PtRuAgCoNi高熵合金納米顆粒高效電催化氧化5-羥甲基糠醛
楊艷,何博文,馬華隆,楊森,任州宏,秦天,盧發貴,任力聞,張熠霄,王天富,*,劉晰,*,陳立桅,*
1上海交通大學化學化工學院,物質科學原位中心,轉化分子前沿科學中心,上海 200240
2上海交通大學環境科學與工程學院,上海 200240
1 Introduction
5-Hydroxymethylfurfural (HMF), which is derived from renewable biomass1is an important bio-sourced intermediates for the preparation of a wide range of chemicals2.Among all the value-added chemicals produced by HMF, 2,5-furandicarboxylic acid (FDCA) has been used as a precursor for the production of polyethylene furandicarboxylate (PEF)3.Bio-based PEF has excellent gas barrier properties, recyclability and mechanical properties4, and thus being considered as a promising substitute for petroleum-based polyethylene terephthalate (PET).Compared to thermal catalytic process, electrocatalytic conversion of HMF into FDCA provides a more environmentalfriendly and economical method to sustainably product valueadded chemicals under mild conditions5, therefore attracting increasing attention from both industrial and academic perspectives.
Cheap transition metal, including Ni, Co, Fe, Cu, V, Mo, Mn,have been used in electro-oxidation of HMF into FDCA6.Their sulfide and phosphite compounds display unique catalytic performance7,8, but suffer poor stability since the surfides or phosphites are prone to be converted into their oxide counterparts under alkaline conditions9,10.Some noble metals,like Pt and Ru, have also been used due to their good electrochemical stability, but only 16% and 6.48% of FDCA yield were obtained, respectively11,12.In order to improve both catalytic activity and stability for electro-oxidation of HMF,alloying noble metals and cheaper transition metals is considered to be an effective strategy to achieve synergy-enhanced catalytic performance for the FDCA production.
High entropy alloys are defined as the mixing of five or more elements at the nanoscale in equal or near molar ratios (recently reported as 5%-35% (atom fraction) for each constituent)13.Compared with monometal and traditional alloys, high entropy alloys show better physical and chemical properties due to the synergistic interaction among metal elements, and display outstanding performance in electrocatalysis14, such as hydrogen evolution reaction (HER)15, oxygen evolution reaction (OER)16and oxygen reduction reaction (ORR)17.Solution-phase synthesis method is widely used in the synthesis of nanomaterials with controllable size, morphology and crystal phase.However, due to increasing difficulty in balancing diverse reduction kinetics of different metal precursors18, limited works have been done in synthesis of metallic nanometarials containing more than three metal elements in solvent phase.Additionally,surfactants used in solution-phase synthesis will cover parts of active sites of synthesized nanomaterials and therefore reduce its catalytic performance19.Various methods have been applied to remove these surfactants, including annealing in gas20, acid washing21, and UV-ozone irradiation22, but all these methods show certain limitations or negative influences on the microstructure and catalytic performance of supported catalysts23.
In this work, we developed a low-temperature solvothermal method to synthesize single-phase PtRuAgCoNi high entropy alloy nanoparticles with an averaged diameter of about 9 nm and applied a facile treatment to remove the surfactant without changing the particle structure and composition24.The active carbon-supported PtRuAgCoNi high entropy alloy nanoparticles with surfactants showed better activities in the electrochemical conversion of HMF to FDCA than commercial Pt/C catalyst.After calcination at lower temperature (185 °C), the catalytic performance of the surfactant-free high entropy nanoparticles was enhanced markedly.Therefore, PtRuAgCoNi high entropy alloy nanoparticles could be a promising electrocatalyst for production of FDCA from electro-oxidation of HMF.
2 Experimental
2.1 Chemicals and materials
Sodium citrate (97%), potassium bromide (98%), ethylene glycol (99%), and Nafion solution (5%, mass fraction) were all purchased from Aladdin Industrial Co., Ltd.Oleylamine (RG).Polyvinylpyrrolidone (PVP) with an averageMW = 40000 was procured from Shanghai Titan Scientific Co., Ltd.Platinum bis(acetylacetonate) (Pt(acac) (97%), ruthenium(Ⅲ)2,4-pentanedionate (Ru(acac)3) (97%), cobalt(III) acetylacetonate(Co(acac)3) (97%), nickel(II) acetylacetonate (Ni(acac)2), and AgNO3were purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd.All reagents were used directly without further purification.The water used for all the experiments was deionized (DI) to achieve a resistivity of 18.2 MΩ·cm at room temperature.
2.2 Synthesis of five-element PtRuAgCoNi alloy nanoparticles
To prepare the five-element PtRuAgCoNi alloy nanoparticles,3.9 mg Pt(acac)2, 4.0 mg of Ru(acac)3, 1.7 mg of AgNO3, 2.6 mg of Co(acac)3and 2.6 mg of Ni(acac)2(Pt : Ru : Ag : Co : Ni molar ratio is 1 : 1 : 1 : 1 : 1), 100 mg of PVP, 80 mg of sodium citrate and 50 mg of KBr, were dissolved in 20 mL of ethylene glycol while stirring, and the mixture was sonicated until transparent.The resulting homogeneous solution was transferred to a 20 mL Teflon-lined stainless-steel autoclave.The sealed vessel was then heated at 200 °C for 12 h in the oven before it was cooled to room temperature.The synthetic five-element alloy nanoparticles was obtained by high-speed centrifugation and washed three times with anhydrous ethanol, which was denoted as PtRuAgCoNi-PVP.Then, the obtained nanoparticles(5 mg) were loaded on activated carbon (20 mg), which was denoted as PtRuAgCoNi-PVP/C (20%, mass fraction).
2.3 Surfactant removal of five-element PtRuAgCoNi alloy nanoparticles
The PtRuAgCoNi-PVP ethanol solution was transferred into a 50 mL round bottom flask, and 10 mL of ethanol, 5 mL of oleylamine and 10 mL of toluene were added.Afterwards, it was refluxed at 60 °C for 8 h.The solid sample, which was collected after centrifugation, was denoted as PtRuAgCoNi-OAm.Then,the obtained nanoparticles (5 mg) were loaded on activated carbon (20 mg), which was denoted as PtRuAgCoNi-OAm/C(20% (w)).Afterwards, the obtained sample was heated in air at 185 °C for 5 h or 300 °C for 5 h to obtain desired supported nanoalloy catalysts, which are denoted as PtRuAgCoNi-OAm/C-185 and PtRuAgCoNi-OAm/C-300, respectively.
2.4 Electrochemical measurements
The prepared catalysts (1 mg) were ultrasonically dispersed in 500 μL of deionized water, 500 μL of ethanol, and 50 μL of Nafion solution (5%, mass fraction) to form a homogeneous ink.To fabricate the working electrode, 100 μL of the electrocatalyst ink was dropped onto the surface of a piece of carbon paper (1 cm2) and dried at room temperature.Electrochemical measurements were carried out with a CHI 760E electrochemical workstation (Shanghai Chenhua Instrument Company, China) in a standard three-electrode cell configuration using the prepared catalysts as the working electrode, Pt mesh as the counter electrode, and Ag/AgCl (3 mol·L-1KCl, aqueous) as the reference electrode.The polarization curves were recorded at a scan rate of 50 mV·s-1.In order to highlight the properties of the alloy material, commercial Pt/C (20% (w), purchased from Johnson Matthey) was tested under the identical condition for comparison.
2.5 Materials characterization
XRD patterns were obtained using a Bruker D8 Advance diffractometer with CuKαand operating at 40 kV/40 mA.Transmission electron microscopy (TEM) images and EDS mapping were collected on a Hitachi HF5000 microscope operated at 200 kV.X-ray photoelectron spectroscopy (XPS)profiles were obtained on Axis Ultra DLD.Contents of metal elements in the samples were determined by inductively coupled plasma-mass spectrometry (ICP-MS).Infrared spectra were obtained through a FT-IR Spectrometer (Spectrum 100).Quantifications of the reactant and products were done by using High performance liquid chromatography (HPLC, FL2200-2).
3 Results and discussion
The PtRuAgCoNi-PVP (PtRuAgCoNi alloy nanoparticles coated with the surfactant PVP) high entropy alloy nanoparticles were synthesized by reduction of M(acetylacetonate)x(M = Pt,Ru, Co and Ni) and AgNO3with PVP and sodium citrate in ethylene glycol solution25.A TEM image of the synthesized PtRuAgCoNi-PVP high entropy alloy nanoparticles is displayed in Fig.1a.It is evident that the PtRuAgCoNi-PVP high entropy alloy nanoparticles have a spherical morphology with a mean size 9.04 ± 0.17 nm (Fig.1b).A selected area electron diffraction(SAED) pattern of the PtRuAgCoNi-PVP nanoparticles shows legible rings corresponding to the (111), (002), (022) and (113)crystallographic planes of face-centered cubic (FCC) crystal structure (Fig.1c).An X-ray diffraction (XRD) profile of the prepared PtRuAgCoNi-PVP/C (PtRuAgCoNi-PVP loaded on activated carbon, which was denoted as PtRuAgCoNi-PVP/C)alloy nanoparticles was used to characterize the crystal structure of the materials.As shown in Fig.1d, a main peak at 2θ= 42.5°observed in the XRD pattern is associated with the (111) plane of the face-centered cube, but its diffraction angle is 0.1° higher than that of FCC Pt (JCPDF file No.96-101-1108), which should be attributed to the polycrystalline nature of the five-element metal alloy nanoparticles or the synergy between the metal elements26(seeing Figs.S1 and S2, Supporting Information).Both the XRD and SAED results confirm that these five elements are stabilized in a FCC structure.
Scanning/Transmission electron microscope energydispersive X-ray spectroscopy (STEM-EDS), inductively coupled plasma-mass spectrometry (ICP-MS) and X-ray photoelectron spectroscopy (XPS) were employed to characterize the element distributions and valence states of PtRuAgCoNi-PVP/C alloy nanoparticles.STEM-EDS elemental mapping (Fig.1e and Fig.S3) demonstrates that the synthesized metal nanoparticles have a core-shell microstructure in which the outer shell is rich in Pt, but Ru, Ag, Co and Ni distribute throughout the particle.ICP-MS confirms the molar ratio of the Pt/Ru/Ag/Co/Ni composition is 16/34/13/20/14(Table S1, Supporting Information), which does not deviate far from our design (1 : 1 : 1 : 1 : 1).XPS was used to investigate the chemical state of the elements in PtRuAgCoNi-PVP/C.As shown in the Pt 4fXPS spectrum of PtRuAgCoNi-PVP/C (Fig.1f), the peaks center at approximately 71.7 and 75.1 eV are assigned to Pt 4f7/2and Pt 4f5/2, respectively, which are likely due to the metallic state of Pt27.In the Ag 3dspectrum (Fig 1g), two peaks at 364.4 and 370.4 eV assigned to Ag 3d5/2 and Ag 3d3/2,respectively, are lower than those of metallic Ag28.The XPS spectrum of Co shows that both metallic (781.6 eV) and divalent cobalt (787.3 and 803.2 eV) are present in the alloy nanoparticles(Fig.1h).The Ru 3pspectra (seeing Fig.S4a) exhibits metallic Ru (3p3/2, 462.8 eV)29.The Ni 2pspectra (seeing Fig.S4b)display divalent Ni (861.3 and 879.1 eV)30.We can speculate that intrinsic charge transfer might occur among Ag, Ni and Co.This phenomenon is consistent with the XPS spectra of CrMnFeCoNi high entropy alloy nanoparticles reported in literature31.

Fig.1 Characterizations of the structure and morphology of the PtRuAgCoNi-PVP and PtRuAgCoNi-PVP/C alloy nanoparticles.
During the synthesis of the PtRuAgCoNi high entropy alloy nanoparticles, PVP acts as a surfactant, which covers surface of the nanoparticles to inhibit nanoparticle overgrowth and aggregation32.However, a larger amount of surface active sites is also occupied by surfactants, leading to the reduction of their catalytic performance.Since PVP has a stronger affinity for surface than other small-molecule surfactants, it is more difficult to remove it without damage to microstructure and morphology.The complete removal of PVP by calcination requires more than 450 °C, which makes the morphology, size, structure, and catalytic performance of the material inevitably change during this process33.Accordingly, we developed a universal two-step method of removing surfactants.Firstly, small molecular oleylamine were used for ligand exchange to replace large PVP molecules in the sample, then the obtained nanoparticles were deposited on carbon (denoted as PtRuAgCoNi-OAm/C) and subsequently calcined at low temperature (185 °C) in air to get surfactants-free nano alloys (denoted as PtRuAgCoNi-OAm/C-185).In order to examine the influence of calcination temperature on morphology and catalytic performance of the nano alloy, the prepared PtRuAgCoNi-OAm/C was also calcinated at 300 °C (which was denoted as PtRuAgCoNi-OAm/C-300) for comparison.
Fourier infrared (FTIR) spectroscopy, XRD and TEM-EDS were employed to examine the effectiveness of the applied twostep method for the removal of PVP.As displayed in Fig.2a,FTIR spectrum of PtRuAgCoNi-OAm exhibits characteristic peaks associated with the C-H stretching vibrations of oleylamine molecules34.After the nanomaterials were calcined at 185 or 300 °C, the stretching vibration peaks of C-H bond disappeared, indicating that the surfactant was completely removed from the nanoparticles.Fig.2b demonstrates that the crystal structure of the material retains a single-phase FCC structure after calcination at 185 or 300 °C.A TEM image of PtRuAgCoNi-OAm/C-185 (Fig 2c and Fig.S3) confirms that there is no change in the morphology and microstructure of the high entropy alloy nanoparticles after calcination at 185 °C.EDS mapping of Fig.2d further demonstrates that the element distributions remain almost identical.However, when the calcination temperature increased to 300 °C, A STEM image and corresponding EDS mapping (Fig.2e) shows that the microstructure of nanoparticles is completely destroyed and broken nanoparticles are sintered into larger particles with varied elemental compositions.
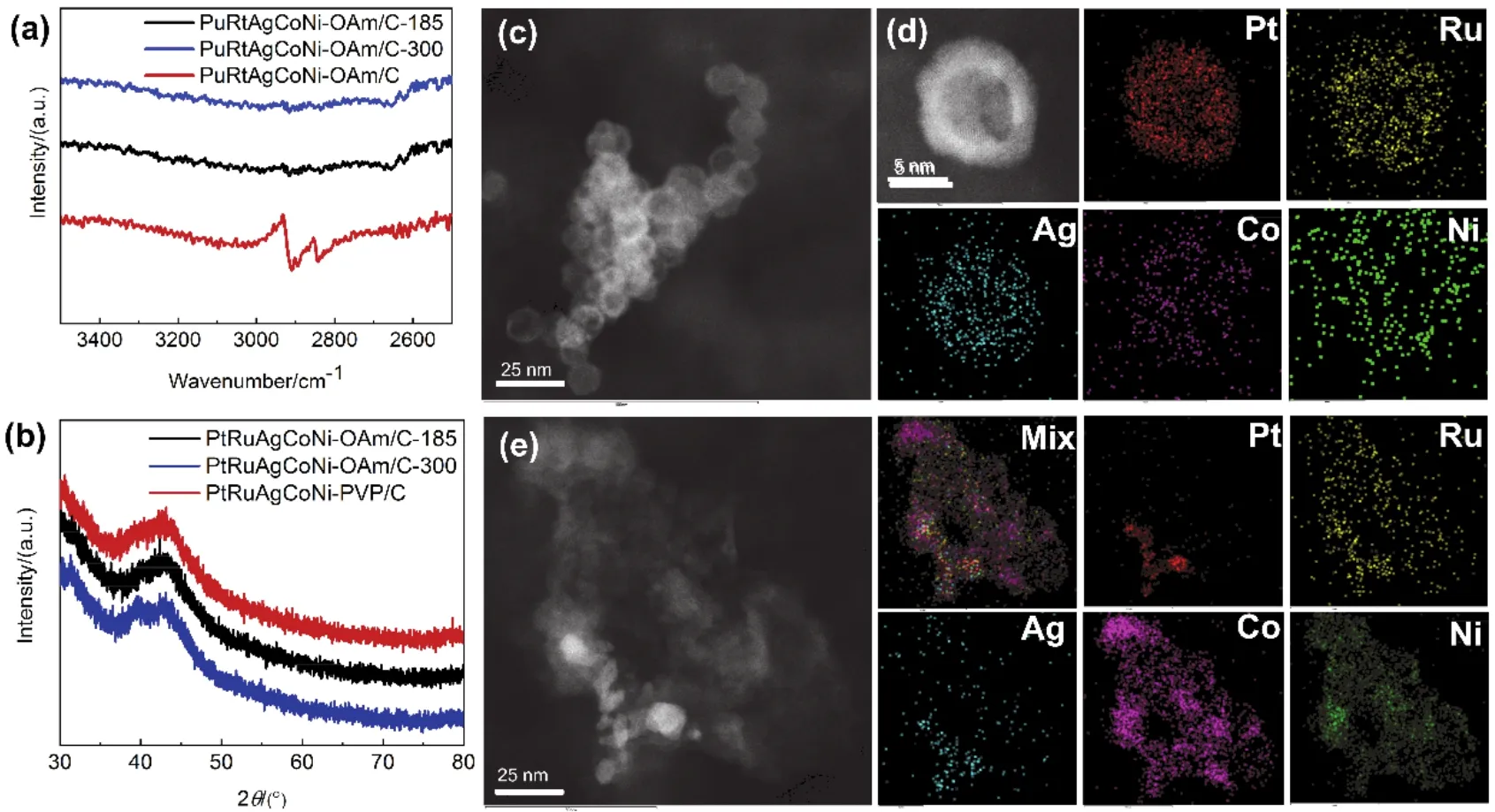
Fig.2 Characterizations of the structure, morphology and element distributions of the high-entropy alloy after the surfactant was removed.
The electrocatalytic performance of the PtRuAgCoNi-PVP/C,PtRuAgCoNi-OAm/C, PtRuAgCoNi-OAm/C-185 and PtRuAgCoNi-OAm/C-300 was evaluated by linear sweep voltammetry (LSV).In order to evaluate the catalytic performance of these prepared materials, commercial Pt/C(20%) catalyst was used for comparison.These catalysts for HMF oxidation was studied in a three-electrode cell, and a nitrogen-saturated 1 mol·L-1KOH electrolyte solution was used in all tests.The prepared catalyst was mixed into ink solution and dropped onto carbon paper as the working electrode for the electrocatalytic oxidation of HMF.LSV of all the tested materials show similar change trend toward the OER reaction in the absence of HMF (Fig 3a).Comparing Fig.3a, b, it can be proved that OER is negligible in the potential between 1.2 and 1.5 Vvs.RHE, where HMF oxidation is facilitated by all catalyst materials (Fig.3b).For comparison catalytic performance of all catalyst materials for electrocatalytic oxidation of HMF, two performance criteria were taken into consideration, which are the current density at a potential of 1.4 Vvs.RHE and the potential to attain a current density of 1 mA·cm-2.The PtRuAgCoNi-OAm/C-185 catalyst achieved a current density of 3.79 mA·cm-2at 1.4 Vvs.RHE and 1.0 mA·cm-2at 1.06 Vvs.RHE (in the presence of 50 mmol·L-1HMF).The overpotential for HMF oxidation is decreased by 378 mV as compared to the OER at current density 1.0 mA·cm-235.Among these materials tested,PtRuAgCoNi-OAm/C-185 is the most active electrocatalyst for the oxidation of HMF.A detailed comparison of the activities of all catalysts is highlighted in Table 1.

Fig.3 Electrocatalytic performance of the high entropy alloys nanoparticles.

Table 1 A comparison of the OER and HMF oxidation activities of PtRuAgCoNi-OAm/C-185, PtRuAgCoNi-OAm/C-300,PtRuAgCoNi-OAm/C, PtRuAgCoNi-PVP/C, commercial Pt/C (data taken from LSVs of Fig.3).
Subsequently, Tafel plots were used to analyze the kinetics for the reaction36.The Tafel slope of each catalyst is shown in Fig.3c.The Tafel slope of PtRuAgCoNi-OAm/C-185 is 45.2 mV·dec-1, which is much lower than that of the other four catalysts, suggesting that PtRuAgCoNi-OAm/C-185 exhibits improved kinetics for HMF electrooxidation.Furthermore, we used electrochemical impedance spectroscopy (EIS) to study the electrochemical behavior at the interface between the electrode and electrolyte37.Comparing with all tested catalyst, theRct(charge transfer resistance) of PtRuAgCoNi-OAm/C-185 is the smallest, which means that the catalyst has the fastest charge transfer rate (Fig.3d).The Tafel slope andRctindicate that PtRuAgCoNi-OAm/C-185 is more favorable for catalytic oxidation of HMF.
Based on the above electrochemical performance study, these five catalyst materials only undergo HMF oxidation reactions without water oxidation at 1.40 Vvs.RHE.At this potential, the products of the electrocatalytic reaction were analyzed by highperformance liquid chromatography (HPLC).The HMF conversion rate, FDCA yield and Faraday efficiency of all specimens are presented in Fig.4a and 4b, respectively.The HMF conversion rate, FDCA yield and Faraday efficiency of PtRuAgCoNi-OAm/C-185 is 55.2%, 46.0% and 45.1%,respectively, which are much higher than those obtained for surfactant decorated catalysts (PtRuAgCoNi-OAm/C andPtRuAgCoNi-PVP/C).This enhanced activity is due to the exposure of active sites caused by removal of surfactants.In addition, the results in Fig.4a, b also indicate that the commercial Pt/C shows the lowest reaction rate and energy conversion efficiency (16.6% FDCA yield and 14.8% Faraday efficiency), which also implies the advantages of high entropy alloy catalyst.A STEM image and corresponding element mapping (Fig.5) of the used PtRuAgCoNi-OAm/C-185 electrocatalyst confirm stability of PdRuAgCoNi-OAm/C-185 as the microstructure and elemental composition of the electrocatalyst is unchanged under the reaction condition.Therefore, the better catalytic performance of PtRuAgCoNi alloy nanoparticles should be attributed to the synergy effect among noble metals and cheap transition metals, which not only improves its catalytic performance, but also reduces the amount of noble metals.Meanwhile, once the calcination temperature increased from 185 to 300 °C, the nanoparticles are broken and its electrocatalytic performance dropped markedly, which suggests that the unique catalytic performance of PtRuAgCoNi-OAm/C-185 should be assigned to its high entropy nature.
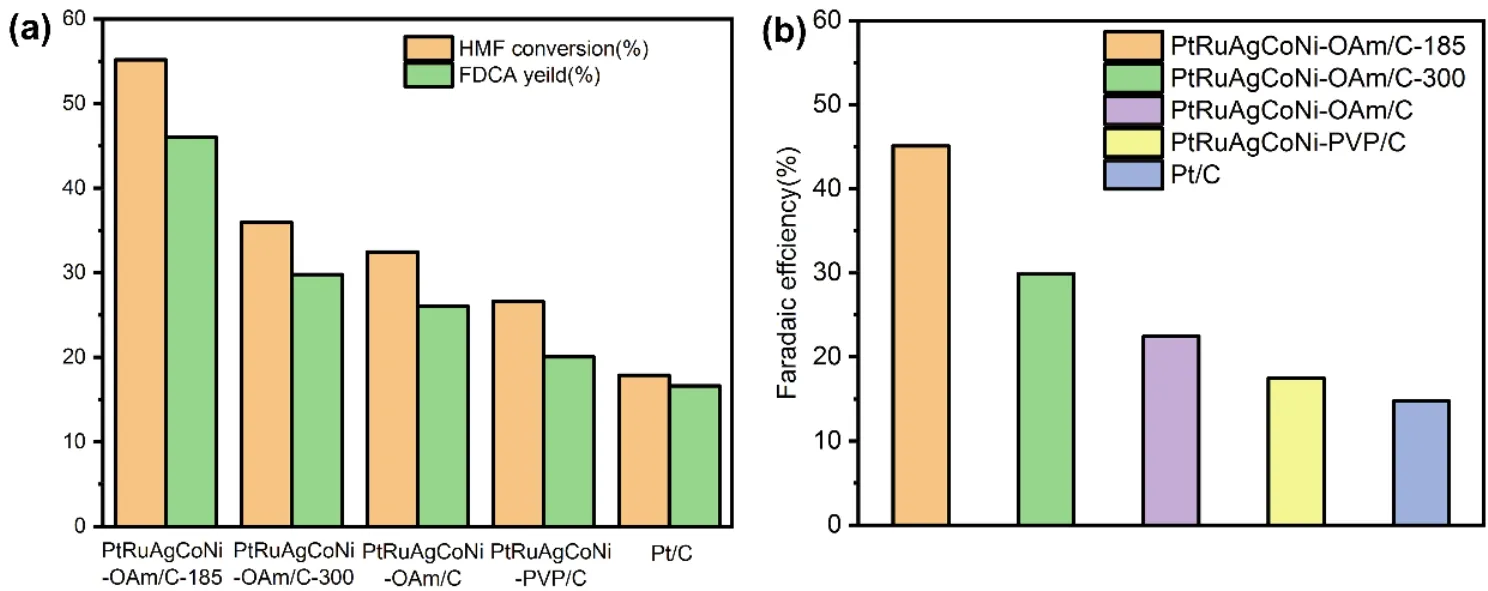
Fig.4 HPLC analysis of the products of the reaction within 3 h.
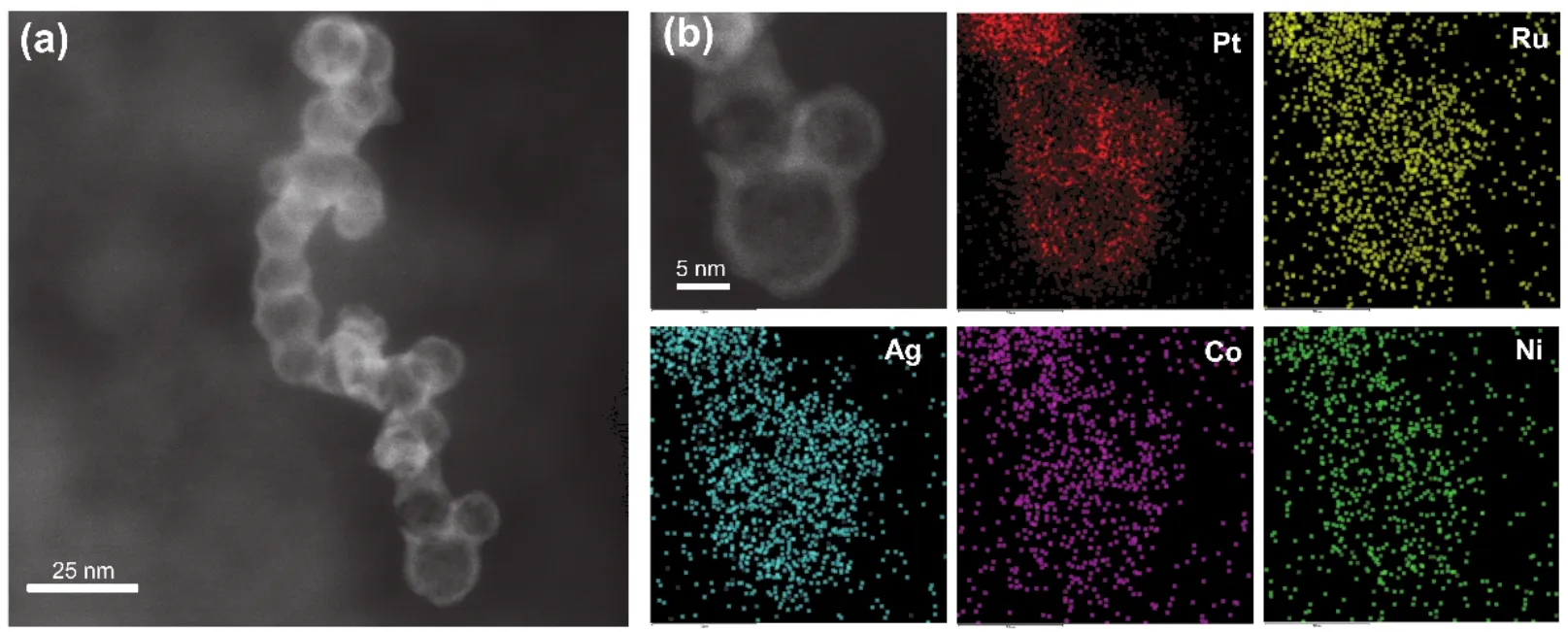
Fig.5 HAADF-STEM image (a) and element map (b) of PtRuAgCoNi-OAm/C-185 after reaction 3 h for oxidation HMF.
4 Conclusions
In summary, we synthesized PtRuAgCoNi high entropy alloy nanoparticles with a uniform morphology and size distributions using low-temperature solvothermal method.Small molecule substitution and low-temperature calcination were used to remove the surfactant from nanoparticles without changing their morphology or element distribution.After the complete removal of the surfactants under mild conditions, the catalytic activity of the PtRuAgCoNi high entropy alloy with an unchanged structure and morphology was significantly enhanced.Compared with Pt/C catalyst, PtRuAgCoNi high entropy alloy shows better catalytic performance of electrocatalytic oxidation of HMF,validating the important significance of the high entropy structure to the catalytic activity.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.

