Molecular mechanisms of lesioninduced axonal sprouting in the corticofugal projection: the role of glial cells
Leechung Chang,Nobuhiko Yamamoto
After injury of the central nervous system (CNS),neuronal circuits are known to remodel for functional recovery.In general,there are two strategies for the remodeling: axonal regeneration and sprouting (Figure 1A).Axonal regeneration is re-growth of injured neurons themselves,but axons hardly regenerate in the adult mammalian CNS (Silver and Miller,2004).On the other hand,axonal sprouting is new growth from intact or spared neurons to the denervated target by forming axon collaterals,compensating for damaged circuits.This process is a more effective way for circuit remodeling,as it does not necessarily require long axonal elongation.A wellknown example of axonal sprouting is the ectopic projections of the corticofugal projections after unilateral injury of the motor cortex (Figure 1B).Originally,layer 5 neurons in the cortex project ipsilaterally to the midbrain and hindbrain,and contralaterally to the spinal cord.After one side of the cortex is injured,axon sprouting from cortical neurons on the intact side restores connections to the denervated brainstem and spinal cord (Tsukahara,1981a,b).The elaborate electrophysiological study revealed that ectopic contralateral projections are formed from the intact cortex to the denervated red nucleus in the midbrain,which is involved in the voluntary muscular movement (Tsukahara,1981a,b).Subsequent studies have further shown that compensatory remodeling occurs in various parts of the CNS,and contributes to functional recovery(Schwab and Strittmatter,2014).Despite its importance in the recovery,there has been limited progress toward understanding the molecular mechanism of spontaneous axonal sprouting;how the formation of axonal sprouting is coordinated after the injury.Recently,we have demonstrated that factors expressed in the denervated midbrain are involved in the remodeling process,and are produced by glial cells including astrocytes or microglia (Chang et al.,2022).In this perspective,we will briefly present our current understanding of the mechanisms of lesion-induced axonal sprouting of the corticofugal projections,and a view of how glial cells are involved in this process.
In the mature CNS,axonal growth is basically suppressed,due to a growth-inhibitory environment and the cells’ low intrinsic growth property (Silver and Miller,2004;Curcio and Bradke,2018).As axonal growth-inhibitory factors such as Nogo,myelin-associated glycoprotein and chondroitin sulfate proteoglycan are abundant in the adult CNS,experimental approaches to eliminate these external axon-inhibitory factors and facilitate axonal sprouting after CNS injury have been previously explored.Indeed,this approach efficiently increases axonal sprouting in rodents (Schwab and Strittmatter,2014).Meanwhile,axonal sprouting occurs after adult CNS injury to some extent,which indicates the existence of “built-in” mechanisms to initiate axonal sprouting spontaneously.Therefore,along with strategies to eliminate obstacles in the adult stage,facilitating these mechanisms may also be effective to promote axonal sprouting.
The following two possibilities are raised for the mechanisms of axonal sprouting.First,endogenous growth properties may be enhanced in intact neurons to drive axonal sprouting after the lesion.Indeed,a comparison of transcriptomes between neurons with and without sprouting after unilateral corticospinal tract lesion showed that lysophosphatidic acid signaling modulators such as lipid phosphate phosphatase-related protein type 1 and inositol polyphosphate-5-phosphatase K are elevated in the sprouting neurons and contribute to the enhancement of axon growth property(Fink et al.,2017),indicating that sprouting axons have more ability to overcome the inhibitory environment.Furthermore,boosting endogenous axon growth properties by deletion of phosphatase and tensin homolog which is an intrinsic inhibitor of axon growth (Geoffroy et al.,2015),and by overexpression of transcription factors related to axon growth capability (Blackmore et al.,2012)have been shown to promote axonal sprouting after injury in adult mice.
Second,“sprouting factors”,certain factors expressed in the denervated target,may induce axonal sprouting via receptors expressed in intact axons.In accordance with this view,transcriptome analysis in the unilateral lesion of the corticospinal pathway showed that some neurotrophic and growth-promoting factors are upregulated in the denervated spinal cord (Kaiser et al.,2019).To explore the sprouting factors,we focused on the infant stage,in which massive axonal sprouting occurs compared to the adult stage (Tsukahara,1981a;Chang et al.,2022).Our analysis showed that several growth-promoting factors are dramatically upregulated in the denervated midbrain after unilateral cortical ablation at postnatal day 6 of mice,including extracellular matrix molecules such as osteopontin (Spp1)and plasminogen activator,which is involved in the extracellular cleavage of brain-derived neurotrophic factor into the mature form (Chang et al.,2022).To identify the sprouting factors,the receptors in layer 5 cortical neurons for the candidate growth-promoting factors were then ablated using CRISPR/Cas9 mediated knockout combined with anin uteroelectroporation technique.The result demonstrated that the newly formed ectopic projections to the contralateral midbrain were markedly reduced when TrkB and integrin beta 3 were knocked out;these are the receptors for mature brain-derived neurotrophic factor and Spp1,respectively.Thus,it is likely that these ligand molecules expressed in the denervated midbrain act as sprouting factors in the remodeling of the cortical projection (Chang et al.,2022;Figure 1C).Remarkably,TrkB knockout led to an overall decrease in ectopic projections,while integrin beta 3 knockout specifically diminished axonal projections to the ventrolateral part of the midbrain,indicating that multiple sprouting factors may be involved and be coordinated to accomplish axonal remodeling (Chang et al.,2022).
Which cell type produces these sprouting factors in the denervated midbrain after injury?Interestingly,they were found to be expressed by astrocytes and/or microglia;Spp1 was produced by both astrocytes and microglia,and the plasminogen activator was exclusively produced by astrocytes (Chang et al.,2022).Moreover,glial cells became reactive in the denervated region in response to axon degeneration (Kaiser et al.,2019;Chang et al.,2022).These reactive glial cells could undertake the synthesis of sprouting factors,which may create a permissive environment for axonal remodeling.The role of glial cells in axonal remodeling after injury has been controversial.In general,reactive glial cells are known to form a glial scar at the injury site by producing inhibitory molecules such as myelin-associated inhibitors and chondroitin sulfate proteoglycan,thereby inhibiting axonal regeneration (Silver and Miller,2004).On the other hand,a beneficial role of astrocytic scars in regeneration has also been reported (Anderson et al.,2016),which is in accordance with the case of the denervated midbrain.Such opposing roles of glial cells in axon remodeling may be attributed to their activation states.In fact,the activation states of glial cells are highly variable and may depend on the context of injury.For example,ischemic injury produces neurotrophic astrocytes,whereas an inflammatory insult produces a more toxic subtype (Liddelow and Barres,2017).The states of microglial activation are also heterogeneous,which is determined by the surrounding environment(Masuda et al.,2020).The activation states may depend on whether glial cells are located at the injury site or at the remote denervation site.As reactive glial cells in the denervated region produce sprouting factors (Figure 1C),they may have phenotypes more skewed to a beneficial profile,compared to those at the injury site.
In the sprouting factor model,one important point is that the factors should be expressed in the vicinity of axons that undergo sprouting.In the context of axon guidance,the distance between the growth cones of developing axons and their target has been a long-standing question.Even diffusible substances released from the target are thought to act only on growth cones that are located within a few hundred micrometers(Tessier-Lavigne and Goodman,1996).In lesioninduced axonal sprouting processes such as ectopic contralateral cortical projection to the denervated midbrain,the molecules responsible must spread broadly from the denervated to the intact side.From this point of view,it is appropriate that these molecules are expressed by glial cells,which are highly mobile and can migrate over long distances (Figure 1C).Indeed,after unilateral cortical ablation,reactive astrocytes and microglial cells are broadly dispersed in the denervated midbrain,from the cerebral peduncle(where a bundle of efferent fibers degenerate after the lesion is incurred) to the region near the midline (Chang et al.,2022).How are glial cells distributed in the appropriate position to induce axonal sprouting? As glial cells may recognize the degenerated axonal debris resulting from the lesion,such material may act as a migration cue for directing the glial cells to the proper locations.
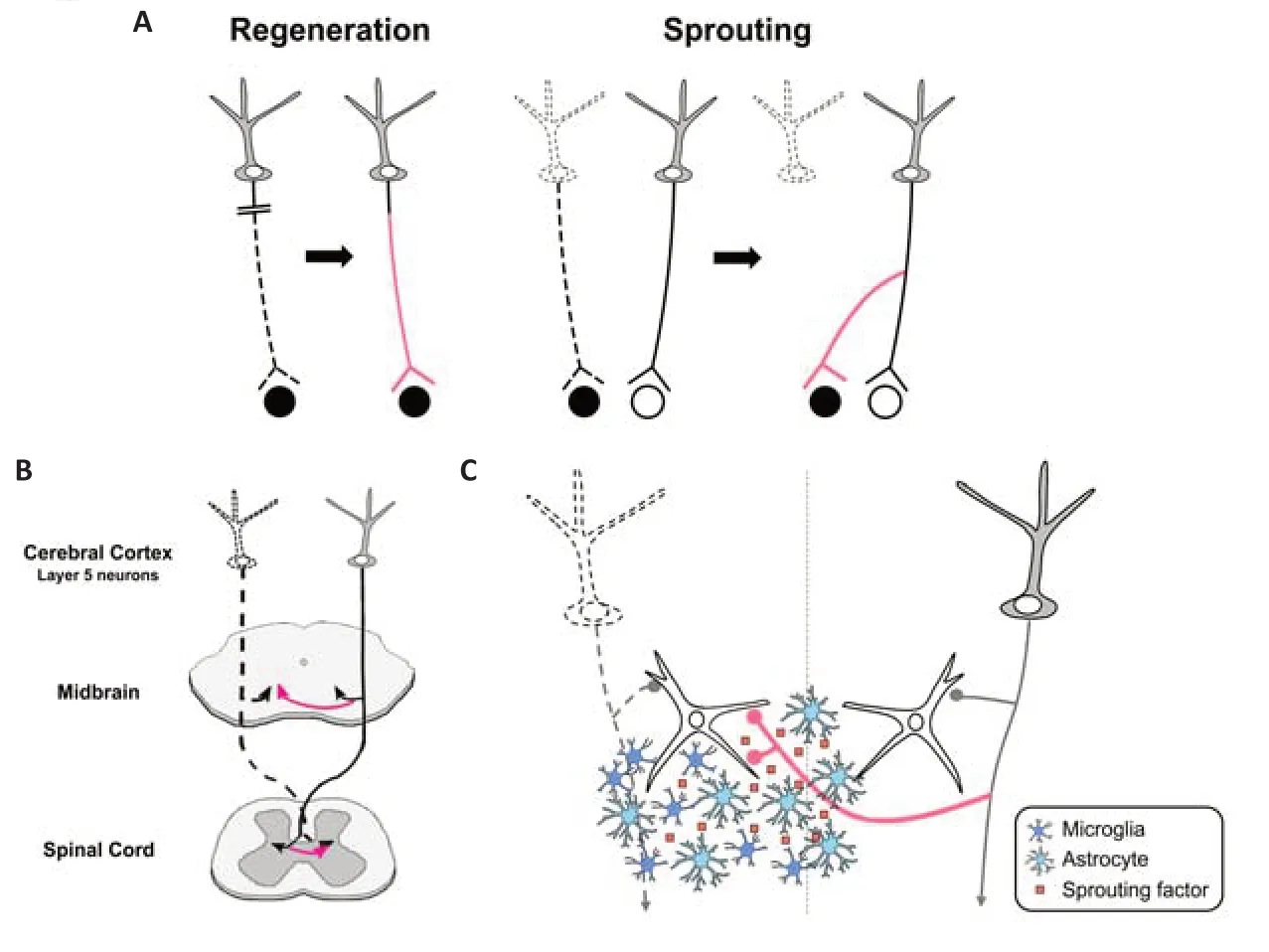
Figure 1|Mechanisms of axonal remodeling in the central nervous system.
Finally,we would like to emphasize that the difference between the adult and infant stages has to be taken into consideration.Further studies are necessary to determine whether our proposed mechanism is also effective for enhancing axonal remodeling in the adult stage.As described above,the abundance of axon growth-inhibitory factors and the lower growth capacity of neurons themselves are the major hurdles for axonal remodeling at the adult stage (Silver and Miller,2004;Curcio and Bradke,2018).Moreover,glial cell states in adults are distinct from those at the developmental stages and may be more involved in shaping the inhibitory environment (Li et al.,2020).To sum up,both abolition of suppressive factors and amplification of intrinsic factors may be required to maximize axonal sprouting and also for future therapeutic applications.
We would like to thank the Otsuka Toshimi Scholarship Foundation for scholarship support to LC.We thank Dr.Ian Smith (Elite Scientific Editing,UK) for the critical reading of the manuscript.
This work was supported by MEXT KAKENHI on Dynamic Regulation of Brain Function by Scrap&Build System,No.16H06460 (to NY) and JSPS KAKENHI Grant Nos.19H03325 (to NY) and 20J13844 (to LC).
Leechung Chang,Nobuhiko Yamamoto*
Laboratory of Cellular and Molecular Neurobiology,Graduate School of Frontier Biosciences,Osaka University,Suita,Osaka,Japan
*Correspondence to:Nobuhiko Yamamoto,PhD,nmtyama@gmail.com.
https://orcid.org/0000-0002-1916-932X(Nobuhiko Yamamoto)
Date of submission:June 20,2022
Date of decision:August 25,2022
Date of acceptance:September 23,2022
Date of web publication:November 18,2022
https://doi.org/10.4103/1673-5374.360168
How to cite this article:Chang L,Yamamoto N(2023) Molecular mechanisms of lesion-induced axonal sprouting in the corticofugal projection: the role of glial cells.Neural Regen Res 18(6):1259-1260.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Yuanquan Song,Children’s Hospital of Philadelphia,USA.
Additional file:Open peer review report 1.
- 中國神經再生研究(英文版)的其它文章
- The relationship among amyloid-β deposition,sphingomyelin level,and the expression and function of P-glycoprotein in Alzheimer’s disease pathological process
- Translational bioengineering strategies for peripheral nerve regeneration: opportunities,challenges,and novel concepts
- The role of crm-1 in ionizing radiation-induced nervous system dysfunction in Caenorhabditis elegans
- Chitosan conduits enriched with fibrin-collagen hydrogel with or without adipose-derived mesenchymal stem cells for the repair of 15-mm-long sciatic nerve defect
- Argon preconditioning protects neuronal cells with a Toll-like receptor-mediated effect
- Glial cell line-derived neurotrophic factor and brainderived neurotrophic factor regulate the interaction between astrocytes and Schwann cells at the trigeminal root entry zone