu(111). Reproduced with permission from Ref.[42]. Copyright 2018, Springer Nature. [(k)and(l)]Generation of cyclo[18]carbon on bilayer NaCl on Cu(111)based on two kinds of molecular precursors. (m)Formation of a linear polyyne chain via bond cleavage within the cyclic unit of C24O6. [(k)and(m)]Reproduced with permission from Ref.[43]. Copyright 2019, AAAS.(l)Reproduced with permission from Ref.[44]. Copyright 2020,American Chemical Society.
In 2018, polyyne moieties were fabricated on surface and structurally characterized by scanning probe microscopy(SPM)for the first time by Pavliˇceket al.[42]By a combination of STM and nc-AFM techniques,the molecular precursor was precisely manipulated to induce the skeletal rearrangement at the molecular level, and simultaneously, the geometry of reactants, intermediates, and the final products were monitored with atomic resolution as shown in Figs. 2(a)–2(j). Accordingly,tri-,tetra-,hexa-,and octaynes were generated from the reductive rearrangement of the precursors (i.e., 1,1-dibromo alkenes)on bilayer NaCl supported by Cu(111)at 5 K.Interestingly,the skeleton rearrangement was triggered along with the cleavage of the C–Br bonds of precursors by the atomic manipulation using an STM/AFM tip. The nc-AFM images of the intact precursors,intermediates,and polyynes are shown in Figs.2(b)–2(d)and 2(f)–2(j). Following such a strategy,relatively long polyyne moieties, up to the octayne Ph–(C≡C)8–Ph,have been successfully fabricated with atomic precision.
More interestingly, cyclic polyynes (cyclo[n]carbons)could also be prepared by this method. In 2019, Kaiseret al.[43]further applied these techniques to synthesize a cyclo[18]carbon allotrope from a cyclocarbon oxide molecule,C24O6(Fig. 2(k)). C24O6molecules were deposited onto Cu(111) precovered with bilayer NaCl islands at 5 K. By atomic manipulation,carbon monoxides were eliminated from C24O6, thus forming cyclo[18]carbon. The nc-AFM image of cyclo[18]carbon showed nine bright protrusions, revealing a polyynic structure with alternating single and triple bonds. Similarly, cyclo[18]carbon was also proved to be accessible by dehalogenation of a bromocyclocarbon precursor,C18Br6,as shown in Fig.2(l),with a much higher yield of cyclo[18]carbon(64%)compared to that in the case using C24O6(13%).[44]Furthermore, a linear polyyne chain could also be produced by breaking the bonds within the cyclic unit from the C24O6molecule(Fig.2(m)). The precision of the synthesis achieved by this approach opens a new window for the onsurface fabrication of carbon-rich materials and atomic-scale devices.
3. On-surface synthesis of cumulenes
Compared to polyynes, cumulenes have been less studied because their stability dramatically decreases in the presence of more consecutive double bonds.[10,47]Such instability has been undoubtedly a major obstacle in their synthesis and characterizations. Followed by the strategies in the preparation of long polyyne moieties,[20,21]the synthesis and stabilization of long cumulenes have been successfully achieved through rotaxination[48]and increase of steric bulk of end groups.[49]Additionally,on-surface synthesis also provides a convenient alternative approach for the fabrication of cumulene-containing nanostructures with atomic precision.
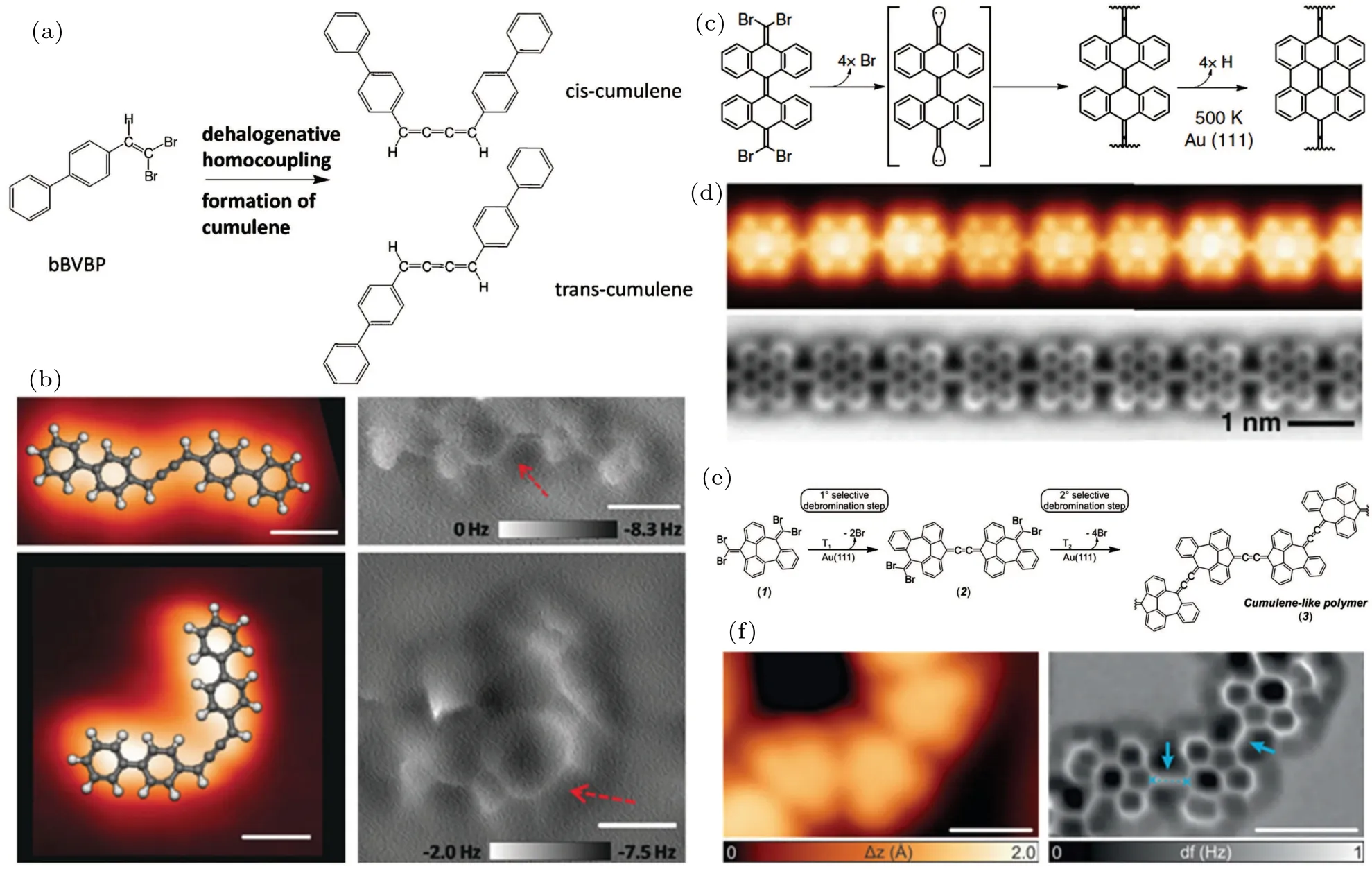
Fig.3. On-surface synthesis of cumulenes. [(a)and(b)]On-surface synthesis of cis-and trans-cumulene compounds by dehalogenative C–C homocoupling reactions of alkenyl gem-dibromides. Reproduced with permission from Ref.[50]. Copyright 2017,Wiley-VCH.[(c)–(f)]Synthesis of different types of cumulene-containing polymers on Au(111). [(c)and(d)]Reproduced with permission from Ref.[51]. Copyright 2020,Springer Nature. [(e)and(f)]Reproduced with permission from Ref.[52]. Copyright 2020,Wiley-VCH.
Sunet al.[50]firstly designed and reported the on-surface synthesis of cumulene moiety based on a dehalogenative homocoupling reaction of alkenyl gem-dibromides. As illustrated in Fig.3(a),they designed a 4-(2,2-dibromovinyl)-1,1’-biphenyl(bBVBP)molecular precursor functionalized with an alkenyl gem-dibromide group.Upon deposition onto Au(111)surface at room temperature, the bBVBP molecules were activated by the removal of halogen substituents and underwent C–C homocoupling reactions, formingcis- andtranscumulene products. The nc-AFM images showed sharp lines with a homogeneous contrast connecting the two biphenyl groups, which unambiguously demonstrated the formation of three consecutive C–C double bonds(Fig.3(b)).
Such a C(sp2)–Br2substitution strategy has been widely applied to the on-surface synthesis of cumulene-containing nanostructures ever since this seminal work. Torreet al.[51]reported the synthesis of cumulene-bridged bisanthene polymers (CBBPs) by the dehalogenative homocoupling of 4Br-BiA precursors on Au (111) at 500 K, which were endowed with =CBr2functionalities (Fig. 3(c)). Highresolution STM and nc-AFM images of CBBPs are shown in Fig.3(d). Interestingly,the cumulenic bridges in CBBPs were further fused to pentalene bridges after annealing at 650 K,leading to defectfreeπ-conjugated ladder polymers. Besides, Urgelet al.[52]further extended the strategy to the synthesis of 1D cumulenecontaining polymers (CCPs) composed ofn-membered rings(n= 5, 6, 7) on Au (111) using similar functional groups(Fig. 3(e)). It is worth noting that the highly nonplanar conformation of the dibromomethylenes-functionalized precursors on Au (111) leads to the separated steps in selective debromination and coupling, and consequently, a selective tailto-tail/head-to-head monomer sequence in the polymer (as shown in Fig. 3(f)). The consecutive C–C double bonds are highlighted by blue arrows in the nc-AFM image. Moreover, other similar cumulene-containing nanostructures have also been systematically studied recently.[53–56]The investigations of theseπ-conjugated polymers linked by cumulene bridge open new avenues in the field of on-surface synthesis with prospects for applications in molecular electronics.
4. The on-surface synthesis of organometallic polyynes
Organometallic polyyne,a chain composed of alternating sp-hybridized carbon atoms and metal atoms, is a promising candidate for future electronic and optical devices due to its regulable electronic, optical, and magnetic properties by the incorporation of different transition metals.[57–59]Similar to the case of carbyne,the high chemical reactivity and extreme instability have been blocking the synthesis and characterization of organometallic polyynes,which may be enlightened or solved by applying an on-surface synthesis strategy.

Fig.4. On-surface synthesis of acetylenic Cu-carbyne. [(a)and(b)]Illustration showing the formation of acetylenic Cu-carbyne on Cu(110)through dehydrogenative coupling of ethyne precursors. (c) Large-scale nc-AFM image and the corresponding STM image of acetylenic Cu-carbynes. (d)Equally scaled high-resolution nc-AFM image, STM image, DFT-optimized model, STM simulation and line-scan profile of a single acetylenic Cucarbyne chain on Cu(110). Reproduced with permission from Ref.[60]. Copyright 2016,American Chemical Society.
In 2016, Sunet al.[60]firstly reported the synthesis of organometallic Cu-polyynes(i.e.,acetylenic Cu-carbynes)by dehydrogenation of ethyne (C2H2) molecules and coupling with copper atoms on Cu (110) as shown in Fig. 4. After deposition of C2H2onto Cu (110) held at 450 K, acetylenic Cu-carbyne chains were efficiently synthesized and extended along the close-packed [1ˉ10] direction of the substrate. The high-resolution nc-AFM images showed the characteristic protrusions of C–C triple bonds between neighboring two Cu atoms, which appeared as bright dots in the STM image, yet were not resolved in the nc-AFM image. These features further confirmed the formation of organometallic polyynes.This synthetic strategy would prompt the synthesis and characterization of other 1D organometallic polyynes with various incorporated metal atoms as well as periodic polyyne moieties.
Inspired by the above strategy, the organometallic Aupolyynes (i.e., diacetylenic Au-carbynes) were successfully obtained through on-surface debrominative coupling of C4Br4molecule with a cumulene moiety (Br2C=C=C=CBr2) on Au(111).[61]Interestingly,thein-situskeleton rearrangement from a cumulene moiety to a diyne one (Br–C≡C–C≡C–Br)was directly triggered by cleaving two C–Br bonds within a C4Br4viaSTM tip manipulation. Thereafter, the complete debromination of C4Br4molecules was realized by further thermal treatment,with the formation of 1D diacetylenic Aucarbynes as shown in Fig. 5. Note that two discrete characteristic protrusions as indicated by blue arrows in the nc-AFM images corresponded to two adjacent C–C triple bonds.Moreover, the bandgap of a diacetylenic Au-carbyne on Au (111)was experimentally determined to be~2.0 eV by scanning tunneling spectroscopy (STS), indicating a semiconducting characteristic for potential applications in future molecular electronic devices.
Very recently,a new kind of organometallic polyynes,triacetylenic Ag-carbyne,has been successfully synthesizedviaan unexpected ring-opening reaction of completely debrominated hexabromobenzene (C6Br6) molecules on Ag (111) by Gaoet al.[62]As illustrated in Fig. 6, the whole scenario can be described as follows: a complete debromination of C6Br6molecules occurred at 300 K on Ag (111), resulting in the formation of unstable C6ring intermediates followed by subsequent transformation into the C6polyynic chainsviaa ring-opening process; afterward, the C6polyynic chains polymerized into triacetylenic Ag-carbynes. The nature of the polyynic segment within chains was clearly revealed by the nc-AFM image,showing three discrete characteristic protrusions of C–C triple bonds, as indicated by the yellow arrows. The debromination and ring-opening processes were demonstrated by extensive density functional theory (DFT)calculations. In addition,Yuet al.[63]further investigated the thermal-induced transformation between acetylenic Ag/Cucarbyne and diacetylenic ones. They theoretically predicted that the bandgap of organometallic polyynes would decrease with the increasing number of C–C triple bonds involved.It was also revealed by DFT calculations that the bandgaps would be metal-dependent with the order of Ag-carbyne>Cu-carbyne>Au-carbyne. Moreover,metalated carbyne ribbons with different incorporated metals might also be synthesized by using surface-assisted elimination reactions of methane tetrabromide molecular precursors and their subsequent polymerization. The bandgap of metalated carbyne ribbons would vary with its width based on theoretical calculations. These regulable electronic properties of organometallic polyynes thus provide a promising prospect for next generation semiconducting materials.

Fig.5. On-surface synthesis of diacetylenic Au-carbyne. (a)Schematic illustration showing the formation of diacetylenic Au-carbyne from C4Br4. (b)STM image showing the formation of Au-carbyne chains on the Au(111)surface by heating the sample pre-covered with C4Br4 molecules to 300 K.(c) Equally scaled high-resolution STM image and the corresponding DFT-optimized model of a single diacetylenic Au-carbyne chain on Au (111).(d)Close-up STM images and the Laplace filtered nc-AFM images of the single chain, double chain, and triple chain, respectively. Reproduced with permission from Ref.[61]. Copyright 2020,American Chemical Society.

Fig.6. On-surface synthesis of triacetylenic Ag-carbyne. (a)Schematic illustration showing the formation of triacetylenic Ag-carbyne from C6Br6. (b)A large-scale STM image showing the formation of triacetylenic Ag-carbynes on the Ag(111)surface by depositing C6Br6 molecules on the sample held at 300 K.(c)Constant-height nc-AFM image and the corresponding STM image of triacetylenic Ag-carbynes. (d)From top to bottom: an STM image,a simulated STM image,and top-and side-view DFT models of a single Ag-carbyne on Ag(111). Reproduced with permission from Ref.[62].Copyright 2022,American Chemical Society.
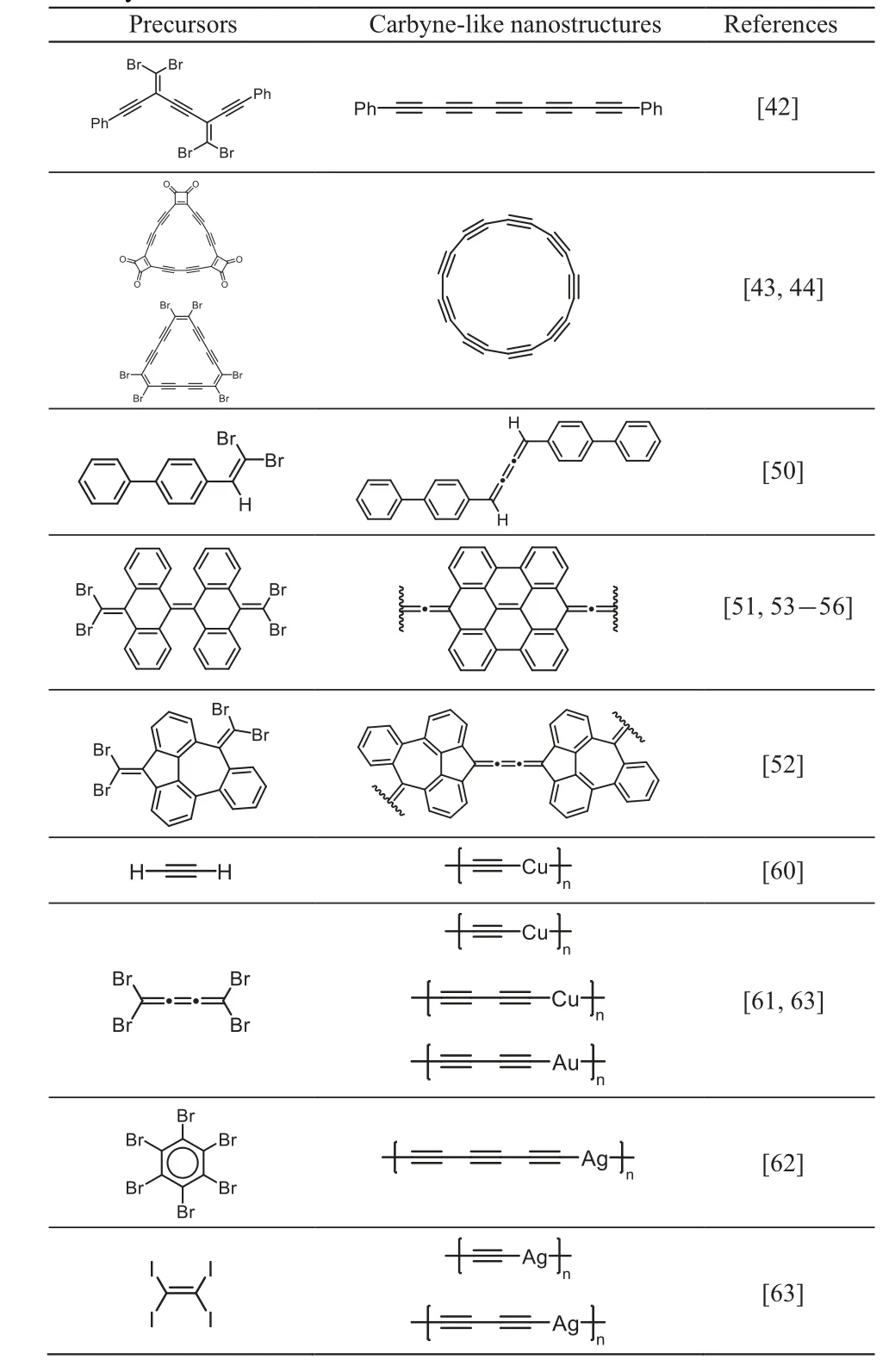
Table 1 Representative carbyne-like nanostructures synthesized via the onsurface synthesis method.
5. Conclusion and perspectives
In summary, we have briefly reviewed recent advances in the on-surface synthesis of one-dimensional carbynelike nanostructures with sp-hybridized carbons, including polyynes,cumulenes,and organometallic polyynes(Table 1).On-surface synthesis strategy has exhibited its great potential for the preparation of nanostructures with atomic precision which are not accessible through conventional solution chemistry. Nonetheless, there are still many difficulties as well as challenges ahead. For instance, some precursors are too reactive to survive before the corresponding reactions start on noble metal surfaces, which prevents obtaining such interesting nanostructures. In addition, intrinsic carbyne structures,instead of metalated carbynes,are yet to be synthesized at the atomic scale, which may require a new synthetic approach.Moreover,the on-surface synthesis of novel nanostructures is currently restricted to metal surfaces,which limits its characterization and further application to a certain extent. For all these reasons, design of new precursors, exploration of new synthetic strategies, approach to transferring products from metal surfaces to other substrates,and even direct synthesis on semiconducting substrates deserve to be explored in the near future.
Acknowledgement
Project supported by the National Natural Science Foundation of China(Grant Nos.22125203 and 21790351).
- Chinese Physics B的其它文章
- Fault-tolerant finite-time dynamical consensus of double-integrator multi-agent systems with partial agents subject to synchronous self-sensing function failure
- Nano Ag-enhanced photoelectric conversion efficiency in all-inorganic,hole-transporting-layer-free CsPbIBr2 perovskite solar cells
- Low-voltage soft robots based on carbon nanotube/polymer electrothermal composites
- Parkinsonian oscillations and their suppression by closed-loop deep brain stimulation based on fuzzy concept
- Temperature dependence of spin pumping in YIG/NiO(x)/W multilayer
- Interface effect on superlattice quality and optical properties of InAs/GaSb type-II superlattices grown by molecular beam epitaxy

