Thermally enhanced photoluminescence and temperature sensing properties of Sc2W3O12:Eu3+phosphors
Yu-De Niu(牛毓德), Yu-Zhen Wang(汪玉珍), Kai-Ming Zhu(朱凱明), Wang-Gui Ye(葉王貴),Zhe Feng(馮喆), Hui Liu(柳揮), Xin Yi(易鑫), Yi-Huan Wang(王怡歡), and Xuan-Yi Yuan(袁軒一)
Beijing Key Laboratory of Optoelectronic Functional Materials&Micro-nano Devices,Department of Physics,Renmin University of China,Beijing 100872,China
Keywords: photoluminescence,Sc2W3O12:Eu3+,negative lattice expansion,thermal-enhanced luminescence
1.Introduction
Lanthanide ions-doped luminescence phosphors have been widely used in displays,[1]lighting,[2]and sensors.[3]Therein optical thermometry based on luminescence materials has attracted considerable attention due to the fast response, non-contact, and high sensitivity.[4,5]By employing the fluorescence intensity ratio (FIR) of thermally coupled energy levels (TCELs) or non-thermally coupled energy levels (NTCELs), the temperature sensing properties could be obtained.[6-8]The potential of luminescence materials to be applied to optical thermometers was evaluated by absolute sensitivity (Sa) and relative sensitivity (Sr).[7,9]For Eu3+-doped materials,several pairs of emissions have been executed for FIR technique due to the abundant energy levels of Eu3+ions.[10,11]Lianget al.revealed that5D1/5D→7F1TCELs(541 nm/590 nm) and (5D→7F2)/(5D1→7F1) NTCELs(625 nm/541 nm)of Eu3+ions in LiNbO3single crystals can be devoted to the optical temperature sensor based on the FIR method,and the maximum values ofSaare 7×10-4K-1and 24×10-4K-1,respectively.[12]In addition,Nikoli′cet al.developed (5D1→7F1)/(5D→7F2) NTCELs (533 nm/611 nm)in Gd2O3:Eu phosphors for thermography,and the maximum value ofSawas 7×10-4K-1at 800 K.[13]
However, the current phosphors used for optical temperature measurement inevitably face the problem caused by thermal quenching.With the increase of temperature,the luminescence intensity of phosphors would be significantly reduced.[14]This will seriously hinder the application of optical thermometry in high-temperature environment.So far, many efforts have been devoted to resisting the thermal quenching.[15-17]Negative thermal expansion(NTE)luminescence materials have triggered off much concern due to the resistance of volume expansion with temperature increasing,which is a distinct advantage for practical applications.[18]In particular, the energy collection by activators could be promoted by the reversible lattice shrinkage and deformation, resulting in the thermal-enhanced emissions.[18,19]For instance,Zouet al.reported that the luminescence of NTE upconversion Yb2W3O12:Er crystal increases more than 12 times when temperature reaches to 573 K.[19]Besides, Zhouet al.demonstrated that the NTE effect of Zr(WO4)2:Eu phosphors can effectively inhibit the emission loss of thermal quenching.Comparing with the scenario at room temperature,the luminescence intensity can be enhanced by 130%at 373 K.[20]Furthermore, the investigation on luminescence properties of lanthanide ions-doped NTE Sc2W3O12material was carried out.Liet al.reported Eu3+-doped Sc2W3O12phosphors with good thermal-quenching resistance at low temperature(97 K-280 K).[21]Recently,Wanget al.demonstrated that the emission intensities of Sc2W3O12:Eu3+(x=0.01-0.10)are abnormally enhanced with temperature increasing up to 473 K.[22]However, the application of Sc2W3O12:Eu3+phosphor in the field of optical temperature measurement is seldom reported.
In this work, we successfully synthesize Sc2W3O12phosphors with high Eu3+concentration in a range of 6 mol%-34 mol%.The luminescent thermal properties of Sc2W3O12:Eu3+with various concentrations and their applications in the field of optical temperature measurement are systematically investigated.Under the excitation at 264 nm,the bright red emission of Sc2W3O12:Eu3+phosphors exhibit their good luminescences thermally enhanced from 373 K to 548 K.Specifically, the Sc2W3O12:6-mol% Eu3+phosphors present 147.8% of initial intensity at 473 K.Besides, owing to the different responses of emissions at 592 nm (5D→7F1)and 613 nm(5D→7F2)to temperature,Sc2W3O12:Eu3+phosphors show excellent temperature sensing properties based on the FIR technique.The estimated maximumSaandSrare 6.872×10-3K-1and 3.063%K-1at 298 K,respectively.In addition, this thermally enhanced luminescence performance of phosphors reduces the difficulty in implementing the luminescence detection at high temperature.These results verify the availability of lanthanide ions-doped NTE luminescence materials against thermal quenching,and demonstrate their applications in optical thermometers.
2.Experiment
2.1.Sample preparation
The Sc2W3O12phosphors were prepared by hightemperature solid-state reaction.The Sc2O3(scandium oxide,99.99%,Aladdin),WO3(tungsten trioxide,99.99%,Aladdin)and Eu2O3(europium oxide, 99.99%, Aladdin) were used as raw materials.After being weighed according to stoichiometry ratio, the raw materials were stirred in planetary ball mill for 12 h.After the obtained slurry is dryed, the mixture was pressed into thin discs each with a diameter of 15 mm and a thickness of 2 mm,and they were sintered in a box furnace at 1100°C for 4 h.The sintered samples can be used for subsequent tests after being carefully ground in an agate mortar.
2.2.Characterization
The x-ray diffraction (XRD) of all samples were identified by the D8 ADVANCE x-ray diffractometer of the Bruker corporation with Cu-Kαradiation(λ=1.5406 °A).Morphologies were characterized on a field-emission scanning electron microscope(FE-SEM,JSM-6700 F).Photoluminescence(PL)and PL excitation(PLE)spectra were measured by using a fluorescence spectrophotometer(F-7000,Hitachi)equipped with a high-temperature fluorescence controller(TAP-02).
3.Results and discussion
3.1.Phase and morphology analysis
Figure 1(a)shows the XRD results of Sc2W3O12:x-mol%Eu3+phosphors (x= 6, 10, 14, 18, 22, 26, 30, 34).The diffraction peaks of samples are well matched with the standard patterns of Sc2W3O12(PDF#89-4690),demonstrating the successful synthesis of Sc2W3O12pure phase.[23]

Fig.1.(a) XRD patterns of Sc2W3O12:x-mol% Eu3+ (x=6, 10, 14, 18, 22, 26, 30, 34) at room temperature (RT).(b) Schematic diagram of crystal structure of orthorhombic Sc2W3O12 and Eu3+ ions substitution diagram.(c) Results of Rietveld refinement of XRD pattern of Sc2W3O12:6-mol%Eu3+ phosphors.(d)Cell volume of Sc2W3O12 with various Eu3+ doping concentrations.
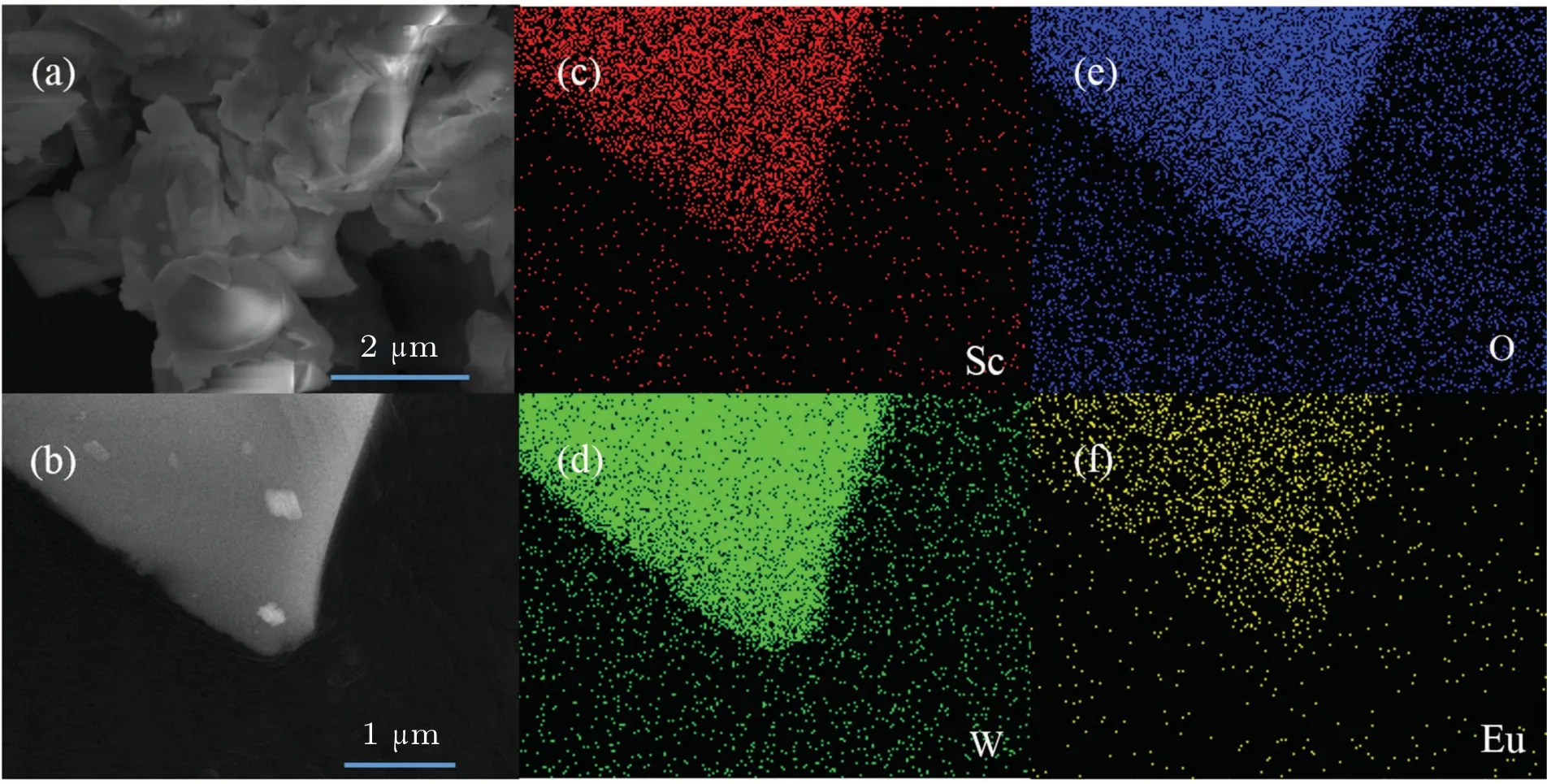
Fig.2.(a)and(b)SEM images of Sc2W3O12:22-mol%Eu3+phosphors.(c)-(f)Element mapping images of Sc,W,O,and Eu snatched from the region indicated in panel(b),respectively.
Figure 1(b) displays the crystal structure of Sc2W3O12,consisting of tetrahedral WO4and octahedral ScO6units connected by oxygen.The Rietveld refinement results indicate the orthorhombic structure with space group ofPnca(No.60)of Sc2W3O12:Eu3+phosphors at room temperature as shown in Fig.1(c) and Fig.S1.The corresponding fitting baseline of Fig.1(c)is given in Fig.S2.According to the refinement,we calculate the lattice parameters and corresponding volumes of various Eu3+-doped Sc2W3O12phosphors and the results are presented in Fig.1(d)and Fig.S3,respectively.Obviously,the volume of the crystal gradually increases with the augment of doping concentration of Eu3+ions.This can be attributed to the fact that Eu3+(0.95 °A)with larger radius occupies the position of Sc3+(0.885 °A).[24]
The morphologies are recorded by SEM images in Fig.2(a).The average size of Sc2W3O12:Eu3+particles ranges from 1μm to 5μm.Figures 2(c)-2(f)show the element mappings of Sc,W,O,and Eu3+in Sc2W3O12:22-mol%Eu3+phosphors snatched from the region indicated in Fig.2(b),respectively.It can be observed that the elements are uniformly distributed in Sc2W3O12: Eu3+phosphors.
3.2.PL properties at room temperature
In order to study the luminescence properties of Sc2W3O12:Eu3+phosphors, we measure the PLE spectra of samples with different doping concentrations of Eu3+ions at room temperature.The measurements are monitored at 613-nm emission, and the results are presented in Fig.3(a).In general, the excitation spectra of samples can be mainly divided into two parts.The broadband in the ultraviolet region(200 nm-350 nm)derives from the charge transfer band(CTB)of tungstate ions.[20,21,25]And the other sharp weak peaks at 318 nm,360 nm,384 nm,393 nm,and 464 nm originate from the transitions7F→5D4,7F→5L7,7F→5L6,7F→5D3, and7F→5D2of Eu3+ions,respectively.Figure 3(b)illustrates the emission peaks of samples with different Eu3+concentrations under 264-nm excitation.And the peaks located at 592 nm,613 nm,and 653 nm result from the5D→7F1,5D→7F2,and5D→7F3transition of Eu3+ions respectively.With the increase of Eu3+concentration,the emission intensities initially increase and reach their corresponding maximum values whenx=22, and then decrease due to the concentration quenching effect.The emission intensities of 592 nm and 613 nm are normalized to the emission of Sc2W3O12:22-mol% Eu3+phosphors,and their variations with Eu3+concentration is depicted in Fig.3(c).It can be observed that the emission intensity of 592 nm varying with Eu3+concentration is basically consistent with that of 613 nm.To explore the interaction type of the activator in Sc2W3O12:Eu3+phosphors,the Dexter theory is employed in Fig.3(d):[26-30]
whereIis the integral fluorescence intensity,xrepresents the doping concentration of Eu3+,Ais a constant,andθrefers to the eigenvalue related to the interaction type (θ= 3, 6, 8, 10 corresponding to exchange, dipoledipole, dipole-quadrupole, quadrupole-quadrupole interactions, respectively).[28]According to the fitting result, theθvalue is about 3.0199, suggesting that the concentration quenching of activators in Sc2W3O12:Eu3+phosphors is derived from exchange interaction.

Fig.3.(a) PLE (emitted at 613 nm) and (b) PL (excited by 264 nm) spectra of Sc2W3O12:x-mol% Eu3+ (x=6, 10, 14, 18, 22, 26, 30, 34)phosphors at room temperature.(c)Curves of normalized luminescence intensity at 592 nm and 613 nm varying with doping concentrations.(d)Plot of ln(I/x)versus lnx,and the slope of the fitting line is 1.0065.
3.3.Temperature-dependent PL performances
Taking the Sc2W3O12:6-mol%Eu3+sample for example,the negative thermal expansion behavior of the Sc2W3O12matrix is demonstrated.The Rietveld refinement results(Fig.S4)of variable temperature XRD patterns presented in Fig.4(a)verify the NTE of sample,and the refined lattice parameters as well as the cell volume values are illustrated in Figs.4(b)and 4(c), respectively.Notably, thein-situXRD patterns demonstrate the stable orthorhombic phase of Sc2W3O12at various temperatures.And the lattice of the sample shrinks in the direction of theaaxis andcaxis, and expands slightly in the direction ofbaxis from room temperature to 573 K.Consequently, the cell volume expands until 373 K and further contracts with temperature rising, which indicates the NTE of Sc2W3O12:6-mol% Eu3+phosphors ranging from 373 K to 573 K.As is well known, changes in the crystal structure of materials will inevitably affect its luminescent properties.The temperature-dependent emission spectra of Sc2W3O12:6-mol%Eu3+phosphors under the excitation at 264 nm are delineated in Fig.4(d).Figure 4(e) shows that the emission intensity at 613 nm maintains 86.61% of initial intensity at 373 K, then abnormally rapidly increases with temperatures reaching up to 548 K and the highest emission intensity arrives at 147.8% of initial intensity at 473 K.Moreover, the emission at 592 nm presents a similar trend to that at 613 nm.It is noteworthy that the luminescence intensity attenuating with temperature rising from RT to 373 K can be ascribed to the thermal quenching, and the enhanced luminescence is due to the NTE effect that occurs in a temperature range from 373 K to 548 K.In general, the bridging oxygen of Sc-OW undergoes the transverse vibrations, leading to the coupled tilting of framework polyhedra.[23]As a result,the crystal structure of Sc2W3O12:Eu3+phosphors grows denser and the energy collection of activator is promoted, demonstrating the stronger thermal-quenching resistance of luminescence.[19,22]In addition,we measure the PL spectra of Sc2W3O12:x-mol%Eu3+(x=10, 14, 18, 22, 26, 30) phosphors (Fig.S5).As shown in Fig.4(f), it is obvious that the thermal-quenching resistance of Sc2W3O12:Eu3+decreases with the increase of Eu3+concentration at 298 K-548 K.At low Eu3+concentrations(6 mol%-10 mol%), the PL intensity of the sample can maintain about 85% of initial intensity (in the green dashed box of Fig.4(f)).However, at medium Eu3+concentrations(14 mol%-18 mol%,in the orange dashed box)and high doping concentrations (26 mol%-30 mol%, in the blue dashed box),the PL intensities of the sample can be reduced to about 80% and 67% of initial intensity, respectively.On the other hand, the onset temperature at which luminescence enhancement begins to appear also tends to increase with the Eu3+doping concentration increasing.
To investigate the temperature sensing properties of Sc2W3O12:x-mol% Eu3+phosphors, the contributions of TCEL’s emissions at 592 nm and 613 nm are measured by the FIR technique.The FIR valueRis defined asR=I592nm/I613nm, and depicted in Fig.5(a) and Fig.S6.The relationship betweenRvalue and temperature can be given by the following function:[31]
Here,A,B,andCare the parameters related to the sample.After being fitted by a mono-exponential function, the absolute sensitivity and relative sensitivity are calculated.Generally,these two indicators are employed to evaluate the temperature sensing properties of luminescence materials.They can be estimated by the following equations:[32-34]
The calculated sensitivities are shown in Fig.5(b)and Fig.S6.The corresponding mathematical expressions are listed in Table S1.Comparing the maximum values ofSaandSrof samples under different Eu3+concentrations in Fig.5(c), we can see that the sensitivity of the sample decreases with the increase of Eu3+concentration.When the concentration reaches 22%, the maximum sensitivity begins to increase again.Of all samples in this work, the sensitivity of the sample with 6-mol% Eu3+is the highest, and its maximum values ofSaandSrcan reach 6.872×10-3K-1and 3.063%K-1at 298 K,respectively.In addition, the values ofSaandSrmaintain the downward trends with temperature increasing, which implies possible preferable temperature sensing properties in low-temperature region.

Fig.4.(a)Temperature-dependent XRD patterns of Sc2W3O12:6-mol%Eu3+phosphors.(b)Lattice constants and(c)cell volume values of sample changing with temperatures.(d)Temperature-dependent PL spectra of Sc2W3O12:6-mol%Eu3+under 264-nm excitation.(e)Relative intensity at 592-nm and 613-nm emissions of Sc2W3O12:6 at.%Eu3+ varying with temperatures.(f)Relative intensity at 613 nm of Sc2W3O12:x-mol%Eu3+ (x=6,10,14,18,22,26,30)varying with temperatures,with inset showing enlarged part of curves at temperatures in a range of 350 K-475 K.
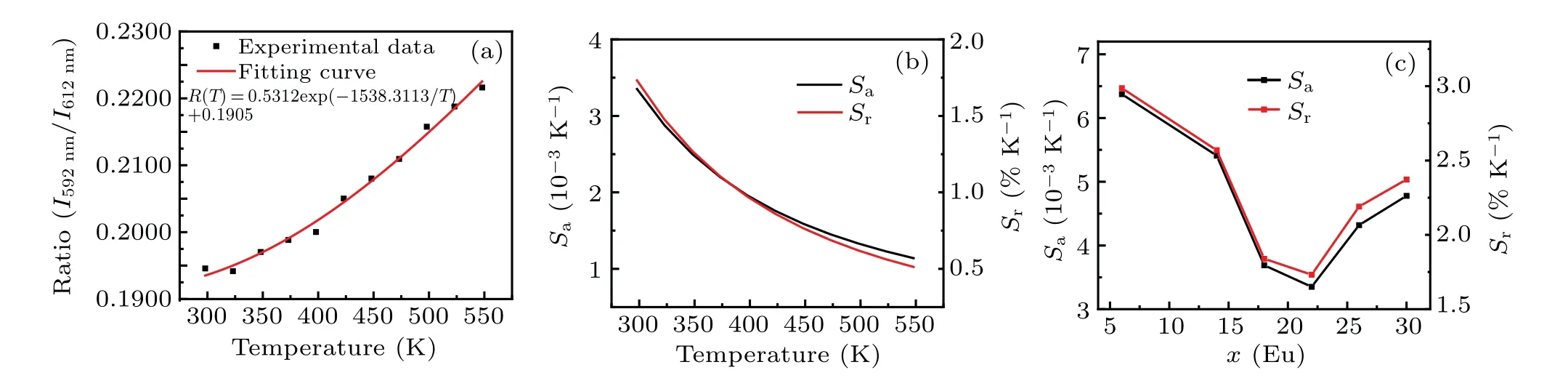
Fig.5.The temperature-dependent(a)PL intensity ratio of 593 nm and 613 nm with fitting results and(b)absolute sensitivity(Sa)and relative sensitivity(Sr)values of Sc2W3O12:22-mol%Eu3+ phosphors,and(c)maximum values of Sa and Sr varying with doping concentrations.
4.Conclusions
In this work, we synthesized negative thermal expansion Sc2W3O12:Eu3+phosphors by the conventional solidstate method.The XRD results reveal that the pure orthorhombic phase can be maintained when being doped with various concentrations of Eu3+ions or elevating temperature.Furthermore, the SEM images and elemental mappings confirm the uniform distributions of Sc, W, O, and Eu ions in samples.Under the 264-nm irradiation, the bright red emission can be observed and the quenching concentration of Eu3+ions is 22 mol%due to the exchange interaction.When the temperature increasing from 298 K,the bridging oxygen of Sc-O-W undergo transverse vibrations,leading to the coupled tilting of framework polyhedron.And the lattices of Sc2W3O12:Eu3+phosphors expand until 373 K and then contract with temperature rising up to 548 K.The emission intensity is abnormally rapidly enhanced with increasing temperature, which is derived from the enhanced energy collection of activators by lattice shrinkage and deformation.The capability of anti-thermal quenching decreases with increasing Eu3+doping concentration.In addition, resulting from the different responses of emissions at 592 nm (5D→7F1) and 613 nm (5D→7F2) to temperature,Sc2W3O12: Eu3+phosphors can be used as optical thermometers based on the FIR technique.The estimated maximum values ofSaandSrof Sc2W3O12:6-mol% Eu3+phosphors are 6.872×10-3K-1and 3.063%K-1at 298 K,respectively,These results demonstrate that the Sc2W3O12:Eu3+phosphors can be used as optical thermometers due to the good temperature sensing properties and thermal-enhanced luminescence performance.
Acknowledgement
Project supported by the National Natural Science Foundation of China(Grant No.51872327).
- Chinese Physics B的其它文章
- Analysis of cut vertex in the control of complex networks
- Atlas of dynamic spectra of fast radio burst FRB 20201124A
- Investigating the characteristic delay time in the leader-follower behavior in children single-file movement
- Micro-mechanism study of the effect of Cd-free buffer layers ZnXO(X =Mg/Sn)on the performance of flexible Cu2ZnSn(S,Se)4 solar cell
- Heterogeneous hydration patterns of G-quadruplex DNA
- Analysis of refraction and scattering image artefacts in x-ray analyzer-based imaging

